Abstract
The Bruton’s Tyrosine Kinase (BTK) inhibitor ibrutinib has transformed the treatment of chronic lymphocytic leukemia (CLL), leading to unprecedented improvements in progression-free and overall survival for all patients, including those with poor prognostic features. The side effect profile of ibrutinib is unique compared with chemoimmunotherapy and includes atrial fibrillation, increased bleeding risk, and arthralgias/myalgias. Although common, arthralgias/myalgias and their management are poorly described. We identified 214 patients with CLL treated with ibrutinib (as a single agent or in combination) from 2011-2018 at the University of Pennsylvania. In this cohort, 36% (76/214) of patients developed arthralgias/myalgias during follow-up with a median onset of 34.5 months. Most events (79%) were Grade 1 or 2. Risk factors for developing arthralgias/myalgias included younger age at start of ibrutinib, female sex, and ibrutinib use as first treatment. Twenty-eight percent of patients with grade 1 or 2 toxicity continued ibrutinib and had resolution of symptoms. Dose holds were frequently used to manage this toxicity and this strategy was more successful than dose reduction. Sixty-two percent of patients with grade 3 toxicity ultimately discontinued ibrutinib. Supportive care measures such as discontinuing statins or use of non-steroidal anti-inflammatory drugs (NSAIDs), acetaminophen, or corticosteroids were not used frequently enough in this cohort to evaluate their efficacy. Additional studies to determine the mechanism of ibrutinib-related arthralgias/myalgias are needed to develop optimal management strategies.
Keywords: Ibrutinib, toxicity, arthralgias, myalgias, musculoskeletal, chronic lymphocytic leukemia
Micro-Abstract:
Ibrutinib-associated arthralgias/myalgias are a frequent adverse event which can occur late in the treatment course and are mostly Grade 1 or 2. For patients with Grade 1 or 2 arthralgias/myalgias, dose reductions can frequently mitigate the side effects and allow patients to continue on therapy, while the majority of patients with Grade 3 toxicity ultimately discontinue therapy. Further study is needed to determine their pathogenesis in order to develop best practice management strategies.
INTRODUCTION
The treatment of chronic lymphocytic leukemia (CLL) has been transformed by the use of inhibitors of B-cell receptor signaling. Ibrutinib is a first-in-class Bruton’s Tyrosine Kinase (BTK) inhibitor that has resulted in improved progression-free survival (PFS) and overall survival (OS) compared to monoclonal antibodies1, alkylating agents2, and chemoimmunotherapy combinations.3,4 As a result, the United States Food and Drug Administration has approved ibrutinib for treatment of CLL as a single agent in front-line and relapsed-refractory settings 1,2,5–7 as well as in combination with chemoimmunotherapy8 and obinutuzumab3,9.
Ibrutinib has a unique adverse effect profile compared to chemoimmunotherapy, with toxicities that include atrial fibrillation10–12, increased bleeding risk,13,14, diarrhea, 15 fatigue, hypertension16, and cytopenias.17 In addition, arthralgias/myalgias is common among CLL patients treated with ibrutinib in both upfront and relapsed/refractory (R/R) settings and their incidence have been noted in both clinical trials and clinical practice. In clinical trials and retrospective cohort studies, arthralgias and myalgias were reported in 11-36% of patients.1,2,18–21 In terms of ibrutinib intolerance, arthralgias and myalgias has also been common reasons for discontinuation of ibrutinib 22,23 However, the mechanisms for ibrutinib-associated arthralgias/myalgias remain unknown. Moreover, there have been no detailed descriptions regarding their presentation and limited guidance24 for their management.
The objective of this study was to determine the incidence, timing, and risk factors for arthralgias/myalgias among CLL patients treated with ibrutinib in clinical practice. In addition, we evaluated management strategies and their outcomes.
METHODS
Study Design
We conducted a retrospective cohort study of CLL patients treated with ibrutinib at the University of Pennsylvania between January 1, 2011 and December 31, 2018. Demographic information, medical diagnoses, laboratory results, medication prescriptions, and pathology and radiology reports were collected from patients’ electronic medical records. The study was approved by the University of Pennsylvania Institutional Review Board.
Study Patients
Patients were included if they had a diagnosis of CLL and were treated with ibrutinib. Patients could have received treatment with at least one dose of ibrutinib in the front-line or relapsed/refractory setting, or in combination with another agent. Patients were excluded if they: 1) started ibrutinib prior to establishing care at the University of Pennsylvania, or 2) received chimeric antigen receptor T (CART) cell therapy within 3-12 months of initiating ibrutinib treatment since the effects of CART cells on arthralgias/myalgias remain unknown.
Outcomes
The primary outcome was arthralgias/myalgias, defined as the presence of arthralgias or myalgias attributed to ibrutinib per treating physician. Grading was performed using Common Terminology Criteria for Adverse Effect (CTCAE) version (Grade 1-3). If more than one episode was recorded, the highest grade was used. We evaluated arthralgias/myalgias as a composite outcome because of the difficulty in separating joint and periarticular soft tissue and/or muscle symptoms. As secondary outcomes, we compiled and evaluated the various management strategies utilized for arthralgias and/or myalgias, which included dose adjustments, medication holds, and discontinuation, as well as the use of acetaminophen, NSAIDs, corticosteroids, and Coenzyme Q10 (CoQ10), and their effectiveness for alleviating symptoms. Overall response rate, progression free survival and overall survival were evaluated as other secondary outcomes of interest.
Data Collection
Demographic and clinical data (age, sex, race, ethnicity, date of CLL diagnosis, date of start of ibrutinib, number of prior therapies) were collected from patients’ electronic medical records. Candidate prognostic factors included immunoglobulin heavy chain rearrangement (IGHV) mutation status, fluorescence in situ hybridization (FISH) testing, karyotype, next generation sequencing, Rai stage, history of joint pain and history of rheumatologic disease as defined by International Classification of Diseases, Ninth and Tenth Revisions (ICD-9 and ICD-10) diagnoses and antecedent arthritis diagnosis. Medication data including concomitant statin use and potency as defined by the American College of Cardiology, as well as ibrutinib dose reductions/dose holds, use of NSAIDs/corticosteroids, and the supplement CoQ10 were also captured. Electronic medical record data were abstracted by a medical oncologist (J.M.R.), who adjudicated the presence of musculoskeletal toxicity events, date of onset, and their grade (via CTCAE criteria). Response to ibrutinib was assessed by 2018 International Workshop on CLL guidelines.25
Statistical Analysis
We first calculated the unadjusted incidence rate of arthralgias/myalgias. The Kaplan-Meier estimator was used in a time to event analysis for the development of an initial event of arthralgias/myalgias. Follow-up for development of arthralgias/myalgias ended at the earliest time of reported arthralgias/myalgias per physician notes, date of discontinuation of ibrutinib (for other toxicity or progression), at date of data extraction, or at the time of death. Overall response rate was determined based on best response by International Workshop on CLL guidelines.25 Progression free survival (PFS) was calculated based on the start date of ibrutinib therapy to the date of documented progression, next treatment, or last follow up. PFS was stratified by frontline ibrutinib use. Overall survival was calculated based on the start date of ibrutinib to the date of last follow up or date of death. Log-rank tests were used to test for differences in the distribution of time to onset of arthralgias/myalgias in relation to individual covariables of interest including: older age (age ≥65, female sex, ibrutinib dose (420 mg, 280 mg, 140 mg), ibrutinib as frontline therapy (vs. R/R), history of joint disease defined by ICD9 code, history of autoimmune disease defined by ICD9 code, Rai stage, and concurrent statin prescription.
Multivariable Cox regression analysis was used for adjusted analyses of potential risk factors for incident arthralgias/myalgias. Variables were selected for inclusion in the final multivariable model based on statistical significance of the log rank test included age, sex, ibrutinib as frontline therapy, and history of autoimmune disease. Statistical significance was evaluated at the two-sided alpha = 0.05 level. We also performed univariate analyses to examine the risk of developing Grade 3 arthralgias/myalgias utilizing the same variables chosen multivariate analysis of the entire cohort. Analyses were performed using Stata version 14.2 (Stata Corporation; College Park, TX).
RESULTS
Characteristics of CLL Patients Treated with Ibrutinib
We identified 214 eligible patients for this study. The cohort (Table 1) was predominantly male (70%) and Caucasian (94%). Sixty-two percent received ibrutinib as front-line therapy (n=134), and the starting dose was 420 mg in 92% of patients (n=196). Eighty-six percent received ibrutinib as monotherapy (n=185), while 14% received ibrutinib in combination with another agent, most commonly rituximab (76%, n=22). Twenty-one percent of patients had 17p deletion (42/196) and 33% had 11q deletion (40/121). Fifty-nine percent were IGHV unmutated (58/98). Thirty-five percent were on a concurrent statin, of whom 34% (n=26) had received high dose statin (as defined by American College of Cardiology26). Seventeen percent had a documented history of prior joint issues and 8% had a history of a rheumatologic disorder.
Table 1:
Characteristics of chronic lymphocytic leukemia patients at the time of initiation of ibrutinib treatment at the University of Pennsylvania between January 1,2011 and December 31,2018 (n=214).
| Characteristic | Overall (n=214) |
|---|---|
| Median age, years (IQR) | 67.3 (41-89) |
| Sex, n (%) | |
| Male | 149 (70%) |
| Race/ethnicity, n (%) | |
| Caucasian | 202 (94%) |
| African American | 7 (3%) |
| Hispanic | 2 (2%) |
| Other | 1 (1%) |
| Ibrutinib dose, n (%) | |
| 420 mg | 196 (92%) |
| 280 mg | 11 (5%) |
| 140 mg | 7 (3%) |
| Ibrutinib front line, n (%) | 134 (62)% |
| Median Number of Prior therapies (IQR)* | 1 (1-7) |
| Ibrutinib monotherapy, n (%) | 185 (86%) |
| Unfavorable cytogenetics, n(%) | |
| del(17p) | 42 of 196 tested (21%) |
| del(11q) | 40 of 121 tested (33%) |
| IGHV**, n(%) (98 tested) | |
| Unmutated | 58(59%) |
| Mutated | 31(32%) |
| Borderline | 8(8%) |
| Polygenic | 1(1%) |
| Complex Karyotype, n(%) (148 tested) | 25 (16%) |
| LDH > ULN*** (118 tested) | 88 (75%) |
| Rai stage, n (%) | |
| 0-2 | 109 (51%) |
| >3 | 105 (49%) |
| Concurrent statin use, n (%) | 75 (35%) |
| Statin intensity **** | |
| Low | 20 (27%) |
| Moderate | 29 (39%) |
| High | 26 (34%) |
| History of autoimmune disease | 18 (8%) |
Among non-first line patients
IGHV, Immunoglobulin heavy-chain variable region gene
ULN, upper limit of normal
Among patients treated with statins, statin intensity per AHA/ACC Guidelines1
Incidence of Arthralgias/Myalgias
Among the 214 patients treated with ibrutinib, 76 patients (36%) developed a new or worsening of arthralgias/myalgias while receiving treatment with ibrutinib (26.4 months median follow up, IQR 15-38 months). The incidence rate was 0.26 events per person year. Forty-seven percent of patients treated in the front-line setting developed arthralgias/myalgias, as compared to 30% treated for relapsed/refractory disease. Seventy-nine percent of incident arthralgia/myalgia events were grade 1-2 (mild or moderate, n=60), and 21% were grade 3 (severe, n=16). The Kaplan-Meier estimate of the median time to development of arthralgias/myalgias was 34.5 months (Figure 1). For patients treated with frontline ibrutinib, the Kaplan-Meier estimate of median time to development of arthralgias/myalgias was 19.9 months and was greater than the maximum observed follow-up time for patients with relapsed/refractory disease (Figure 2). Of the 76 patients experiencing arthralgias/myalgias, median time to development was 7 months (IQR 2-16.7 mo).
Figure 1:
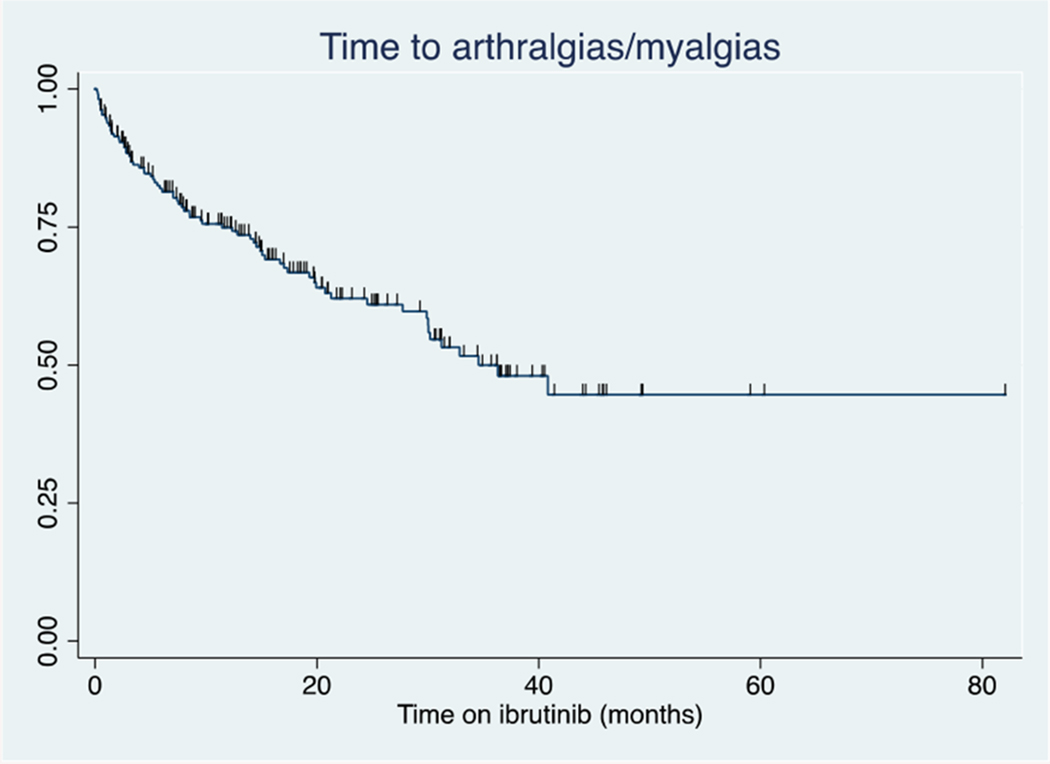
Time to development of incident arthralgias/myalgias
Figure 2:
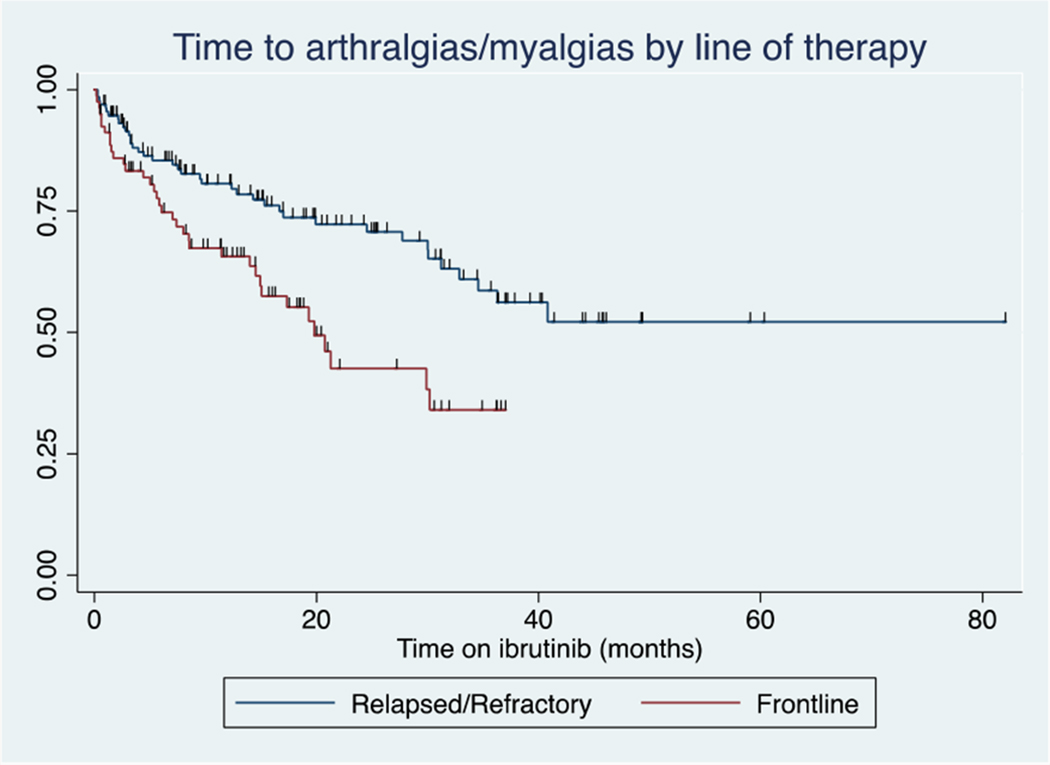
Arthralgias/myalgias by treatment line
Risk Factors for Arthralgias/Myalgias
In univariable analysis, age (<65 years), sex, ibrutinib use in the frontline and history of autoimmune disorders were associated with developing arthralgias/myalgias (Appendix 1). Concurrent statin use was not significantly associated with incident arthralgias/myalgias in univariable analyses. In univariate analysis, female sex was the only covariate associated with developing Grade 3 musculoskeletal toxicity (HR 2.73; 95% CI 1.02-7.34).
In a model adjusted for age (≥ 65 years vs. <65 years), sex, frontline ibrutinib use and history of autoimmune disease (Table 2), incident arthralgias/myalgias were associated with age <65 years (1.59; 95% CI,1.01-2.5) female sex (HR, 2.0; 95% CI, 1.31-3.35) and ibrutinib use in the frontline setting (HR, 1.89 95% CI, 1.19-3.01). (Table 2). History of autoimmune disease was non-statistically significantly associated with time to development of arthralgias/myalgias (HR 1.78; 95% CI 0.91-3.49). (Table 2).
Table 2:
Risk factors for incident musculoskeletal toxicities
| Risk Factor | Adjusted Hazard Ratio (95% Confidence Interval) |
|---|---|
| Age <65 years | 1.59 (1.01-2.5) |
| Female sex | 2.0 (1.31-3.35) |
| Front-line ibrutinib | 1.89 (1.19-3.01) |
| History of autoimmune disease | 1.78 (0.91-3.49) |
Management Strategies and Outcomes
Management strategies and outcomes for arthralgias/myalgias are described in Table 3. Seventeen percent (7/41) were managed by holding ibrutinib (median hold duration, 12 days; IQR 3-17), and 37% (15/41) by ibrutinib dose reduction. Twenty-nine percent (12/41) utilized both strategies and 17% (7/41) stopped ibrutinib altogether without re-challenge. Dose hold without dose reduction was successful in 14% (1/7) of patients, while dose reduction alone alleviated symptoms in 60% (9/15). Statins were discontinued in 8% (6/41) patients, with improvement in symptoms noted in 83% of patients (5/6). Eleven percent (8/76) of patients received corticosteroids of which the median dose was 40 mg (range 20-50 mg prednisone or equivalent) and the median duration 5.5 days (range 5-80 days). Eight percent (6/76) received CoQ10 in conjunction with dose holds and dose reductions with symptomatic improvement in 63% (5/8) and 50% (3/6), respectively. Twenty-seven percent (20/76) of patients received NSAIDs and 12% (9/76) used acetaminophen with improvement in 50% (10/20) and 22% (2/9) of patients, respectively. Eight percent (6/76) of patients required opioid analgesics for pain management.
Table 3:
Arthralgias/Myalgias Management and Outcomes
| Arthralgias/Myalgias Management and Outcomes (n=76) | |||
|---|---|---|---|
| Intervention | Improved | Discontinued | |
| No Change in Management | 47% (35/76) | 19% (19/35) | |
| Dose Reduction | 42% (15/41) | 60% (9/15) | 13% (2/15) |
| Ibrutinib Hold | 17% (7/41) | 40% (1/7) | 28% (2/7) |
| Hold and dose reduction | 29% (12/41) | 8% (1/12) | 83% (10/12) |
| Discontinued without re-challenge | 17% (7/41) | ||
References:
Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 Pt B):2889-2934.
Arthralgias/myalgias resolved in 55% (42/76) of patients during the follow up period (median follow up 26.4 months). Overall, no changes in ibrutinib therapy were made in 51% (39/76) of patients and 54% (21/39) had spontaneous resolution of symptoms. Of these patients, 71% (15/21) had Grade 1 arthralgias/myalgias. The most frequent management strategy for the 16 patients with Grade 3 arthralgias/myalgias was the combination of dose hold and dose reduction, which improved symptoms in 25% (2/8) patients. Ultimately, 63% of patients (10/16) with Grade 3 arthralgias/myalgias discontinued ibrutinib. The overall discontinuation rate for arthralgias/myalgias was 22% (17/76). Management and outcomes did not differ if arthralgias/myalgias occurred before or after 6 months.
Outcomes
In this cohort, 79/214 (36%) patients discontinued ibrutinib: 17/214 (8%) for toxicity related to arthralgias/myalgias; 33/214 (15%) for other toxicity; 19/214 (9%) for disease progression; 9/214 (4%) for transformation; 3/214 (1%) for other reasons. Overall response rate (ORR) for the entire cohort was 82% [relapsed/refractory 78% (105/134); frontline 90% (72/80)). At 24 months, 77% (n=165) of the whole cohort did not have disease progression. Of patients who developed arthralgias/myalgias, seventy-six percent (58/76) of patients had a partial response or partial response with lymphocytosis at the onset of symptoms. ORR was 79% (60/76) at the time of development of arthralgias/myalgias. There was no difference in PFS if patients were treated with ibrutinib for relapsed/refractory disease or in the frontline setting in this cohort (HR 0.6, CI 0.32-1.13 p=0.177). Overall survival of the cohort was not reached at a median follow up of 26.4 months.
DISCUSSION
In this largest reported series focused on ibrutinib-associated arthralgias/myalgias, we observed a high incidence of arthralgias/myalgias, which is consistent with results previously reported in multi-center retrospective and clinical trial populations (approximately 30-40%).1,2,20,23,27 The majority of the patients in our cohort had grade 1-2 toxicity (79%), with only 21% of patients developing grade 3 arthralgias/myalgias. The median time to myalgias/arthralgias was 34.5 months by Kaplan-Meier analysis, which is later than prior reports21 and indicates that this is a toxicity that should be monitored for closely throughout the duration of ibrutinib treatment. We discovered several risk factors associated with developing arthralgias/myalgias including younger age, female sex, and use of ibrutinib as first treatment. While the number of patients who developed arthralgias/myalgias who ultimately discontinued ibrutinib appears high (22% [17/76]), the total discontinuation rate for the whole cohort is similar to the discontinuation rate for all toxicities (8%[17/214]).
The associations of younger age (HR 1.59) and female sex (HR 2.0) with incident arthralgias/myalgias is surprising and may suggest that ibrutinib may exacerbate arthralgias/myalgias in patients who are prone to autoimmune phenomena 28,29 The reason for this sexual dimorphism remains unknown, though there is evidence to suggest that women may be more immunocompetent and have increased immune reactivity30. Additionally, while history of autoimmune disease not statistically significant (p=0.08) in this mutlivariable model, our cohort may have been underpowered to detect this effect due to the small number of patients with a known autoimmune condition in the cohort (n=18). Female sex was the only risk factor for developing Grade 3 arthralgias/myalgias. Although the mechanism remains unknown, this may represent an off-target effect of ibrutinib as suspected with other ibrutinib-associated toxicities such as atrial fibrillation10,31. A recent report has demonstrated that approximately two-thirds of patients with ibrutinib-induced arthralgias/myalgias who were treated with the next-generation BTK, acalabrutinib, did not experience recurrent symptoms supporting the hypothesis that arthralgias/myalgias toxicity is unlikely related to BTK inhibition32.
We observed several management strategies used for ibrutinib-related arthralgias/myalgias in this cohort. For some patients, the arthralgias/myalgias appeared to be self-limited, particularly for those with mild (grade 1-2) arthralgias/myalgias toxicity. In this cohort, dose reductions were the most successful strategy with more than half of patients experiencing resolution of symptoms. Dose hold was utilized for patients with severe (grade 3) toxicity, as recommended by package guidelines in 81% of these patients. Ultimately, 63% of patients with grade 3 or severe toxicity discontinued ibrutinib, indicating that this subgroup of patients may be intolerant to ibrutinib. The impact on long-term outcomes for this subgroup should be studied further since PFS is improved with long-term use of ibrutinib compared to those who discontinue for any reason 18,20,33,34 The use of analgesics was overall low, possibly due to underreporting of over-the-counter medication use 35 Notably, 50% of patients who used NSAIDs reported improvements in arthralgias/myalgias. However, prior reports have recommended avoiding these drugs because they may exacerbate the risk of bleeding 24 In our cohort, no patient had a serious bleeding event.
Outcomes in our cohort were similar to those reported in prior clinical trials and observational studies 1,21,22,27,36,37. ORR was higher for patients treated in the frontline setting (90% vs. 78%). Median PFS was not reached for the entire cohort, nor was OS likely due to the relatively short median follow up (26.4 months). It has previously been shown that the outcomes for patients who discontinue ibrutinib because of toxicity are better than those in patients who discontinue for disease progression or Richter’s transformation. 38,39,20,40 Comparisons of patient outcomes who discontinue ibrutinib for specific toxicities have never been reported. It is unclear whether this is related to the underlying mechanism of toxicity but warrants further study.
Based on our observations, we propose the following algorithm for the management of ibrutinib-associated arthralgia/myalgias (Figure 3): if arthralgias/myalgias are grade 1 or 2 and do not interfere with activities of daily living (as reported by the patient), we recommend continuing ibrutinib at current dose and monitoring closely for symptom progression, as cases can resolve spontaneously. If symptoms affect activities of daily living, clinicians should consider a dose reduction. If there is no improvement at a lower dose, consider a further dose reduction or a dose hold until improvement in symptoms. If symptoms recur with re-challenge after a dose hold, consider permanent discontinuation of ibrutinib and use of alternative CLL directed therapies. For patients with grade 3 arthralgias/myalgias, we recommend dose hold until resolution of symptoms. If symptoms resolve, we recommend re-challenging with a lower dose of ibrutinib. If symptoms do not recur, we suggest continuing the reduced dose of ibrutinib rather than attempting to escalate. If symptoms recur, discontinue ibrutinib and consider other CLL-directed therapies.
Figure 3:
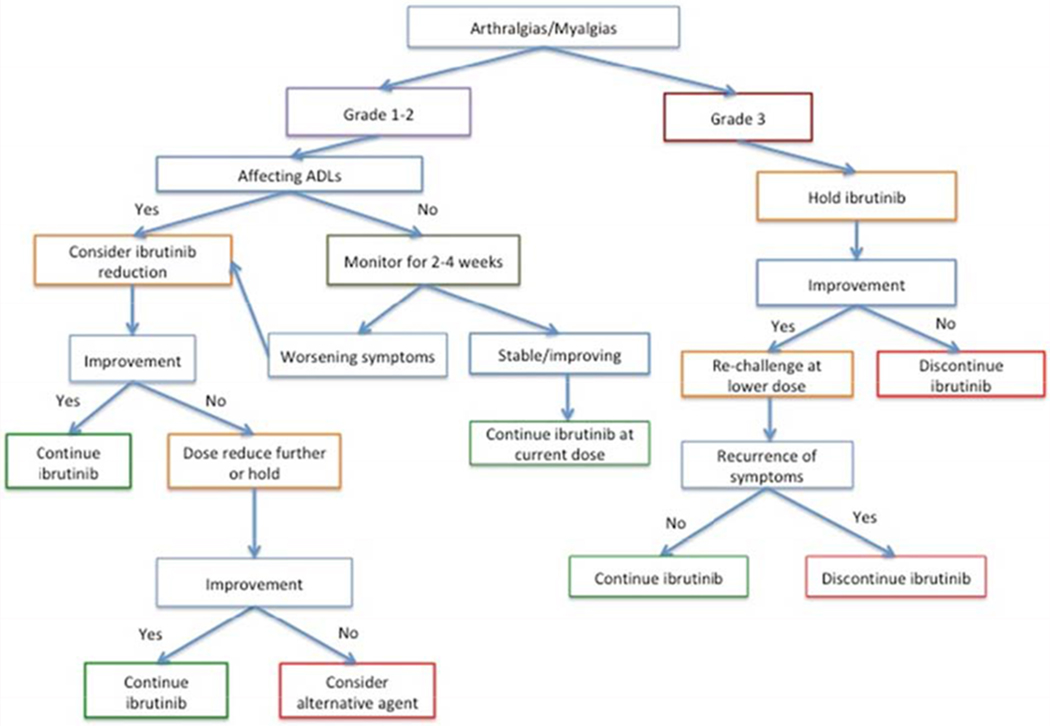
Proposed Algorithm for Ibrutinib-associated arthralgias/myalgias
Our study has several potential limitations. First, our cohort was from a single medical center and was predominantly Caucasian and male. Thus, the results may not be generalizable in other settings or other demographic groups. Second, misclassification or under-reporting of arthralgias/myalgias may have occurred. We minimized the risk of outcome misclassification by having a medical oncologist review the medical records of all patients to adjudicate and grade incident arthralgias/myalgias toxicity events. However, it is still possible that some events might have been missed if they were not reported by the patient or documented by the clinician. Finally, use of over-the-counter medications may have been underreported.
In conclusion, our study highlights the frequency of ibrutinib-related arthralgias/myalgias, occurring in 36% of treated patients. Time to developing arthralgias/myalgias was 34.5 months after starting ibrutinib. Of patient’s experiencing arthralgias/myalgias, most developed at 7 months. Approximately half of grade 1-2 events were self-limited, and dose reductions were successful in managing this toxicity for patients with persistent symptoms. The majority of patients with grade 3 toxicity ultimately had to discontinue ibrutinib. Younger age, female sex, use of ibrutinib as first treatment and response to ibrutinib were significant risk factors for arthralgias/myalgias. Additional studies are needed to understand the mechanisms of ibrutinib-related arthralgias/myalgias and to develop optimal management strategies to maximize responses to ibrutinib.
Clinical Practice Points:
Ibrutinib is a first-in-class BTK inhibitor which is FDA approved for use for the treatment of chronic lymphocytic leukemia in the frontline and relapsed refractory settings. Arthralgias/myalgias are a common side effect, but there are limited descriptions on their management. We identified 214 patients with CLL treated with ibrutinib at a single center. In this cohort, 36% (76/214) of patients developed arthralgias/myalgias during follow-up with a median onset of 34.5 months. Most events (79%) were Grade 1 or 2. Risk factors for developing arthralgias/myalgias included younger age at start of ibrutinib, female sex, and ibrutinib use as first treatment. From this cohort, we propose the following management algorithm: for Grade 1-2 toxicity, if symptoms are not affecting activities of daily living, continue ibrutinib at the current dose and monitor closely for symptom progression, as cases can resolve spontaneously. If symptoms affect activities of daily living, consider a dose reduction. If there is no improvement at a lower dose, consider a further dose reduction or a dose hold until improvement in symptoms. If symptoms recur with re-challenge after a dose hold, consider permanent discontinuation and use of alternative CLL directed therapies. For patients with grade 3 arthralgias/myalgias, we recommend dose hold until resolution of symptoms. If symptoms resolve, we recommend re-challenging with a lower dose of ibrutinib. If symptoms do not recur, we suggest continuing the reduced dose of ibrutinib rather than attempting to escalate. If symptoms recur, discontinue ibrutinib and consider other CLL-directed therapies.
Acknowledgements:
Rebecca Hubbard, PhD for her biostatistics mentorship
Funding: This work was supported by University of Pennsylvania Pharmacoepidemiology T32 (T32 GM075766-11) and a Conquer Cancer Foundation Young Investigator Award (15032)
Disclosures:
Joanna M. Rhodes: Consultancy: AstraZeneca. Anthony R. Mato: Consultancy: TG Therapeutics, Abbvie, Pharmacyclics, Johnson & Johnson, Regeneron, AstraZeneca, Celgene; Research Funding: TG Therapeutics, Abbvie, Pharmacyclics, Johnson & Johnson, Regeneron, Portola, DTRM, and Acerta; Data Safety and Monitoring Board: TG Therapeutics. Elise A. Chong: No disclosures. Jacqueline A. Barrientos: Consulting: Gilead, AstraZeneca, Janssen, Sandoz, Genentech, Bayer, Celgene, Research Funding: Pharmacyclics, Abbvie, AstraZeneca, Gilead James N. Gerson: Consultancy: AbbVie, Pharmacyclics, Seattle Generics; Stefan K. Barta: Honoraria: Jannssen Daniel J. Landsburg: No disclosures. Sunita Dwivedy Nasta, MD: No disclosures. Jakub Svoboda, MD: Research funding: Seattle Genetics, BMS, Merck, Pharmacyclics, TG, Novartis, Incyte, Consultancy: Seattle Genetics, BMS, Celgene, AstraZeneca; Alison W. Loren: No disclosures. Vincent LoRe III: No disclosures. Stephen J. Schuster: Patent: Novartis; Research funding: Novartis, Celgene, Genentech, Merck, Pharmacyclics, Acerta, Gilead; Honoraria: Nordic Nanovector, Pfizer, AstraZeneca, Loxo Oncology, Acerta, Celgene; Membership on an entity’s Board of Directors of advisory committees: Novartis, Nordic Nanovector, Pfizer
Appendix 1:
Univariate Cox Regression Analysis
| Risk Factor | Adjusted Hazard Ratio (95% Confidence Interval) |
|---|---|
| Age <65 years | 1.92 (1.16-3.21) |
| Female sex | 2.15 (1.34-23.45) |
| Front-line ibrutinib | 2.31(1.41-3.79) |
| History of autoimmune disease | 1.82 (0.91-3.64) |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Byrd JC, Brown JR, O’Brien S, et al. Ibrutinib versus Ofatumumab in Previously Treated Chronic Lymphoid Leukemia. New England Journal of Medicine. 2014;371 (3):213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burger JA, Tedeschi A, Barr PM, et al. Ibrutinib as Initial Therapy for Patients with Chronic Lymphocytic Leukemia. N Engl J Med. 2015;373(25):2425–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shanafelt TD, Wang V, Kay NE, et al. A Randomized Phase III Study of Ibrutinib (PCI-32765)-Based Therapy Vs. Standard Fludarabine, Cyclophosphamide, and Rituximab (FCR) Chemoimmunotherapy in Untreated Younger Patients with Chronic Lymphocytic Leukemia (CLL): A Trial of the ECOG-ACRIN Cancer Research Group (E1912). Blood. 2018;132(Suppl 1):LBA-4–LBA-4. [Google Scholar]
- 4.Woyach JA, Ruppert AS, Heerema NA, et al. Ibrutinib Regimens versus Chemoimmunotherapy in Older Patients with Untreated CLL. N Engl J Med. 2018;379(26):2517–2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369(1):32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farooqui MZ, Valdez J, Martyr S, et al. Ibrutinib for previously untreated and relapsed or refractory chronic lymphocytic leukaemia with TP53 aberrations: a phase 2, single-arm trial. Lancet Oncol. 2015;16(2):169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Brien S, Jones JA, Coutre SE, et al. Ibrutinib for patients with relapsed or refractory chronic lymphocytic leukaemia with 17p deletion (RESONATE-17): a phase 2, open-label, multicentre study. Lancet Oncol. 2016;17(10):1409–1418. [DOI] [PubMed] [Google Scholar]
- 8.Chanan-Khan A, Cramer P, Demirkan F, et al. Ibrutinib combined with bendamustine and rituximab compared with placebo, bendamustine, and rituximab for previously treated chronic lymphocytic leukaemia or small lymphocytic lymphoma (HELIOS): a randomised, double-blind, phase 3 study. Lancet Oncol. 2016;17(2):200–211. [DOI] [PubMed] [Google Scholar]
- 9.Moreno C, Greil R, Demirkan F, et al. Ibrutinib plus obinutuzumab versus chlorambucil plus obinutuzumab in first-line treatment of chronic lymphocytic leukaemia (iLLUMINATE): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20(1):43–56. [DOI] [PubMed] [Google Scholar]
- 10.Brown JR, Moslehi J, OB S, et al. Characterization of atrial fibrillation adverse events reported in ibrutinib randomized controlled registration trials. Haematologica. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farooqui M, Valdez J, Soto S, Bray A, Tian X, Wiestner A. Atrial Fibrillation in CLL/SLL Patients on Ibrutinib. Blood. 2015;126(23):2933–2933. [Google Scholar]
- 12.Leong DP, Caron F, Hillis C, et al. The risk of atrial fibrillation with ibrutinib use: a systematic review and meta-analysis. Blood. 2016;128(1):138–140. [DOI] [PubMed] [Google Scholar]
- 13.Lipsky AH, Farooqui MZ, Tian X, et al. Incidence and risk factors of bleeding-related adverse events in patients with chronic lymphocytic leukemia treated with ibrutinib. Haematologica. 2015;100(12):1571–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caron F, Leong DP, Hillis C, Fraser G, Siegal D. Current understanding of bleeding with ibrutinib use: a systematic review and meta-analysis. Blood Advances. 2017;1(12):772–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown JR, Hillmen P, O’Brien S, et al. Extended follow-up and impact of high-risk prognostic factors from the phase 3 RESONATE study in patients with previously treated CLL/SLL. Leukemia. 2018;32(1):83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roeker LE, Maryam Yazdy, Lisa Gashonia, JUlie Goodfriend, Mayur Narkhede, Joanna Rhodes, Kaitlin Kennard, Hannah Morse, Colleen Dorsey, Kristen Battiato, Marissa Peterson, Joseph Carver, Stephen Schuster, Bruce Chest, Anthony Mato Hypertension in Pateints Treated with Ibruitnib for Chronic Lymphocytic Leukemia (CLL) EHA Library 2019:PF386. [Google Scholar]
- 17.Imbruvica Prescribing Information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/205552s002lbl.pdf Accessed October 4, 2017.
- 18.Mato AR, Roeker LE, Allan JN, et al. Outcomes of front-line ibrutinib treated CLL patients excluded from landmark clinical trial. Am J Hematol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mato A, Jahnke J, Li P, et al. Real-world treatment and outcomes among older adults with chronic lymphocytic leukemia before the novel agents era. Haematologica. 2018; 103(10):e462–e465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forum UC. Ibrutinib for relapsed/refractory chronic lymphocytic leukemia: a UK and Ireland analysis of outcomes in 315 patients. Haematologica. 2016;101(12):1563–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Brien S, Hillmen P, Coutre S, et al. Safety Analysis of Four Randomized Controlled Studies of Ibrutinib in Patients With Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma or Mantle Cell Lymphoma. Clin Lymphoma Myeloma Leuk. 2018;18(10):648–657 e615. [DOI] [PubMed] [Google Scholar]
- 22.Mato AR, Hill BT, Lamanna N, et al. Optimal Sequencing of Ibrutinib, Idelalisib, and Venetoclax in Chronic Lymphocytic Leukemia: Results from a Multi-Center Study of 683 Patients. Ann Oncol. 2017. [DOI] [PubMed] [Google Scholar]
- 23.Mato AR, Nabhan C, Thompson MC, et al. Toxicities and outcomes of 621 ibrutinib-treated chronic lymphocytic leukemia patients in the United States: a real-world analysis. Haematologica. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stephens DM, Byrd JC. How I manage ibrutinib intolerance and complications in patients with chronic lymphocytic leukemia. Blood. 2019;133(12):1298–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hallek M, Cheson BD, Catovsky D, et al. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood. 2018; 131 (25):2745–2760. [DOI] [PubMed] [Google Scholar]
- 26.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 Pt B):2889–2934. [DOI] [PubMed] [Google Scholar]
- 27.Mato AR, Allan JN, Pagel JM, et al. Front-line ibrutinib therapy for chronic lymphocytic leukemia (cll) in the real world: Responses, toxicity, outcomes and subsequent therapies. In: American Society of Hematology; 2017. [Google Scholar]
- 28.Eaton WW, Rose NR, Kalaydjian A, Pedersen MG, Mortensen PB. Epidemiology of autoimmune diseases in Denmark. J Autoimmun. 2007;29(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ngo ST, Steyn FJ, McCombe PA. Gender differences in autoimmune disease. Front Neuroendocrinol. 2014;35(3):347–369. [DOI] [PubMed] [Google Scholar]
- 30.Hewagama A, Patel D, Yarlagadda S, Strickland FM, Richardson BC. Stronger inflammatory/cytotoxic T-cell response in women identified by microarray analysis. Genes Immun. 2009;10(5):509–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caldeira D, Alves D, Costa J, Ferreira JJ, Pinto FJ. Ibrutinib increases the risk of hypertension and atrial fibrillation: Systematic review and meta-analysis. PLoS One. 2019;14(2):e0211228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Awan FT, Schuh A, Brown JR, et al. Acalabrutinib monotherapy in patients with chronic lymphocytic leukemia who are intolerant to ibrutinib. Blood Advances. 2019;3(9): 1553–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mato AR, Nabhan C, Barr PM, et al. Outcomes of CLL patients treated with sequential kinase inhibitor therapy: a real world experience. Blood. 2016;128(18):2199–2205. [DOI] [PubMed] [Google Scholar]
- 34.Mato AR, Nabhan C, Thompson MC, et al. Toxicities and outcomes of 616 ibrutinib-treated patients in the United States: a real-world analysis. Haematologica. 2018; 103(5):874–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Delaney JA, Biggs ML, Kronmal RA, Psaty BM. Demographic, medical, and behavioral characteristics associated with over the counter non-steroidal anti-inflammatory drug use in a population-based cohort: results from the Multi-Ethnic Study of Atherosclerosis. Pharmacoepidemiol Drug Saf. 2011;20(1):83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burger JA, Tedeschi A, Barr PM, et al. Ibrutinib as Initial Therapy for Patients with Chronic Lymphocytic Leukemia. New England Journal of Medicine. 2015;373(25):2425–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mato AR, Allan JN, Pagel JM, et al. Front-Line Ibrutinib Therapy for Chronic Lymphocytic Leukemia (CLL) in the Real World: Responses, Toxicity, Outcomes and Subsequent Therapies. Blood. 2017;130(Suppl 1 ):3011–3011. [Google Scholar]
- 38.Mato AR, Tam CS, Allan JN, et al. Disease and patient characteristics, patterns of care, toxicities, and outcomes of chronic lymphocytic leukemia (CLL) patients treated with venetoclax: a multicenter study of 204 patients. In: American Society of Hematology; 2017. [Google Scholar]
- 39.Mato AR, Roeker LE, Allan JN, et al. Outcomes of front-line ibrutinib treated CLL patients excluded from landmark clinical trial. 2018;93(11):1394–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Byrd JC, Furman RR, Coutre SE, et al. Three-year follow-up of treatment-naive and previously treated patients with CLL and SLL receiving single-agent ibrutinib. Blood. 2015;125(16):2497–2506. [DOI] [PMC free article] [PubMed] [Google Scholar]


