Abstract
Objective.
The purpose of this study was to characterize lower extremity passive muscle stiffness in a young, healthy, athletic population. It was hypothesized that males would exhibit greater stiffness than females and that hamstring stiffness would increase with increased passive hamstring stretch.
Methods.
Male (n=52, age 16.0±1.3 years, height 180.3±7.9 cm, weight 73.1±11.8 kg) and female (n=89, age 15.6±1.3 years, height 169.7±8.1 cm, weight 65.2±13.2 kg) high school basketball athletes were recruited for this study. SWE was used to measure shear wave velocity (m/s) of the biceps femoris muscle at three leg positions (40%, 60%, and 80%) of the maximum passive 90–90 straight-leg raise position for each leg. Hamstring stiffness (kPa) was quantified from the SWE elastogram using custom processing software.
Results.
Hamstring stiffness was significantly greater for males than females at every position on both the dominant and non-dominant limbs (p<0.05). Hamstring stiffness was greater on the non-dominant limb than the dominant for females at the 40% position. Stiffness at 60% was greater than stiffness at 40% for males on both the dominant and non-dominant limbs. However, stiffness at 60% was greater than stiffness at 80% on the male non-dominant limb. Females demonstrated higher stiffness at 40% than both 60% and 80% for the dominant and non-dominant limbs.
Conclusion.
Healthy male basketball players had higher hamstring muscle stiffness than female players. Future studies may investigate what factors contribute to the large variability observed in muscle stiffness, resulting in mixed results on the effects of leg dominance and stretching positions.
Keywords: musculoskeletal ultrasound, sex differences, skeletal muscle, biomechanics, shear wave elastography
INTRODUCTION
Shear wave elastography (SWE) is an emerging ultrasound imaging technology that quantifies tissue stiffness properties. SWE provides a quantitative measure of mechanical and elastic tissue properties, which can augment diagnoses provided by gray-scale ultrasound images.[1, 2] Briefly, acoustic radiation force is used to generate a shear wave from the transducer into the muscle tissue. The shear wave propagates perpendicularly to the primary ultrasound wave at different velocities depending on tissue type.[3] Shear wave propagation velocity is measured and through simple mathematical manipulation can be converted to muscle shear modulus and muscle tissue stiffness.[4] Understanding muscle mechanical properties is useful information for both clinical practice as well as biomechanical research on musculoskeletal injuries. Skeletal muscle tissue properties can be attributed to contributions from both passive and active stiffness and tissue elasticity, which are also major determinants of muscle performance and force generation.[5]
Muscle stiffness is affected by several factors.[6–8] Shear modulus values differ between various skeletal muscles, even synergistic muscles that act to perform a single action.[6, 9] For example, the hamstrings, which consist of the semitendinosus, semimembranosus, and biceps femoris muscles to perform hip extension and knee flexion, differ in shear modulus values. The shear elastic modulus values for the biceps femoris were higher than the shear elastic modulus values of the semintendinosus.[6, 9] Another factor that affects shear modulus is passive stretch; SWE measures of muscle stiffness increase with a greater passive stretch of the hamstrings in young adults.[6] Moreover, static stretching alters the shear modulus of hamstring muscles.[7, 9] After a five minute static stretching program, flexibility of the muscle tendon unit increased and the shear elastic modulus of the semitendinosus, semimembranosus, and biceps femoris muscles decreased.[9] In the upper extremity, shear modulus values increase from 90 degrees of elbow flexion to full extension, further confirming that muscle stiffness increases with greater joint angle, which produces greater tension in the muscle.[10] In addition to intrinsic factors affecting shear modulus of muscle tissue, regression analysis of shear modulus values of the biceps brachii identified age and sex as significant predictors for muscle shear modulus.[10, 11] Shear modulus values increased with advanced age and tended to be higher for females than males of the same age.[10] However, these studies were performed on the biceps brachii in a sample of adults ranging from 21 to 94 years old and were not specific to an athletic population. In the lower extremity musculature, studies have reported mixed findings on the effect of sex.[8, 12] No sex differences were reported in shear modulus of the tibialis anterior during active muscle contraction[12]; however, another study reported that males had a higher shear modulus of the relaxed rectus femoris, soleus, and lateral head of the gastrocnemius muscles than females.[8] Stiffness of the vastus medialis and lateral muscles in males and females yielded mixed results that were dependent on both muscle and body position.[13] Therefore, the effect of sex on shear modulus values remains inconclusive but for lower extremity muscle it appears that males may have higher stiffness than females. Minimal data exist regarding the effect of limb dominance on shear wave modulus and muscle stiffness, particularly in the lower extremity musculature. No difference in muscle stiffness due to limb dominance was observed in the biceps brachii using SWE technology.[11] Using myotonometry to assess lower extremity muscle stiffness, the non-dominant leg on average showed greater muscle stiffness than the dominant leg.[14] However, this finding has not been confirmed using SWE technology.
While previous studies identified factors that influence muscle stiffness as measured by SWE technology, passive hamstring muscle stiffness has not been reported in the literature for a young, healthy athletic cohort. Muscle injuries are the most common sport-related injury, with hamstring injuries accounting for up to 29% of all injuries in various sports.[15] Moreover, hamstring muscle strains have high rates of re-injury.[15, 16] Therefore, the purpose of this study was to characterize how lower extremity passive muscle stiffness in a young, healthy, athletic population differs between age and sex. It was hypothesized that males would exhibit greater stiffness than females. Further, it was hypothesized that there would be no differences in hamstring stiffness between the dominant and non-dominant leg and that hamstring stiffness would increase with increased passive hamstring stretch.
METHODS
The study was approved by the Mayo Clinic Institutional Review Board (IRB 17–003905). Informed consents were obtained from each high school basketball player and his/her parent if under 18 years old. Male (n=52) and female (n=89) subjects were significantly different for height (180.3±7.9 cm vs. 169.7±8.1 cm) and weight (73.1±11.8 kg vs. 65.2±13.2 kg), but not for age (16.0±1.3 years vs. 15.6±1.3 years). A clinician assessed hamstring flexibility using a passive knee extension test and a digital inclinometer; ipsilateral hip and knee flexion both positioned at 90° were considered neutral.[17] 40%, 60%, and 80% of the maximum flexibility were calculated. The greater trochanter and femoral condyle were then marked and the midpoint was identified by the clinician for repeatable placement of the ultrasound transducer both within and between subjects. While lying supine, the clinician moved and held the athlete’s leg at each position to assess biceps femoris (a hamstring muscle) stiffness using SWE at each position (GE LOGIQ E9, 9L-D transducer, GE Healthcare, Wauwatosa, WI). The same researcher (ALM), who was trained by a SWE expert, completed all ultrasound data collection. Three SWE images were acquired at each position. The subject was instructed to relax their muscles and allow the clinician to support their leg completely. Surface electrodes placed on the medial hamstring muscle provided real-time audio feedback to ensure the subject remained relaxed and was not actively contacting their hamstring muscles, to ensure measurement of passive muscle stiffness. Minimal pressure was applied to the ultrasound transducer probe by the researcher in order to prevent tissue compression that would artificially influence the measured tissue stiffness. Muscle stiffness (kPa) was measured using shear wave velocities from the SWE elastogram using custom MATLAB software (Fig. 1).
Fig. 1.

an example of shear wave elastogram used to calculate hamstring muscle stiffness
Analysis was performed between dominant and non-dominant limbs, defined as preferred leg for kicking a soccer ball. Average stiffness from the three SWE images acquired at each position on both sides was used for analysis. Descriptive statistics were computed for all variables. Normality was assessed by fitting a normal distribution to each variable. The distributions of hamstring stiffness at each position were non-normal, indicated by a Shapiro-Wilk W test of the goodness-of-fit of the normal distribution (p<0.0001). All distributions were right-skewed; thus, non-parametric analyses were performed. Wilcoxon-signed rank method was used to analyze between sex comparisons for each position (40%, 60%, 80%) on the dominant and non-dominant sides (p<0.05) (JMP 13, SAS Institute Inc., Cary, North Carolina). Additional matched-pair Wilcoxon-signed rank method tests were used to analyze within sex differences between both dominant and non-dominant sides and between positions.
RESULTS
Intra-session reliability and precision of our SWE protocol demonstrated high reliability (ICC = 0.833–0.925; SEM = 8.2–12.2 kPa). Hamstring stiffness was significantly greater for males than females at every position on both the dominant and non-dominant limbs (p<0.01) (Fig. 2). Hamstring stiffness was significantly different between dominant and non-dominant limbs for females at the 40% position, with higher stiffness observed on the non-dominant limb (p<0.01) (Table 1). No other positions demonstrated significant differences between limbs for either sex. Within-sex differences were observed in hamstring stiffness between separate leg positions. Stiffness at 60% was greater than stiffness at 40% for males on both the dominant (p=0.05) and non-dominant limbs (p=0.04). However, stiffness at 60% was greater than stiffness at 80% on the male non-dominant limb (p=0.02). Females demonstrated higher stiffness at 40% than 60% and 80% for both the dominant and non-dominant limbs (p<0.01). Intra-limb variability was greater in males than females (Fig. 3). This was further demonstrated by wider confidence intervals for males for each position in both limbs. In addition, the absolute difference in confidence interval widths between the dominant and non-dominant limbs was larger for males than females (Table 2).
Fig. 2.

box plots of hamstring stiffness (kPa) by sex, at each leg position; outliers denoted by black points.
Table 1:
Hamstring stiffness (kPa) for each sex; Median (Lower Quantile, Upper Quantile).
| 40% | 60% | 80% | ||||
|---|---|---|---|---|---|---|
| D | ND | D | ND | D | ND | |
| Male | 42.9 (27.3,72.4)+a | 45.7 (29.1, 90.2)+a | 49.4 (18.8, 94.6)+a | 64.9 (26.9, 94.0)+ab | 33.1 (21.2, 97.2)+ | 48.7 (23.9, 69.7)+b |
| Female | 28.4 (20.3, 47.8)*a | 32.6 (23.7, 50.5)a | 25.9 (19.4, 52.6)b | 30.7 (21.1, 54.7)b | 22.3 (18.9, 31.2)ab | 24.6 (20.1, 33.0)ab |
indicates significant difference within-sex between dominant (D) and non-dominant (ND) limbs;
indicates significant difference between-sex for same percentage and limb side;
indicates significant difference within-sex for the same limb side, between positions
Fig. 3.
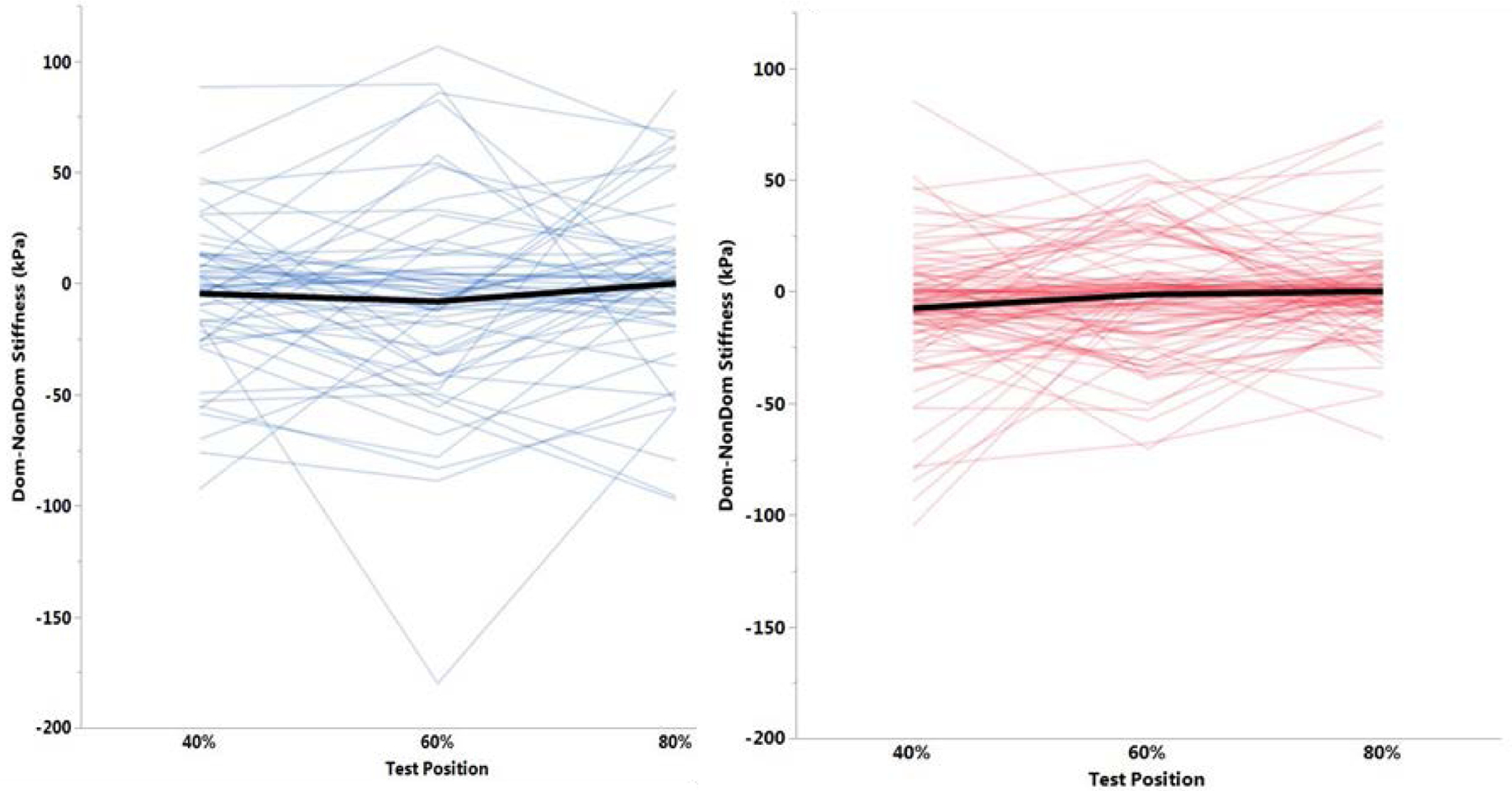
A matched-pair analysis demonstrating intra-limb variability in males (blue) and females (red).
Table 2:
Absolute difference in 95% confidence interval between dominant and non-dominant side in hamstring stiffness (kPa).
| 40% | 60% | 80% | |
|---|---|---|---|
| Male | 16.0 | 8.7 | 30.2 |
| Female | 0.7 | 0.4 | 0.6 |
DISCUSSION
The hypotheses tested were partially supported by the results of the current study. Greater stiffness in male hamstrings than female hamstrings for each position confirmed the primary hypothesis. Previous studies yielded mixed results on differences between sexes, depending on the muscle evaluated.[8, 10–13] A study that included young patients, ranging from sedentary to highly active individuals, reported higher stiffness for males than females in the rectus femoris, soleus, and lateral gastrocnemius muscles.[8] The current results partially support previous findings, as significant differences between limbs were only observed at the 40% position for females. Previous literature demonstrated no significant differences in muscle stiffness due to limb dominance.[11, 14]
Hamstring stiffness was significantly different between positions; however, contrary to the hypothesis, stiffness did not always increase with increased passive stretch of the muscle. Previous studies of SWE demonstrated increased muscle stiffness with increased passive stretch.[6, 18] Theoretically, the 40% position should have the least muscle tension of the three positions assessed, since it was the smallest joint angle of the three test positions. In the current study, females demonstrated significant differences on both limbs between the 40% and 80% positions as well as between the 40% and 60% positions. However, counter to what was anticipated, the 40% position exhibited the greatest muscle stiffness of the three positions. In the current study, males demonstrated a significant increase in stiffness from the 40% to 60% position on the dominant leg, and a significant decrease on the non-dominant leg from the 60% to 80% position. For males, the 60% position produced the highest muscle stiffness. With a greater stretch of the muscle at the 80% position, we observed a tendency of the ultrasound SWE machine to reach maximal capability, demonstrated by longer image acquisition time and poorer SWE elastogram quality. Saturation of the machine, or reaching the maximal shear wave velocity able to be recorded, could result in inaccurate tissue stiffness assessments. This may contribute to why increased stiffness with increased passive muscle stretch was not observed for both sexes. The issue of overloading and shear wave receiving frequency has previously been identified as a limitation of SWE.[19]
Several potential factors may contribute to the differences and variability in patterns of hamstring stiffness observed between males and females. Significant acknowledgement to the inter-subject differences in muscle stiffness has previously been addressed.[20] In accordance, large variability was observed in the current study. As seen in Fig. 2 and 3, there were no male outliers and the male data has much larger variability than female data. However, there were different numbers of outliers for females between the three positions, which provided further evidence of the high inter-individual variability previously demonstrated using SWE. Outliers are those values which are greater than 1.5 times the interquartile range. The interquartile range, or middle 50%, of the male data is much wider than the female interquartile range, further confirming the larger variation among male stiffness values.
It is worth nothing that the testing order was not randomized in this investigation. Each subject was measured in the following order of positions: 80%, 60%, and 40%. Although the time to acquire three SWE images in one position was typically only a few seconds, the subjects’ legs were immediately repositioned for the next trial. It is possible that the muscle properties were altered by the repeated stretching procedures, which started with a maximum flexibility test to determine the appropriate knee angles for the protocol. Short bouts of static stretching have been shown to reduce the shear elastic modulus of the hamstring muscles.[7, 9] Interestingly, the opposite was observed in females in the current study; 40%, the last position tested, had the highest stiffness. SWE is a relatively newer technology and thus there is not an extensive body of literature regarding musculoskeletal applications, particularly in the lower extremity. Since the results of the current study were unanticipated, future investigation may elucidate why the 40% and 60% positions yielded the lowest value of stiffness in the biceps femoris in females and males, respectively.
In addition to sex, limb dominance, and position, age has previously been identified as a contributing factor to muscle stiffness.[6–8, 14] Preliminary analysis demonstrated an overall lack of significant differences in hamstring stiffness with increased age. Males demonstrated differences between the 17 and 15 year old age groups on the non-dominant limb at the 40% position. Seventeen and 14 year old males also demonstrated differences on the dominant limb at the 60% position. It was expected that hamstring stiffness would increase with age, as significant maturational changes occur in the musculoskeletal system between 14 and 18 years of age in both males and females.[21–23] However, sex differences in pubertal timing and different developmental characteristics during pubertal stages may help explain the differences in muscle tissue stiffness between sex for the adolescent age groups. For between-sex and -age group analysis, sample sizes for each subgroup ranged in size from six to 23 subjects. A combination of the small sample sizes and high variability in muscle stiffness measurements may have limited statistical differences between groups. Additional subjects are needed to increase power of the analysis and determine the relationship between hamstring stiffness, sex, and age.
SWE provides a quantitative assessment of mechanical and elastic muscle tissue properties. This clinical tool could monitor normal development, rehabilitation, or the effectiveness of training by quantifying the changes of muscle mechanical and elastic properties in an individual athlete. In addition, establishment of muscle stiffness values for the biceps femoris in healthy is useful in order to compare against stiffness values in patients with pathological conditions. Previous research has reported mixed findings regarding differences in quadriceps muscle stiffness between healthy controls and patients with patellofemoral pain syndrome.[13] However, the study only included 22 healthy subjects, and 11 patients presenting with physical symptoms of patellofemoral pain syndrome. Additional studies are warranted to investigate differences in muscle stiffness between healthy subjects and subjects presenting symptoms of a musculoskeletal pathology. Future prospective studies could help to determine if muscle stiffness differences can be observed between healthy subjects and those who go on to sustain an injury or musculoskeletal disorder.
There were several limitations to the current study. Previous studies reported good reliability of SWE measurements of the biceps femoris during a passive stretch.[24] Although the biceps femoris is a superficial muscle and better reliability has been reported for superficial muscles compared to deep muscles [25, 26], differences in body composition between sexes and age may also contribute to the high variability in muscle stiffness values. During data acquisition of a few individuals with thick subcutaneous fat overlying the biceps femoris muscle, we observed poor shear wave transmission and thus poor shear wave elastograms. This resulted in worse data quality compared to leaner subjects and may not represent true muscle stiffness information in these individuals. Despite these known anatomical variability and technical considerations, we have demonstrated high reliability in our measurements (ICC = 0.833–0.925) similar to previous reported reliability.[24] Moreover, based on anatomical variation, patient-specific ROIs had to be used and transducer alignment at times had to be modified to visualize muscle fibers. Some subjects had larger ROIs which would allow for a more accurate average of muscle tissue stiffness as opposed to a smaller ROI.
This study presents passive hamstring muscle stiffness for a young, healthy, athletic cohort. Differences in passive hamstring muscle stiffness were observed between healthy male and female basketball players, as males demonstrated higher stiffness than females for each position tested in the current study. Future studies may investigate what factors contribute to the large variability observed in muscle stiffness in a healthy, similar-aged population.
Funding
Funding provided by an NBA/GE Collaboration grant. Fellowship funding (ALM) provided by the Mayo Clinic Graduate School of Biomedical Sciences. NIH funding includes: K12HD065987 and L30AR070273 (NDS), T32AR056950 (RH), R01AR055563 (NAB), and R01AR056259 (TEH).
Footnotes
Ethics declarations
The study was approved by the Mayo Clinic Institutional Review Board (IRB 17–003905). Informed consents were obtained from each high school basketball player and his/her parent if under 18 years old.
REFERENCES
- 1.Drakonaki EE, Allen GM, Wilson DJ. Ultrasound elastography for musculoskeletal applications. Br J Radiol. 2012; 85(1019):1435–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klauser AS, Miyamoto H, Bellmann-Weiler R, Feuchtner GM, Wick MC, Jaschke WR. Sonoelastography: musculoskeletal applications. Radiology. 2014; 272(3):622–633. [DOI] [PubMed] [Google Scholar]
- 3.Taljanovic MS, Gimber LH, Becker GW, Latt LD, Klauser AS, Melville DM, et al. Shear-Wave Elastography: Basic Physics and Musculoskeletal Applications. Radiographics. 2017; 37(3):855–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eby SF, Song P, Chen S, Chen Q, Greenleaf JF, An KN. Validation of shear wave elastography in skeletal muscle. J Biomech. 2013; 46(14):2381–2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roberts TJ. Contribution of elastic tissues to the mechanics and energetics of muscle function during movement. J Exp Biol. 2016; 219(Pt 2):266–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Le Sant G, Ates F, Brasseur JL, Nordez A. Elastography Study of Hamstring Behaviors during Passive Stretching. PLoS ONE. 2015; 10(9):e0139272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miyamoto N, Hirata K, Kanehisa H. Effects of hamstring stretching on passive muscle stiffness vary between hip flexion and knee extension maneuvers. Scand J Med Sci Sports. 2017; 27(1):99–106. [DOI] [PubMed] [Google Scholar]
- 8.Akagi R, Yamashita Y, Ueyasu Y. Age-Related Differences in Muscle Shear Moduli in the Lower Extremity. Ultrasound Med Biol. 2015; 41(11):2906–2912. [DOI] [PubMed] [Google Scholar]
- 9.Umegaki H, Ikezoe T, Nakamura M, Nishishita S, Kobayashi T, Fujita K, et al. Acute effects of static stretching on the hamstrings using shear elastic modulus determined by ultrasound shear wave elastography: Differences in flexibility between hamstring muscle components. Man Ther. 2015; 20(4):610–613. [DOI] [PubMed] [Google Scholar]
- 10.Eby SF, Cloud BA, Brandenburg JE, Giambini H, Song P, Chen S, et al. Shear wave elastography of passive skeletal muscle stiffness: influences of sex and age throughout adulthood. Clin Biomech. 2015; 30(1):22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen J, O’Dell M, He W, Du LJ, Li PC, Gao J. Ultrasound shear wave elastography in the assessment of passive biceps brachii muscle stiffness: influences of sex and elbow position. Clin Imaging. 2017; 45:26–29. [DOI] [PubMed] [Google Scholar]
- 12.Souron R, Bordat F, Farabet A, Belli A, Feasson L, Nordez A, et al. Sex differences in active tibialis anterior stiffness evaluated using supersonic shear imaging. J Biomech. 2016; 49(14):3534–3537. [DOI] [PubMed] [Google Scholar]
- 13.Botanlioglu H, Kantarci F, Kaynak G, Unal Y, Ertan S, Aydingoz O, et al. Shear wave elastography properties of vastus lateralis and vastus medialis obliquus muscles in normal subjects and female patients with patellofemoral pain syndrome. Skeletal Radiol. 2013; 42(5):659–666. [DOI] [PubMed] [Google Scholar]
- 14.Um GM, Wang JS, Park SE. An analysis on muscle tone of lower limb muscles on flexible flat foot. J Phys Ther Sci. 2015; 27(10):3089–3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahmad CS, Redler LH, Ciccotti MG, Maffulli N, Longo UG, Bradley J. Evaluation and management of hamstring injuries. Am J Sports Med. 2013; 41(12):2933–2947. [DOI] [PubMed] [Google Scholar]
- 16.Warren P, Gabbe BJ, Schneider-Kolsky M, Bennell KL. Clinical predictors of time to return to competition and of recurrence following hamstring strain in elite Australian footballers. Br J Sports Med. 2010; 44(6):415–419. [DOI] [PubMed] [Google Scholar]
- 17.Reurink G, Goudswaard GJ, Oomen HG, Moen MH, Tol JL, Verhaar JA, et al. Reliability of the active and passive knee extension test in acute hamstring injuries. Am J Sports Med. 2013; 41(8):1757–1761. [DOI] [PubMed] [Google Scholar]
- 18.Maisetti O, Hug F, Bouillard K, Nordez A. Characterization of passive elastic properties of the human medial gastrocnemius muscle belly using supersonic shear imaging. J Biomech. 2012; 45(6):978–984. [DOI] [PubMed] [Google Scholar]
- 19.Martin JA, Biedrzycki AH, Lee KS, DeWall RJ, Brounts SH, Murphy WL, et al. In Vivo Measures of Shear Wave Speed as a Predictor of Tendon Elasticity and Strength. Ultrasound Med Biol. 2015; 41(10):2722–2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Creze M, Nordez A, Soubeyrand M, Rocher L, Maitre X, Bellin MF. Shear wave sonoelastography of skeletal muscle: basic principles, biomechanical concepts, clinical applications, and future perspectives. Skeletal Radiol. 2018; 47(4):457–471. [DOI] [PubMed] [Google Scholar]
- 21.Malina RM, Bouchard C, Bar-Or O. Timing and Sequence of Changes During Adolescence Growth, Maturation, and Physical Activity. Second Edition ed. Champaign, IL: Human Kinetics; 2004:307–333. [Google Scholar]
- 22.Quatman CE, Ford KR, Myer GD, Paterno MV, Hewett TE. The effects of gender and pubertal status on generalized joint laxity in young athletes. J Sci Med Sport. 2008; 11(3):257–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hewett TE, Myer GD, Ford KR. Decrease in neuromuscular control about the knee with maturation in female athletes. J Bone Joint Surg Am. 2004; 86-A(8):1601–1608. [DOI] [PubMed] [Google Scholar]
- 24.Dubois G, Kheireddine W, Vergari C, Bonneau D, Thoreux P, Rouch P, et al. Reliable protocol for shear wave elastography of lower limb muscles at rest and during passive stretching. Ultrasound Med Biol. 2015; 41(9):2284–2291. [DOI] [PubMed] [Google Scholar]
- 25.MacDonald D, Wan A, McPhee M, Tucker K, Hug F. Reliability of Abdominal Muscle Stiffness Measured Using Elastography during Trunk Rehabilitation Exercises. Ultrasound Med Biol. 2016; 42(4):1018–1025. [DOI] [PubMed] [Google Scholar]
- 26.Alfuraih AM, O’Connor P, Hensor E, Tan AL, Emery P, Wakefield RJ. The effect of unit, depth, and probe load on the reliability of muscle shear wave elastography: Variables affecting reliability of SWE. J Clin Ultrasound. 2018; 46(2):108–115. [DOI] [PubMed] [Google Scholar]


