Abstract
The “estrogen hypothesis” suggests that estrogen is a protective factor against psychotic disorders such as schizophrenia. Although the precise protective mechanisms are still unclear, one potential explanation lies in the role that increased estrogens play in mediating hippocampal plasticity, as this may reduce hippocampal dysconnectivity that is characteristically observed in psychosis. In support of this view, later age at menarche- less available estrogen during critical early adolescent development- is related to earlier onset of psychosis and increased symptom severity. Furthermore, if estrogens have protective effects, then we should see this effect in the psychosis risk period in those at clinical high-risk (CHR) for psychosis – i.e., individuals showing attenuated symptoms at imminent risk for transitioning to a psychotic diagnosis. This study examined whether earlier age at menarche would result in more normative hippocampal connectivity in CHR youth; menarche is an easily assessed, developmental marker associated with the availability of estrogens. Resting-state connectivity was examined in sixty female participants (26 CHR and 34 healthy control; age 12–21) using a cross-sectional approach; hippocampal connectivity was found to relate to age at menarche. Later age at menarche in the CHR group related to increased hippocampal dysconnectivity to the occipital cortex (a region with a neurotrophic response to estrogen) compared to the controls. Results suggest that earlier availability of estrogens may have neuroprotective effects on hippocampal plasticity. Findings have relevance for understanding sex differences and etiology, as well as guiding novel treatments.
1. Introduction
The “estrogen hypothesis” posits that estrogens serve as a protective factor against psychotic disorders, but the mechanisms underlying this effect remain poorly understood. This theory highlights the potential importance of estrogens as neuroprotective- promoting healthy connectivity during development (Biegon and McEwen, 1982; Gould et al., 1990; McEwen, 2001). Estrogen has a number of actions on the brain, but the hippocampus is a major site of overlapping impacts of estrogen. Indeed, increased estrogens mediate hippocampal plasticity – through synaptogenesis, (Woolley, 1998) spine density (Gould et al., 1990), and neuroprotective influences (Diaz Brinton et al., 2000). The culmination of estrogen effects may reduce hippocampal dysconnectivity observed in psychosis (Bähner and Meyer-Lindenberg, 2017; Benetti et al., 2009). This hormonal impact has particular relevance for individuals at clinical high-risk (CHR) for psychosis- adolescents and young adults who show emerging low-level psychotic symptoms coupled with a decline in functioning. CHR individuals are in a period characterized by pubertal hormone development (Trotman et al., 2013; Walker et al., 2013), and show significant hippocampal abnormalities (Dean et al., 2016; Mittal and Walker, 2011; Vargas et al., 2018). Furthermore, CHR represent a population at imminent risk for transition to psychosis with as many as 10–30% developing a psychotic disorder in a one to two year period (Hengartner et al., 2017). Despite these intersections, to date, there have been no empirical studies aimed at testing the estrogen hypothesis in CHR individuals. To address this question, this study examined hippocampal resting-state connectivity in CHR and matched healthy control (HC) individuals by comparing time since menarche (duration of estrogen availability) and age at menarche (developmental timing of estrogen availability).
Low-level psychotic symptoms tend to emerge in a prodromal period during late adolescence to early adulthood, which is a developmental period that is also characterized by neurobiological changes and the onset of menarche (Walker et al., 2013). In fact, a growing body of research supports a diathesis-stress model, suggesting that neurobiological development during this period may interact with early risk factors leading to the onset of psychosis (Trotman et al., 2013; Walker et al., 2013). In the case of menarche, however, the prodromal period may also be a window into potential protective factors that reduce or delay the onset of psychosis in women (Seeman, 1996; Trotman et al., 2013). This period is also a unique window to examine both risk and protective mechanisms with fewer confounds that are often associated with psychosis onset (Insel, 2010; Mittal and Walker, 2007). Despite the benefits of examining CHR individuals, evidence for the estrogen hypothesis (Seeman, 1996) has been largely been limited to retrospective investigations in individuals with psychosis. These studies have demonstrated that women show a later and less severe onset of psychosis (Hayes et al., 2012; Hochman and Lewine, 2004; Lewine, 2004; Seeman, 1996) and a second peak of onset during peri-menopause (Huber et al., 2001). Although much of the research on the estrogen hypothesis has been focused on menopause, prior to menopause women with a psychosis diagnosis are more likely to have a psychosis relapse in low estrogen points in their menstrual cycle (Huber et al., 2001; Seeman, 1996) and during estrogen withdrawal post-partum (Mahé and Dumaine, 2001). The few studies that focused on the protective role of menarche in psychosis have largely examined individuals with psychosis after onset, (Kilicaslan et al., 2014; Cohen et al., 1999a) and have yet to examine potential underlying mechanisms.
Studies that have examined the timing of menarche suggest that an earlier age at menarche relates to a later onset of psychosis (Cohen et al., 1999a; Kilicaslan et al., 2014) and less severe symptomatology (Hochman and Lewine, 2004) in retrospective clinical accounts of hospitalized individuals with psychosis (Hochman and Lewine, 2004; Cohen et al., 1999a; Kilicaslan et al., 2014); while informative, these studies did not account for potential biological mechanisms related to menarche. However, based on extant literature on the estrogen hypothesis, early timing of menarche may be beneficial because estrogens exert a protective effect at a critical time prior to psychosis deterioration (Wei and Berman, 2018). Additionally, these studies did not examine individuals with a variety of ages at first menarche or a wide array of current age. Therefore it remains unknown if earlier menarche reflects a neuroprotective impact co-occurring with critical neurodevelopment in early adolescence or if early menarche leads to a greater protective impact against emerging psychosis symptoms with each menstrual cycle (Maric et al., 2005). No study has directly examined whether protective effects are related to the developmental timing of menarche or the duration of estrogen availability, which may have important implications for the mechanisms underlying this protective effect.
As previously noted, there are a number of potential mechanisms by which menarche may have a protective effect during the prodromal period (Becker, 1990; Biegon and McEwen, 1982; Diaz Brinton et al., 2000; Gould et al., 1990), including progesterone and the insulin-like growth factor system. Despite this wide array of potential specific mechanisms, resting state connectivity may be sensitive to the culmination of these effects (Cole et al., 2013; Schaefer et al., 2014). The effect of menarche on resting state connectivity may be particularly relevant for the prodromal period, as functional dysconnectivity has been widely noted in psychosis. In a parallel line of research, psychosis has been characterized as a disorder of dysconnectivity (Friston et al., 2016) resulting from altered neurodevelopmental trajectories (Insel, 2010). Critically, the hippocampus has been widely noted as both a major site of action for estrogens (Diaz Brinton et al., 2000; Gould et al., 1990; Lisofsky et al., 2015; Sato et al., 2007; Woolley, 1998) and dysconnectivity in individuals with psychosis (Bähner and Meyer-Lindenberg, 2017; Benetti et al., 2009; Kraguljac et al., 2016; Lisofsky et al., 2015; Wood et al., 2005). Studies of psychosis have noted hippocampal dysconnectivity between several cortical regions (Kraguljac et al., 2016; Wood et al., 2005;,Bernard and Mittal, 2015; Winton-Brown et al., 2017), including the frontal cortex (Bähner and Meyer-Lindenberg, 2017; Benetti et al., 2009; Samudra et al., 2015). Connectivity of the hippocampus to the frontal cortex changes during adolescence through early adulthood, (Allen et al., 2012; van Duijvenvoorde et al., 2016) and may be particularly impacted by the protective effects of estrogen. Hippocampal abnormalities have also been noted in individuals at clinical high-risk for psychosis (Dean and Mittal, 2015; Vargas et al., 2018). Past studies, however, have not examined the potential for estrogen to impact hippocampal dysconnectivity in CHR individuals (Blankenship et al., 2017; van Duijvenvoorde et al., 2016).
The current paper examines how hippocampal dysconnectivity in CHR individuals relates to age at scan, time since menarche, and age at menarche. Hippocampal connectivity increases over development, but if CHR status reflects growing dysconnectivity over neurodevelopment, then we may see distinct (decreasing or no normative increase) hippocampal connectivity in the CHR group by age at scan. Findings from this analysis will also be used to conceptualize the alterations in hippocampal connectivity related to estrogen in terms of dysconnectivity. A second analysis tests the prediction that earlier neurodevelopmental timing (younger age at menarche) is related to increased hippocampal connectivity. Finally, the last analysis will examine if longer time since menarche relates to increased hippocampal connectivity in CHR individuals.
2. Materials and Methods
2.1. Participants.
Sixty female participants, 26 CHR and 34 healthy control, were recruited through the Adolescent Development and Preventive Treatment (ADAPT) Program. The local institutional review board reviewed and approved all procedures. If participants were 18 years old or older then the participants provided written consent to participate. If participants were under the age of 18, then a parent provided written consent and participants provided written assent. Exclusionary criteria for the neuroimaging protocol included presence of a neurological disorder, lifetime substance dependence, head injury, or the presence of any other contraindication to a magnetic resonance imaging environment. For healthy control participants, exclusion criteria also included the presence of a psychotic disorder in a first-degree relative as this may reflect latent genetic risk. Additionally, participants were excluded for the presence or lifetime history of an Axis I psychotic disorder. All participants are between ages 12–21 (M=18.1, SEM=0.23). All analyses were run with and without individuals taking antipsychotics or birth control, which did not impact the magnitude or direction of the findings reported below, Table 1. To preserve power and transparency, the following results include all subjects for whom data was available.
Table 1.
Sample Demographics, Medications, and Diagnoses
| Study Sample | CHR | Healthy Volunteers | ||
|---|---|---|---|---|
| n=60 | n=26 | n=34 | Group Comparison | |
| Age -Mean (StDev)) | 18.27(2.26) | 18.17(1.92) | 18.34(2.47) | t(58)=0.25, p=.81 |
| SES | Study Sample | CHR | Healthy Volunteers | χ2=7.19, p=.21 |
| >10,000 | 9 | 1 | 8 | |
| 10,000–19,000 | 3 | 1 | 2 | |
| 20,000–39,999 | 15 | 8 | 7 | |
| 60,000–99,999 | 11 | 4 | 7 | |
| 100,000+ | 12 | 3 | 9 | |
| Preferred not to Answer | 10 | 9 | 1 | |
| Race/Ethnicity | Study Sample | CHR | Healthy Volunteers | χ2=6.69, p=.35 |
| Asian | 7 | 2 | 5 | |
| East Asian | 5 | 2 | 3 | |
| Southeast Asian | 2 | 0 | 2 | |
| Black | 3 | 1 | 2 | |
| Central/South American | 14 | 4 | 10 | |
| Hispanic | 14 | 4 | 10 | |
| Non-Hispanic | 0 | 0 | 0 | |
| First Nation | 2 | 2 | 0 | |
| Interracial | 1 | 1 | 0 | |
| Hispanic | 1 | 1 | 0 | |
| Non-Hispanic | 0 | 0 | 0 | |
| White | 31 | 14 | 17 | |
| Medication | % of study sample | CHR | Healthy Volunteers | |
| Antipsychotics | 1.67% | 1 | 0 | |
| Birth Control | 16.67% | 5 | 5 | |
| Antidepressants | 16.67% | 8 | 2 | |
| Antianxiety | 5.00% | 3 | 0 | |
| Any Diagnosis | % of study sample | CHR | Healthy Volunteers | |
| 33.33% | 20 | 6 | ||
| Current Axis 1 Diagnois | % of study sample | CHR | Healthy Volunteers | |
| Generalized Anxiety Disorder | 8.33% | 5 | 0 | |
| Major Depression Disorder | 6.67% | 4 | 0 | |
| Obessive Compulsive Disorder | 5.00% | 3 | 0 | |
| Post-Traumatic Stress Disorder | 5.00% | 3 | 0 | |
| Panic Disorder | 5.00% | 3 | 0 | |
| Social Phobia | 5.00% | 3 | 0 | |
| Bipolar disorder | 3.33% | 2 | 0 | |
| Cannabis Abuse | 3.33% | 2 | 0 | |
| Specific Phobia | 3.33% | 2 | 0 | |
| Dysthmia | 1.67% | 1 | 0 | |
| Alcohol Dependence | 1.67% | 1 | 0 | |
| Agoraphobia | 1.67% | 1 | 0 | |
| Somatoform Disorder | 1.67% | 1 | 0 | |
| Eating Disorder | 1.67% | 1 | 0 | |
| Schizotypal | 1.67% | 1 | 0 | |
| Adjustment Disorder | 1.67% | 1 | 0 | |
| Past Axis 1 Diagnois | % of study sample | CHR | Healthy Volunteers | |
| Major Depression Disorder | 26.67% | 12 | 4 | |
| Alcohol Dependence | 3.33% | 2 | 0 | |
| Alchohol Abuse | 5.00% | 2 | 1 | |
| Cannabuse Dependence | 3.33% | 2 | 0 | |
| Hypochondriases | 1.67% | 1 | 0 | |
| Bipolar disorder | 1.67% | 1 | 0 | |
| Panic Disorder | 3.33% | 1 | 1 |
2.2. Clinical Assessments.
The Structured Clinical Interview for DSM-IV Axis I disorders (SCID) was administered to all subjects to rule out a psychosis diagnosis and to assess history of mood and anxiety disorders in both groups (Gibbon et al., 1997). Similarly, CHR and healthy control participants also completed the Structured Interview for Psychosis-Risk Syndromes (SIPS) in order to diagnose CHR subjects and rule out symptoms in healthy controls (Miller et al., 2003). Finally, all participants completed the Puberty Development Scale which is a 5-item self-report scale that assesses pubertal development and inquires the age of menarche (Carskadon and Acebo, 1993).
2.3. MRI Acquisition.
Images were acquired in a 3T Siemens Tim Trio MRI Scanner (Siemens AG, Munich, Germany with a standard 12-channel head coil, which included a resting-state functional image and structural image for registration. The resting-state sequence was acquired with a T2*-weighted echo-planar functional protocol (33 slices in a 240 mm field of view; 3.8×3.8×3.5 mm voxels; TR=2.00 s; TE=29 ms; Flip Angle=75°), while participants closed their eyes during the 5 min 34 s scan. The structural image was a MPRAGE (sagittal acquisition, 192 interleaved slices; 256 mm field of view; isotropic voxels 1mm3, GRAPPA parallel imaging factor of 2, Time to repetition (TR)=2.530 s; Times to Echo (TE)=1.64 ms, 3.5 ms, 5.36 ms, 7.22 ms, 9.08 ms; flip angle=7°).
2.4. fMRI Data Preparation.
All functional data were preprocessed in FSL v.6, which included brain extraction, a 100s high-pass filtering, and 6-mm FWHM kernel spatial smoothing (Jenkinson et al., 2012). To register and co-register images, functional images were aligned to the structural image, before alignment to the Montreal Neurological Institute 2-mm brain template in a two-step process. At the subject-level, a set of temporal derivative regressors were generated for each subject, which accounted for motion. These regressors were calculated using the ART - artifact detection software, resulting in three translation and three rotation parameters, and confound regressors which noted outliers in brain activation or framewise movement. Brain activation outliers were identified by comparing each voxel to the z-normalized mean signal across all voxels as a function of time; a voxel was considered an outlier if the exceeded a z-score of 3. For motion outliers, a composite measure of total motion, or maximum voxel displacement, across translation and rotation identified any motion outliers as frames where the absolute value of motion exceeded 1-mm.
2.5. Connectivity Analyses.
The study examined whether associations between hippocampal connectivity varied by group along three separate dimensions (age, age at menarche, time since menarche) in three separate analyses. Hippocampal to whole brain connectivity (ROI to voxel) was analyzed as hippocampal connectivity changes in a number of regions over development as well as in response to ovarian hormones and emerging psychosis; accordingly, these analyses should be considered exploratory. Connectivity analyses were conducted in CONN toolbox ROI-to-whole brain analyses v.18.b (Whitfield-Gabrieli and Nieto-Castanon, 2012), which calculated denoising variables that were used in addition to the motion variables discussed above. Additional regressors included grey matter, white matter, cerebrospinal fluid (CSF), and mean global signal regressors by extracting signal from segmenting structural masks for gray matter, white matter, and CSF. Structural images were segmented into gray matter, white matter, and CSF with SPM8 in order to create masks for signal extraction. Then CONN toolbox uses principal components analysis to extract five temporal components from the segmented CSF and white matter, which were entered as confound regression in the subject-level general linear model (GLM). Right and left hippocampal regions of interest (ROI) were defined using the FreeSurfer Atlas (Desikan et al., 2006; Fischl, 2012). The mean time-series, averaged across all voxels within each (right and left) hippocampal seed ROI were used as regression parameters, which were correlated with all other voxels in the brain in seed-tovoxel connectivity analyses. These connectivity values were then used as the outcome variables in a group by age or age at menarche interaction analyses, and at the group level all variables were entered simultaneously.
Group level covariates of interest (age at scan, time since menarche, age at menarche) were mean-centered (z-score) to maximize the sensitivity of the model; mean-centering has been found to be an effective way to reduce co-linearity and increase power for detecting signal in resting-state functional connectivity (Mumford et al., 2015). For the age at menarche analyses, the age at scan variable was entered as a nuisance covariate to identify the independent effects of age of menarche beyond the expected effects related to attenuated psychotic symptoms over time (age at scan). Similarly, in the age at scan analyses, the age at menarche variable was entered as a nuisance covariate to identify the independent effects of age beyond the expected effects related to menarche on hippocampal connectivity. In the time since menarche analyses, the age at menarche and age at scan were both used to calculate the cumulative effect of estrogens, and as a result, no nuisance variables were included. All findings were thresholded at the voxel-level at puncorr< .001 with a cluster-level correction at a false-discovery rate (FDR) of p < .05 (Chumbley and Friston, 2009).
3. Results
3.1. Participants.
This sample included sixty female participants (26 CHR and 34 HC), Table 1. The sample age ranged from 12–21 years of age (M=18.27, SD=2.26), and there were no significant differences in age between groups, t(58)=0.25, p=.81. The sample also included a range of ages of first menarche 11–16 (M=12.43, SD=1.68, skewedness=.20, kurtosis=.25), and there were no significant differences in age at menarche between groups, t(58)=0.57, p=.57, or in terms of time since menarche (range=0–10 years, M=5.84, SD=2.28, skewedness=−1.20, kurtosis=.52), t(58)=.86, p=.87. When compared to healthy controls, the CHR group had significantly greater SIPS Positive (CHR M=11.45, StD=3.45; healthy controls M=0.57, StD=1.1; t(58)=14.54, p<.001) and SIPS Negative (CHR M=10.23, StD=6.68; healthy controls M=0.35, StD=0.75; t(58)=8.31, p<.001) total symptoms scores. During the resting state scan, there was no significant difference between CHR (M=0.28, StD=0.18) and healthy volunteers (M=0.26, StD=0.38) in total framewise displacement (t(60)=0.22, p=.82). For more details on the current sample, see Table 1.
3.2. Hippocampal Connectivity Related to Age by Group.
To assess the impact of age at scan (independent of age at menarche) on hippocampal connectivity across CHR and HC groups, a mean-centered age at scan covariate was compared across groups to predict any connectivity effect of hippocampus (right and left) controlling for the impact of age at menarche as a nuisance covariate. This analysis will help us contextualize the hormonal analyses in the presence of hippocampal dysconnectivity in the CHR group. There was a significant group by age at scan interaction which related to hippocampal connectivity to two frontal clusters, Figure 1A. One cluster was in the superior frontal gyrus/frontal pole (Cluster Size: 167; MNI Coordinates [XYZ]: −18, 48, 46; p-FWE=.01; p-FDR=.01; puncorrected= .005; Figure 1B), and the second cluster was in the ventral medial prefrontal (VMPFC; Cluster Size: 110; MNI Coordinates [XYZ]: −6, 54, −16; p-FWE=.07; p-FDR=.04; puncorrected= .003; Figure 1B). Increased hippocampus to frontal cluster connectivity was related to an older (increased) age at scan in the control group, but not in the CHR group (Figure 1B).
Figure 1.
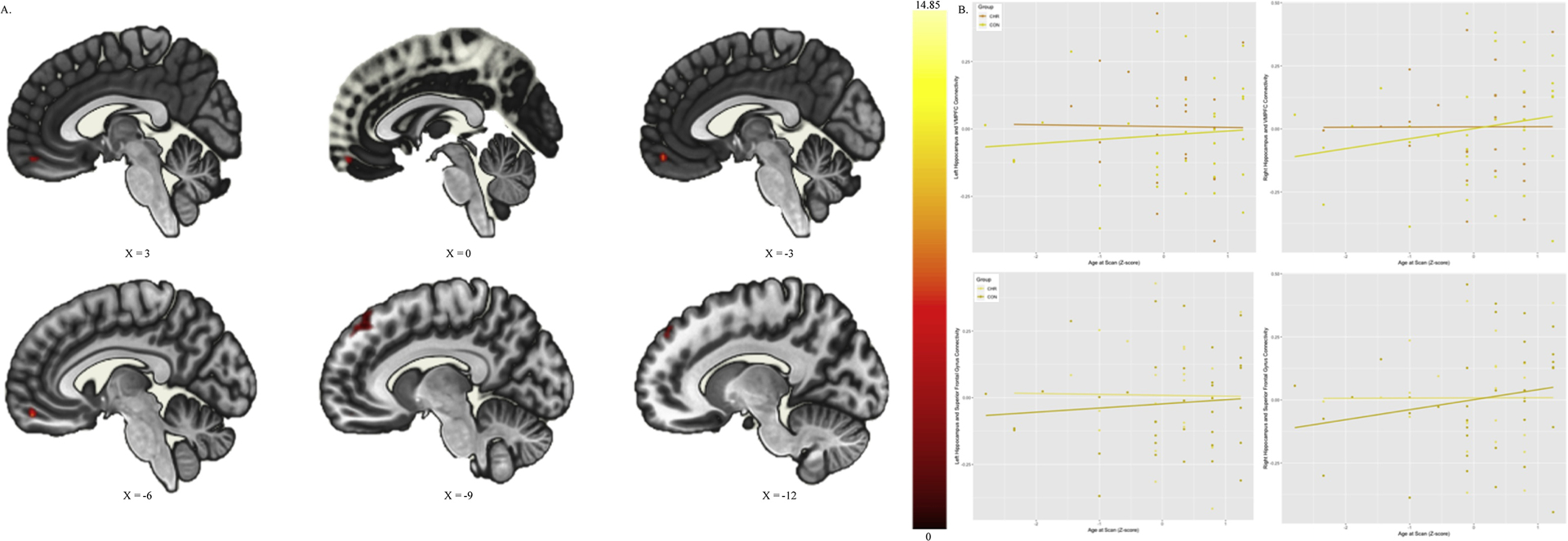
Hippocampal Connectivity Related to Age at Scan by Risk Group: A. Depicts the Superior Frontal Gyrus/Frontal Pole and Ventral Medial Prefrontal Cortex (VMPFC) Cluster where Hippocampal connectivity varies by Group and Age B. Depicts the interaction in connectivity with controls and CHR fit with separate lines
3.3. Hippocampal Connectivity Related to Age at Menarche by Group.
To assess the impact of age at menarche (independent of age at scan) on hippocampal connectivity across CHR and HC groups, a mean-centered age at menarche covariate was compared across groups to predict any connectivity effect of hippocampus (right and left) controlling for the impact of age at scan as a nuisance covariate. There was a significant group by age at menarche interaction which related hippocampal connectivity to an occipital lobe cluster-a major site of neurotrophic estrogen effects (Cluster Size: 187; MNI Coordinates [XYZ]: 34, −80, 20; p-FWE=.007; p-FDR=.009; p=.002; Figure 2A). Increased hippocampus to occipital lobe connectivity was related to an older (increased) age at menarche. Although both groups show a positive relationship between age at menarche and connectivity, this effect was increased in the CHR group (Figure 2B).
Figure 2.
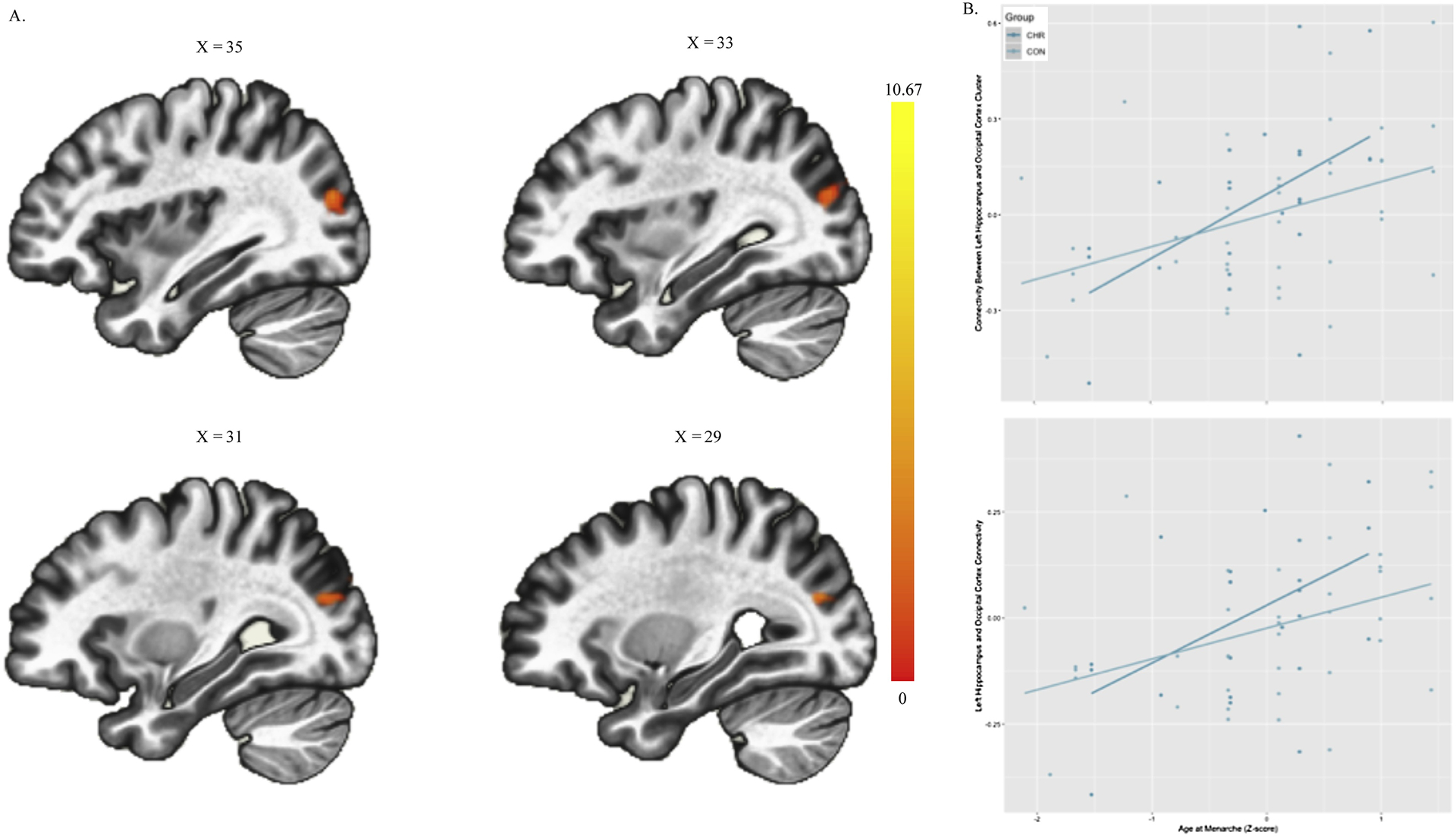
Hippocampal Connectivity Related to Age of Menarche by Risk Group: A. Depicts the Occipital Cluster where Hippocampal connectivity varies by Group and Age at Menarche B. Depicts the interaction in connectivity with controls and CHR fit with separate lines
3.4. Hippocampal Connectivity Related to Time Since Menarche by Group.
To assess the cumulative impact of the availability of estrogens (length of time since menarche) on hippocampal connectivity across CHR and HC groups, a mean-centered time since at menarche covariate was compared across groups to predict any connectivity effect of hippocampus (right and left). There was a no significant group by age at menarche interaction.
4. Discussion
Risk for psychosis is related to hippocampal dysconnectivity; this finding is consistent with previous studies of psychosis (Bähner and Meyer-Lindenberg, 2017; Benetti et al., 2009). Hippocampal connectivity in the CHR group did not show the increase over age that typically occurs over healthy development, which was seen in the healthy group, consistent with the neurodevelopmental hypothesis of psychosis (Trotman et al., 2013; Walker et al., 2013). In females at risk for psychosis, hippocampal dysconnectivity may be mitigated by estrogen’s impact on the brain (i.e., the estrogens hypothesis), but no previous work has directly examined this possibility (Hayes et al., 2012; Seeman, 1996; Trotman et al., 2013). Additionally, the impact of estrogen may be particularly related to the timing of menarche during co-occurring neurodevelopmental processes (age at menarche), which was associated with more normative hippocampal connectivity. The current study is the first to examine if hippocampal dysconnectivity is present in the CHR population, and then examining whether hippocampal connectivity was impacted by either the timing of estrogens availability (age at menarche) or the cumulative effects of estrogens (time since menarche). In summary, there was evidence of hippocampal dysconnectivity in the CHR group, as well as a protective impact of earlier exposure to estrogens (earlier age at menarche). No similar benefits were found in support of a cumulative effect of exposure to estrogens (time since menarche). Collectively, the current study suggests that, despite evidence of hippocampal dysconnectivity (age at scan), earlier timing of estrogens availability (age at menarche) -not the cumulative effect of estrogens availability (time since menarche)- may have protective effects on hippocampal connectivity in women at clinical high-risk for psychosis.
The first analyses confirmed the presence of the expected hippocampal dysconnectivity in the CHR group. The current study found that CHR individuals did not show a normative increase in hippocampal connectivity over development (age at scan), which was seen in the controls as increased hippocampal connectivity to superior and orbital frontal regions over development (age at scan). Typically in adolescent development there is a positive increase in connectivity between the hippocampus and medial/orbitofrontal regions and a positive relationship to superior dorsal frontal regions (van Duijvenvoorde et al., 2016). In the current study the healthy controls group show this expected increase in connectivity by age, converging with previous findings (van Duijvenvoorde et al., 2016). The CHR group, however, showed no age-related increase in hippocampal connectivity to these regions, which may suggest a lack of normative hippocampal connectivity development in the CHR group. Additionally, the current findings are consistent with similar literature implicating frontal-hippocampal connectivity along a risk spectrum in the pathophysiology of psychosis (Benetti et al., 2009; Samudra et al., 2015). Collectively, these findings provide further evidence for the developmental dysconnectivity perspective and emphasize the potential importance of fronto-hippocampal connectivity in CHR for psychosis.
Despite the presence of hippocampal dysconnectivity over development, there was a predicted increased connectivity related to age at menarche (i.e., more normative connectivity) in the CHR group even when accounting for the connectivity related to age. Specifically, earlier age at menarche was associated with more normative hippocampal connectivity with the occipital lobe (another major site of neuroprotective effects of estrogens; Diaz Brinton et al., 2000). This effect was especially pronounced in the CHR group. In the CHR group, early age at menarche was associated with more normative hippocampal-occipital connectivity, than later age at menarche. This finding is consistent with the hypothesis that the developmental timing of menarche may provide protective benefits to hippocampal connectivity (Trotman et al., 2013; Walker et al., 2013; Wei and Berman, 2018). The specific site of connectivity impact, i.e. hippocampal-occipital lobe connectivity increasing with less time since menarche, was unexpected. Despite this effect being unexpected, it is notable that both the hippocampus and occipital lobe are major sites of neuroprotective effects of estrogens (Diaz Brinton et al., 2000); as a result, this connectivity may reflect the added benefit of neuroprotection in the CHR group. This finding suggests that the estrogens may maintain more normative connectivity prior to the early reductions in hippocampal volume (Vargas et al., 2018), but that later menarche may be occurring after volume reductions have resulted in abnormal connectivity.
Time since menarche did not relate to increased hippocampal connectivity, as expected, in the current analyses. Given the results in the age of menarche analyses, these data suggest that the impact on hippocampal connectivity may be specific to the timing of menarche (age at menarche) and not the time since menarche. This finding is in contrast with previous work by Maric et al. (2005), who found that the cumulative effect of estrogens – as measured by bone density- suggested that the cumulative effect of estrogens was reduced in the first episode of psychosis. There are, however, many notable differences between these approaches that may account for this inconsistency. First, it remains possible that, beyond hippocampal connectivity, the cumulative effect of estrogens may relate to early psychosis in other ways (Maric et al., 2005). Second, the cumulative effect of estrogens may reflect both levels and duration of estrogen availability, rather than the duration that estrogens are available only. In fact, Maric et al. (2005) controlled for time since menarche and current age in their reported analyses, and so variance related to the time since may be accounted for in the nuisance variance potentially reflecting estrogens dose over duration. Finally, time since menarche may reflect a smaller effect as menarche is a broader measure of a number of effects related to processes starting at menarche that are often cyclical in nature, rather than a cumulative effect of levels and duration of estrogen levels as in bone density measures. Future work should examine approaches that may address questions of duration and dosage of estrogens in CHR individuals.
In conclusion, the developmental timing of menarche may be a critical feature to the protective impact of estrogens on hippocampal connectivity. This finding emphasizes the need to consider disease etiology within a developmental context (Mittal and Wakschlag, 2017; Trotman et al., 2013; Walker et al., 2013), which can be done even with easily assessed, general metrics of hormonal development (e.g., menarche). This paper also emphasizes the critical importance of examining biological mechanisms; especially as there were no main group differences in age at menarche or duration of menarche. This lack of a group difference in overall menarche may also emphasize that protective factors should be considered within the context of their potential sites of impact. Indeed, the onset of menarche may be occurring near the pial of gray matter volume just prior to normative volume reductions and white matter growth (Giedd et al., 2012, 1999). Additionally, there is evidence that during this prodromal period individuals at risk for psychosis show excessive gray matter pruning and abnormal myelination (Insel, 2010; Trotman et al., 2013; Walker et al., 2013). As a result, the protective effects of estrogens may be interacting with ongoing disease processes in CHR individuals (as evidenced by the age at scan) to mitigate risk for psychosis (Trotman et al., 2013).
Taken together, the current analyses emphasize the importance of timing of menarche, but there are still a number of limitations in the current approach. The current study did not gather information regarding subject’s current stage of menstrual cycle, features of the menstrual cycle (e.g., duration, regularity), or current peripheral hormone levels, which may have an impact on hippocampal connectivity differences (Gould et al., 1990; Lisofsky et al., 2015; Sato et al., 2007; Woolley, 1998). As a result, the current variables should be considered a developmental proxy for hormone levels and caution should be used in attributing these effects to estrogens or hormone levels specifically. Future studies may benefit from larger sample sizes to address questions of cycle stage, estrogen cycle features, along with other factors that may influence estrogen (e.g., body mass index) and relations to symptoms. It is important to note, however, that the CHR group itself is largely defined by the presence of positive symptoms; as a result, one can assume that the effects of group reflect differences in the positive symptom domain with respect to age at menarche and hippocampal connectivity. This assumption may be aided by larger samples (including large consortium investigations such as ProNET and NAPLS) as well as studies including estrogen assays that will be invaluable for further elucidating our understanding in this area. Notably, the current study has a sample size that is similar to or larger than other studies examining menarche in a psychosis sample but it is smaller than the epidemiological examination of menarche and psychosis (Cohen et al., 1999; Maric et al., 2005). As a result, the conclusion of the current study should be replicated in independent samples to ensure that this effect is robust. Although the examined medications (i.e., birth control and antipsychotics) did not impact the magnitude or direction of findings, future studies should consider examining medication free samples.
It is noteworthy that age at menarche is not specific in terms of timing and availability of estrogen. Additionally, estrogen may behave differently across development in terms of neuroprotective effects, and further, other neuroactive factors related to menarche including progesterone and the insulin-like growth factor system may also contribute to alterations in connectivity. As a result, these results could be considered preliminary, and at best, reflective of ovarian hormones more broadly. Future studies should also examine specific assays across multiple hormones/neuroactive factors and multiple stages of adolescence, which would inform us of the particular impact of menarche. For now, the current paper is the first to suggest that there is promising signal in this area that should inspire sorely needed future research in this area.
The current study also highlights a number of potential effects that the current sample is not well suited to examine. Future studies should examine whether estrogen cycle hippocampal synaptogenesis has lasting impacts on hippocampal connectivity. Given that the synaptogenesis effects of estrogen occur cyclically, future studies should measure changes in hippocampal connectivity longitudinally within an individual over multiple points in their estrogen cycle. In addition to examining multiple points in the cycle, future studies should examine multiple sites of estrogens action. Although the current study focused on the hippocampus as a candidate site of mechanism, there are a number of sites of estrogens effect, which should be explored in future studies with larger sample sizes. Additionally, despite the age at scan and age at menarche analyses accounting for variance related to the other variable, it did not illuminate a potential role of estrogens as protective factors on hippocampal connectivity over age. Future studies should consider examining individuals longitudinally to increase sensitivity to the impact of menarche on hippocampal connectivity.
Highlights.
Hippocampal dysconnectivity appears to be present in the prodromal period prior to onset of psychosis.
Earlier availability of estrogens may have neuroprotective effects on hippocampal plasticity in individuals at clinical high risk for psychosis.
Acknowledgments
This work was supported by the National Institutes of Mental Health (Grant R01s. MH094650, MH112545–01, MH103231, MH112545, MH094650, R21/R33MH103231. We have no conflicts to disclose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen P, Luigjes J, Howes OD, Egerton A, Hirao K, Valli I, Kambeitz J, Fusar-Poli P, Broome M, McGuire P, 2012. Transition to Psychosis Associated With Prefrontal and Subcortical Dysfunction in Ultra High-Risk Individuals. Schizophr Bull 38, 1268–1276. 10.1093/schbul/sbr194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bähner F, Meyer-Lindenberg A, 2017. Hippocampal–prefrontal connectivity as a translational phenotype for schizophrenia. European Neuropsychopharmacology 27, 93–106. 10.1016/j.euroneuro.2016.12.007 [DOI] [PubMed] [Google Scholar]
- Becker JB, 1990. Direct effect of 17β-estradiol on striatum: Sex differences in dopamine release. Synapse 5, 157–164. 10.1002/syn.890050211 [DOI] [PubMed] [Google Scholar]
- Benetti S, Mechelli A, Picchioni M, Broome M, Williams S, McGuire P, 2009. Functional integration between the posterior hippocampus and prefrontal cortex is impaired in both first episode schizophrenia and the at risk mental state. Brain 132, 2426–2436. 10.1093/brain/awp098 [DOI] [PubMed] [Google Scholar]
- Bernard JA, Mittal VA, 2015. Dysfunctional Activation of the Cerebellum in Schizophrenia: A Functional Neuroimaging Meta-Analysis. Clinical Psychological Science 3, 545–566. 10.1177/2167702614542463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biegon A, McEwen BS, 1982. Modulation by estradiol of serotonin receptors in brain. J. Neurosci 2, 199–205. 10.1523/JNEUROSCI.02-02-00199.1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenship SL, Redcay E, Dougherty LR, Riggins T, 2017. Development of hippocampal functional connectivity during childhood. Human Brain Mapping 38, 182–201. 10.1002/hbm.23353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carskadon MA, Acebo C, 1993. A self-administered rating scale for pubertal development. Journal of Adolescent Health 14, 190–195. 10.1016/1054-139X(93)90004-9 [DOI] [PubMed] [Google Scholar]
- Chumbley J, Friston K, 2009. False discovery rate revisited: FDR and topological inference using Gaussian random fields. NeuroImage 44, 62–70. 10.1016/j.neuroimage.2008.05.021 [DOI] [PubMed] [Google Scholar]
- Cohen RZ, Seeman MV, Gotowiec A, Kopala L, 1999a. Earlier Puberty as a Predictor of Later Onset of Schizophrenia in Women. AJP 156, 1059–1065. 10.1176/ajp.156.7.1059 [DOI] [PubMed] [Google Scholar]
- Cohen RZ, Seeman MV, Gotowiec A, Kopala L, 1999b. Earlier Puberty as a Predictor of Later Onset of Schizophrenia in Women. AJP 156, 1059–1065. 10.1176/ajp.156.7.1059 [DOI] [PubMed] [Google Scholar]
- Cole DM, Beckmann CF, Oei NYL, Both S, van Gerven JMA, Rombouts SARB, 2013. Differential and distributed effects of dopamine neuromodulations on resting-state network connectivity. NeuroImage 78, 59–67. 10.1016/j.neuroimage.2013.04.034 [DOI] [PubMed] [Google Scholar]
- Dean DJ, Mittal VA, 2015. Spontaneous parkinsonisms and striatal impairment in neuroleptic free youth at ultrahigh risk for psychosis. NPJ Schizophr 1, 14006 10.1038/npjschz.2014.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean DJ, Orr JM, Bernard JA, Gupta T, Pelletier-Baldelli A, Carol EE, Mittal VA, 2016. Hippocampal Shape Abnormalities Predict Symptom Progression in Neuroleptic-Free Youth at Ultrahigh Risk for Psychosis. Schizophr Bull 42, 161–169. 10.1093/schbul/sbv086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ, 2006. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage 31, 968–980. 10.1016/j.neuroimage.2006.01.021 [DOI] [PubMed] [Google Scholar]
- Diaz Brinton R, Chen S, Montoya M, Hsieh D, Minaya J, Kim J, Chu H-P, 2000. The women’s health initiative estrogen replacement therapy is neurotrophic and neuroprotective. Neurobiology of Aging 21, 475–496. 10.1016/S0197-4580(00)00109-3 [DOI] [PubMed] [Google Scholar]
- Fischl B, 2012. FreeSurfer. NeuroImage, 20 YEARS OF fMRI 62, 774–781. 10.1016/j.neuroimage.2012.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K, Brown HR, Siemerkus J, Stephan KE, 2016. The dysconnection hypothesis (2016). Schizophrenia Research 176, 83–94. 10.1016/j.schres.2016.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbon M, Spitzer RL, Benjamin LS, First MB, 1997. Structured Clinical Interview for DSM-5 (SCID-5) [WWW Document]. URL https://www.appi.org/products/structured-clinical-interview-for-dsm-5-scid-5 (accessed 5.2.19). [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL, 1999. Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neuroscience 2, 861–863. 10.1038/13158 [DOI] [PubMed] [Google Scholar]
- Giedd JN, Raznahan A, Mills KL, Lenroot RK, 2012. Review: magnetic resonance imaging of male/female differences in human adolescent brain anatomy. Biology of Sex Differences 3, 19 10.1186/2042-6410-3-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Woolley CS, Frankfurt M, McEwen BS, 1990. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J. Neurosci 10, 1286–1291. 10.1523/JNEUROSCI.10-04-01286.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes E, Gavrilidis E, Kulkarni J, 2012. The Role of Oestrogen and Other Hormones in the Pathophysiology and Treatment of Schizophrenia. Schizophr Res Treatment 2012. 10.1155/2012/540273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengartner MP, Heekeren K, Dvorsky D, Walitza S, Rössler W, Theodoridou A, 2017. Course of psychotic symptoms, depression and global functioning in persons at clinical high risk of psychosis: Results of a longitudinal observation study over three years focusing on both converters and non-converters. Schizophrenia Research 189, 19–26. 10.1016/j.schres.2017.01.040 [DOI] [PubMed] [Google Scholar]
- Hochman KM, Lewine RR, 2004. Age of menarche and schizophrenia onset in women. Schizophrenia Research 69, 183–188. 10.1016/S0920-9964(03)00176-2 [DOI] [PubMed] [Google Scholar]
- Huber TJ, Rollnik J, Wilhelms J, von zur Mühlen A, Emrich HM, Schneider U, 2001. Estradiol levels in psychotic disorders. Psychoneuroendocrinology 26, 27–35. 10.1016/S0306-4530(00)00034-2 [DOI] [PubMed] [Google Scholar]
- Insel TR, 2010. Rethinking schizophrenia. Nature 468, 187–193. 10.1038/nature09552 [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM, 2012. FSL. NeuroImage, 20 YEARS OF fMRI 62, 782–790. 10.1016/j.neuroimage.2011.09.015 [DOI] [PubMed] [Google Scholar]
- KILIÇASLAN EE, EROL A, ZENGİN B, ÇETİNAY AYDIN P, METE L, 2014. Association Between Age at Onset of Schizophrenia and Age at Menarche. Noro Psikiyatr Ars 51, 211–215. 10.4274/npa.y6675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraguljac NV, White DM, Hadley N, Hadley JA, ver Hoef L, Davis E, Lahti AC, 2016. Aberrant Hippocampal Connectivity in Unmedicated Patients With Schizophrenia and Effects of Antipsychotic Medication: A Longitudinal Resting State Functional MRI Study. Schizophr Bull 42, 1046–1055. 10.1093/schbul/sbv228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewine R, 2004. At issue: Sex and gender in schizophrenia. Schizophr Bull 30, 755–762. 10.1093/oxfordjournals.schbul.a007128 [DOI] [PubMed] [Google Scholar]
- Lisofsky N, Mårtensson J, Eckert A, Lindenberger U, Gallinat J, Kühn S, 2015. Hippocampal volume and functional connectivity changes during the female menstrual cycle. NeuroImage 118, 154–162. 10.1016/j.neuroimage.2015.06.012 [DOI] [PubMed] [Google Scholar]
- Mahé V, Dumaine A, 2001. Oestrogen withdrawal associated psychoses. Acta Psychiatrica Scandinavica 104, 323–331. 10.1111/j.1600-0447.2001.00288.x [DOI] [PubMed] [Google Scholar]
- Maric N, Popovic V, Jasovic-Gasic M, Pilipovic N, van Os J, 2005. Cumulative exposure to estrogen and psychosis: a peak bone mass, case-control study in first-episode psychosis. Schizophrenia Research 73, 351–355. 10.1016/j.schres.2004.07.016 [DOI] [PubMed] [Google Scholar]
- McEwen BS, 2001. Invited Review: Estrogens effects on the brain: multiple sites and molecular mechanisms. Journal of Applied Physiology 91, 2785–2801. 10.1152/jappl.2001.91.6.2785 [DOI] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Rosen JL, Cadenhead K, Ventura J, McFarlane W, Perkins DO, Pearlson GD, Woods SW, 2003. Prodromal Assessment With the Structured Interview for Prodromal Syndromes and the Scale of Prodromal Symptoms: Predictive Validity, Interrater Reliability, and Training to Reliability. Schizophr Bull 29, 703–715. 10.1093/oxfordjournals.schbul.a007040 [DOI] [PubMed] [Google Scholar]
- Mittal VA, Wakschlag LS, 2017. Research Domain Criteria (RDoC) Grows Up: Strengthening Neurodevelopmental Investigation within the RDoC Framework. J Affect Disord 216, 30–35. 10.1016/j.jad.2016.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal VA, Walker EF, 2011. Minor Physical Anomalies and Vulnerability in Prodromal Youth. Schizophr Res 129, 116–121. 10.1016/j.schres.2011.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal VA, Walker EF, 2007. Movement abnormalities predict conversion to Axis I psychosis among prodromal adolescents. Journal of Abnormal Psychology 116, 796–803. 10.1037/0021-843X.116.4.796 [DOI] [PubMed] [Google Scholar]
- Mumford JA, Poline J-B, Poldrack RA, 2015. Orthogonalization of Regressors in fMRI Models. PLoS One 10 10.1371/journal.pone.0126255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samudra N, Ivleva EI, Hubbard NA, Rypma B, Sweeney JA, Clementz BA, Keshavan MS, Pearlson GD, Tamminga CA, 2015. Alterations in hippocampal connectivity across the psychosis dimension. Psychiatry Research: Neuroimaging 233, 148–157. 10.1016/j.pscychresns.2015.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Akaishi T, Matsuki N, Ohno Y, Nakazawa K, 2007. β-Estradiol induces synaptogenesis in the hippocampus by enhancing brain-derived neurotrophic factor release from dentate gyrus granule cells. Brain Research 1150, 108–120. 10.1016/j.brainres.2007.02.093 [DOI] [PubMed] [Google Scholar]
- Schaefer A, Burmann I, Regenthal R, Arélin K, Barth C, Pampel A, Villringer A, Margulies DS, Sacher J, 2014. Serotonergic Modulation of Intrinsic Functional Connectivity. Current Biology 24, 2314–2318. 10.1016/j.cub.2014.08.024 [DOI] [PubMed] [Google Scholar]
- Seeman MV, 1996. The role of estrogen in schizophrenia. J Psychiatry Neurosci 21, 123–127. [PMC free article] [PubMed] [Google Scholar]
- Trotman HD, Holtzman CW, Ryan AT, Shapiro DI, MacDonald AN, Goulding SM, Brasfield JL, Walker EF, 2013. The Development of Psychotic Disorders in Adolescence: A potential role for hormones. Horm Behav 64, 411–419. 10.1016/j.yhbeh.2013.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Duijvenvoorde ACK, Achterberg M, Braams BR, Peters S, Crone EA, 2016. Testing a dual-systems model of adolescent brain development using resting-state connectivity analyses. NeuroImage 124, 409–420. 10.1016/j.neuroimage.2015.04.069 [DOI] [PubMed] [Google Scholar]
- Vargas T, Dean DJ, Osborne KJ, Gupta T, Ristanovic I, Ozturk S, Turner J, van Erp TGM, Mittal VA, 2018. Hippocampal Subregions Across the Psychosis Spectrum. Schizophr Bull 44, 1091–1099. 10.1093/schbul/sbx160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker EF, Trotman HD, Goulding SM, Holtzman CW, Ryan AT, McDonald A, Shapiro DI, Brasfield JL, 2013. Developmental mechanisms in the prodrome to psychosis. Dev Psychopathol 25, 1585–1600. 10.1017/S0954579413000783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei S-M, Berman KF, 2018. Ovarian hormones, genes, and the brain: the case of estradiol and the brain-derived neurotrophic factor (BDNF) gene [WWW Document]. Neuropsychopharmacology. 10.1038/s41386-018-0223-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Nieto-Castanon A, 2012. Conn: A Functional Connectivity Toolbox for Correlated and Anticorrelated Brain Networks. Brain Connectivity 2, 125–141. 10.1089/brain.2012.0073 [DOI] [PubMed] [Google Scholar]
- Winton-Brown T, Schmidt A, Roiser JP, Howes OD, Egerton A, Fusar-Poli P, Bunzeck N, Grace AA, Duzel E, Kapur S, McGuire P, 2017. Altered activation and connectivity in a hippocampal–basal ganglia–midbrain circuit during salience processing in subjects at ultra high risk for psychosis. Transl Psychiatry 7, e1245–e1245. 10.1038/tp.2017.174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SJ, Yücel M, Velakoulis D, Phillips LJ, Yung AR, Brewer W, McGorry PD, Pantelis C, 2005. Hippocampal and anterior cingulate morphology in subjects at ultrahigh-risk for psychosis: the role of family history of psychotic illness. Schizophr. Res 75, 295–301. 10.1016/j.schres.2004.10.008 [DOI] [PubMed] [Google Scholar]
- Woolley CS, 1998. Estrogen-Mediated Structural and Functional Synaptic Plasticity in the Female Rat Hippocampus. Hormones and Behavior 34, 140–148. 10.1006/hbeh.1998.1466 [DOI] [PubMed] [Google Scholar]


