Abstract
Extracellular nucleosides and nucleotides have widespread functions in responding to physiological stress. The “purinome” encompasses four G protein-coupled receptors (GPCRs) for adenosine, eight GPCRs activated by nucleotides (P2YRs), seven adenosine 5’-triphosphate(ATP)-gated P2X ion channels, as well as the associated enzymes and transporters that regulate native agonist levels. Purinergic signaling modulators, such as receptor agonists and antagonists, have potential for treating chronic pain. Adenosine and its analogues potently suppress nociception in preclinical models by activating A1 and/or A3 adenosine receptors(ARs), but safely harnessing this pathway to clinically treat pain has not been achieved. Both A2AAR agonists and antagonists are efficacious in pain models. Highly selective A3AR agonists offer a novel approach to treat chronic pain. We have explored the structure activity relationship of nucleoside derivatives at this subtype using a computational structure-based approach. Novel A3AR agonists for pain control containing a bicyclic ring system (bicyclo[3.1.0]hexane) in place of ribose were designed and screened using an in vivo phenotypic model, which reflected both pharmacokinetic and pharmacodynamic parameters. High specificity (>10,000-fold selective for A3AR) was achieved with the aid of receptor homology models based on related GPCR structures. These A3AR agonists are well tolerated in vivo and highly efficacious in models of chronic neuropathic pain. Furthermore, signaling molecules acting at P2X3, P2X4, P2X7 and P2Y12Rs play critical roles in maladaptive pain neuroplasticity, and their antagonists reduce chronic or inflammatory pain, and, therefore, purine receptor modulation is a promising approach for future pain therapeutics. Structurally novel antagonists for these nucleotide receptors were discovered recently.
Keywords: adenosine receptor, P2Y receptor, P2X receptor, pain, agonist, antagonist
Introduction
Chronic pain treatment remains one of the major unsolved medical needs and also accompanies many diseases and pharmacological interventions. Ion channels, G protein-coupled receptors (GPCRs) and kinases are common targets for analgesic drug discovery. However, modulators of purine receptors (either GPCRs or adenosine 5’-triphosphate (ATP)-gated channels) [28,151] are less often considered in pain research, compared to widely used treatments: sodium and calcium channel blockers, γ-aminobutyric acid (GABA) modulators, ligands of opioid and cannabinoid receptors and kinase inhibitors [32]. Nevertheless, the existing treatments are not generally effective in most patients, and the current treatments develop tolerance or have other serious side effect liabilities upon prolonged use. For example, opiates are more effective in acute pain than in chronic pain and can lead to addiction, desensitization and even hyperalgesia. Thus, novel treatment approaches for chronic neuropathic pain are needed.
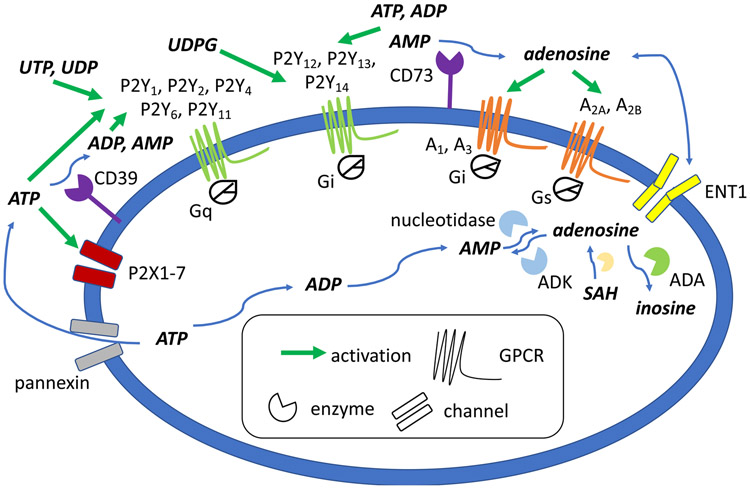
Purine receptors belong to a ubiquitous signaling system in the body that has been termed the “purinome” (Figure 1) [80,185]. ATP and other adenine nucleotides are released during physiological stress, stemming from oxygen and nutrient deprivation, inflammation, cancer, tissue injury, etc., which are ultimately catabolized to adenosine. The immediate response to the stress is activation by ATP of P2X receptors to open ligand-gated cation channels. Each functional P2X ion channel consists of a homomer or heterotrimer of P2X subunits. A more diverse set of adenine and uracil nucleotides activate the eight G protein-coupled P2Y receptors (P2YRs), including nucleoside 5′-diphosphates, 5′-triphosphates and uridine diphosphate (UDP)-sugars. There are eight P2Y receptor subtypes (P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13, P2Y14) and seven P2X receptor subunits (P2X1-7) that comprise an active trimeric channel. The P2X and P2Y receptors tend to boost the immune response in response to nucleotides, with their ligands acting as immediate danger signals [33]. Nucleotide-induced pain is included in this scheme as a beneficial function, as pain is a critical survival mechanism. Subsequent activation of adenosine receptors (ARs), also known as P1 receptors, of which there are four subtypes (A1, A2A, A2B, A3), in general tends to put the brakes on the immune response, and their activation functions endogenously to suppress pain. This signaling system represents a temporal sequence of first activation of proinflammatory P2Rs by nucleotides, followed by the prolonged anti-inflammatory effect of adenosine, mainly formed gradually by enzymatic hydrolysis of released ATP, at the ARs [33]. Thus, in many cases, action of P2Rs increase, and ARs decrease, pain signaling [28,125]. Therefore, often, but not always, P2R antagonists and AR agonists are sought for controlling pain. RNA-seq (RNA-sequencing) analysis has shown that the expression of many of these genes in the purinome is enhanced in the dorsal root ganglia (DRG) and the spinal cord (SC) (Table 1) [146].
Figure 1.
Purinergic signaling pathways for purine nucleosides and nucleotides, and pyrimidine nucleotides. Extracellular ATP and other nucleotides originate from intracellular sources through cell damage, cotransmission, pannexin hemichannels, and other mechanisms. These nucleotides act on P2Y (GPCRs, activated by triphosphates, diphosphates and UDP-sugars) and P2X (ion channels, mainly by ATP) receptors. Ectonucleotidases (CD39, CD73) are largely responsible for the formation, from ATP, of adenosine that activates its four receptors. In general, adenosine receptor agonists and P2X/P2Y receptor antagonists induce pain relief in various models.
Table 1.
Message for purinergic receptors and selected associated enzymes and a transporter in DRG and SC, expressed as transcripts per million (TPM) using RNA-seq data [146]. Other names for enzymes listed below are: ENTPD1 (ectonucleoside triphosphate diphosphohydrolase 1, CD39), NT5E (ecto-5'-nucleotidase, CD73), ENPP1 (ectonucleotide pyrophosphatase/phosphodiesterase 1), SLC29A1 (equilibrative nucleoside transporter 1, ENT1).
| A. | ||
|---|---|---|
| Mouse GENE | DRG | SC |
| ADORA1 | 96.424 | 143.604 |
| ADORA2A | 6.218 | 1.342 |
| ADORA2B | 0.969 | 6.501 |
| ADORA3 | 0.01 | 1.411 |
| P2RY1 | 25.792 | 10.238 |
| P2RY2 | 29.923 | 0.521 |
| P2RY4 | 0.01 | 0.01 |
| P2RY6 | 2.968 | 4.65 |
| P2RY12 | 3.069 | 11.464 |
| P2RY13 | 0.343 | 4.303 |
| P2RY14 | 1.383 | 1.862 |
| P2RX1 | 0.091 | 0.01 |
| P2RX2 | 2.332 | 0.37 |
| P2RX3 | 120.479 | 1.932 |
| P2RX4 | 48.646 | 27.775 |
| P2RX5 | 26.276 | 13.083 |
| P2RX6 | 40.086 | 15.987 |
| P2RX7 | 23.197 | 5.529 |
| ENTPD1 | 9.469 | 96.8 |
| NT5E | 7.621 | 2.776 |
| ENPP1 | 6.814 | 8.653 |
| ADK | 422.075 | 531.732 |
| SLC29A1 | 93.294 | 57.227 |
| B. | ||
| Human GENE | DRG | SC |
| ADORA1 | 16.475 | 48.6 |
| ADORA2A | 2.237 | 8.659 |
| ADORA2B | 0.847 | 1.49 |
| ADORA3 | 65.881 | 33.548 |
| P2RY1 | 1.275 | 0.621 |
| P2RY2 | 1.484 | 0.838 |
| P2RY4 | 0.01 | 0.124 |
| P2RY6 | 3.889 | 1.924 |
| P2RY11 | 15.252 | 15.424 |
| P2RY12 | 23.365 | 10.179 |
| P2RY13 | 4.851 | 3.693 |
| P2RY14 | 6.429 | 2.638 |
| P2RX1 | 0.575 | 2.017 |
| P2RX2 | 0.115 | 0.093 |
| P2RX3 | 53.911 | 0.01 |
| P2RX4 | 21.389 | 24.89 |
| P2RX5 | 36.934 | 1.334 |
| P2RX6 | 22.026 | 11.886 |
| P2RX7 | 1.464 | 18.807 |
| ENTPD1 | 7.077 | 23.276 |
| NT5E | 6.377 | 5.71 |
| ENPP1 | 1.307 | 0.683 |
| ADK | 61.783 | 207.371 |
| SLC29A1 | 29.553 | 23.028 |
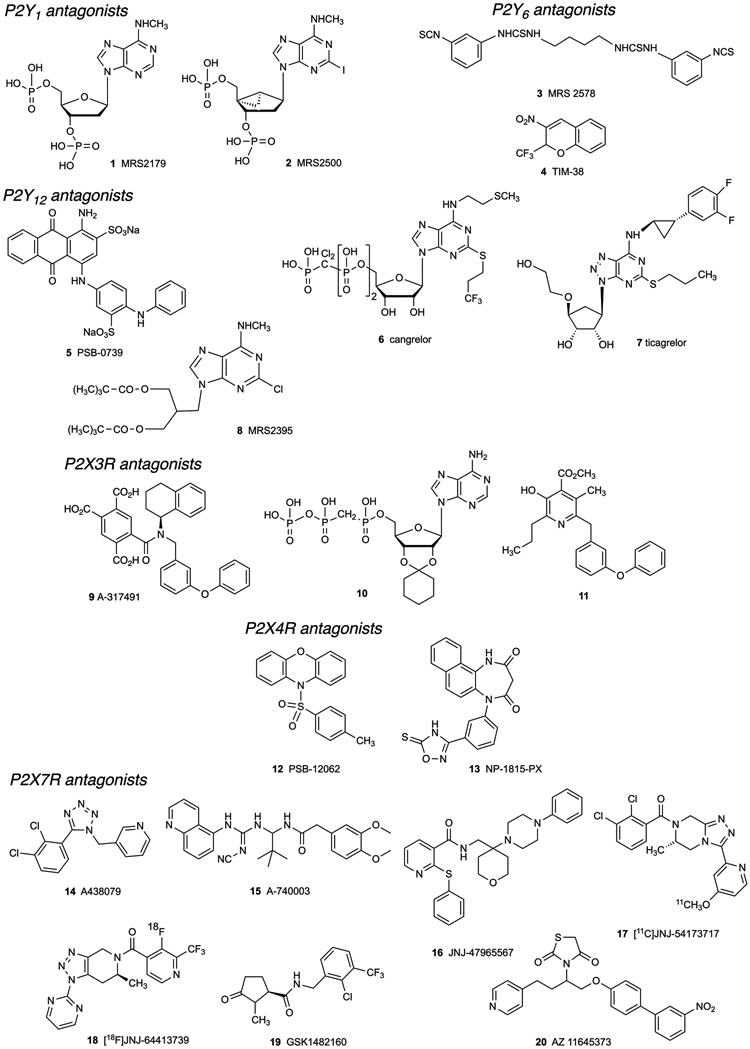
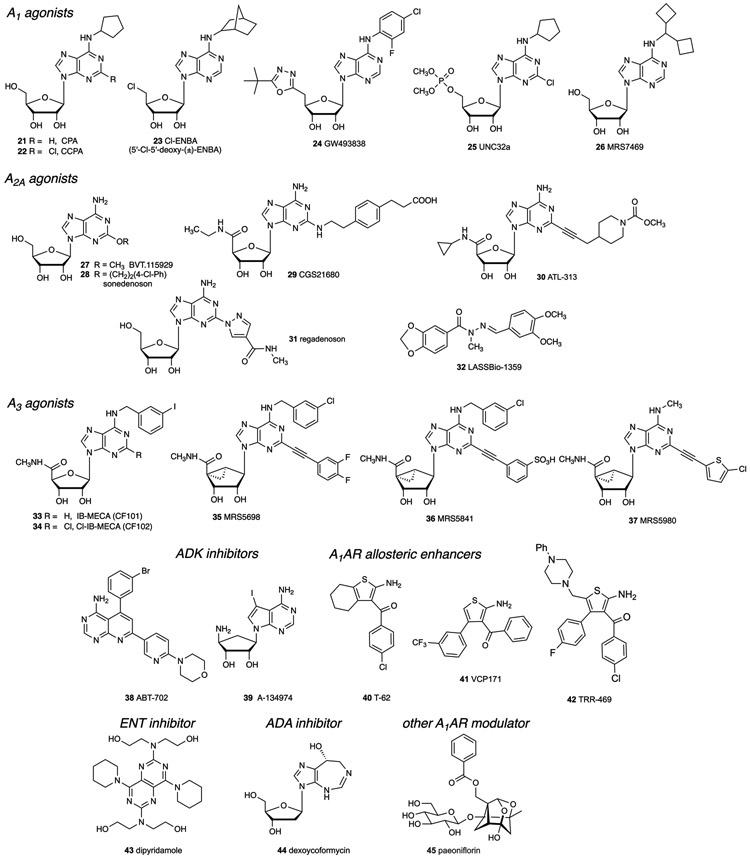
Many compounds are now available as selective modulators of the purinergic system, either as directly acting agonists and antagonists of P2Rs (Figure 2) or ARs (Figures 3 and 4) [80], or as inhibitors of associated enzymes and transporters that regulate native levels of adenosine (Figure 4) or nucleotides. Inhibitors of enzymes involved in processing of adenosine include the widely used adenosine kinase inhibitors ABT-702 38, a pyridopyrimidine, and the nucleoside A-134974 39 (Figure 3) [87]. Inhibitors of these enzymes and transporters, in some cases, are approved drugs. For example, adenosine deaminase (ADA) inhibitor, pentostatin (dexycoformycin) 44, is an anticancer drug. Dipyridamole 43, a clinical vasodilator, inhibits equilibrative nucleoside transporters 1 and 2 (ENT1/2) and also acts as a phosphodiesterase (PDE) inhibitor.
Figure 2.
Structures of representative P2YR and P2XR antagonists that have been used in pain studies.
Figure 3.
Structures of representative AR agonists that have been used in pain studies.
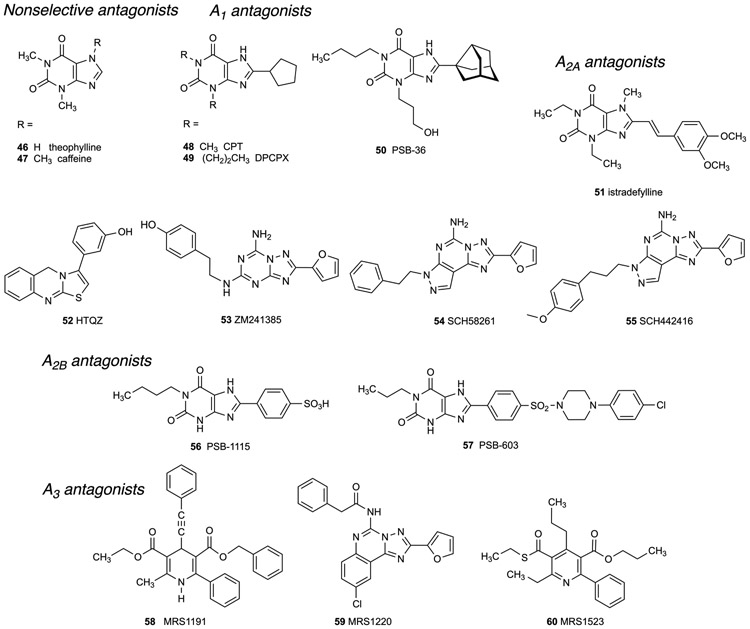
Figure 4.
Structures of representative AR antagonists that have been used in pain studies.
P2XR ligands and their use in relieving chronic pain
Various P2XR antagonists have shown efficacy in pain models [17]. For example, involvement of P2XRs has been explored in acupuncture analgesia and central pain syndrome [107,169]. In neuropathic pain, adrenomedullary chromaffin cells overexpress P2X3 and P2X7 [7]. Early studies of the mechanism of pain induction by ATP focused on the observation that the P2X3R and P2X2/3R are localized and function in neurotransmission at the DRG (Table 1), the main conduit for pain signaling by the peripheral nerve nociceptors [37,112]. ATP is produced by inflammatory cells, tumor cells, endothelial cells, sympathetic neurons, and Merkel cells in the skin and other cells. A local elevation of the extracellular levels of ATP can act to stimulate peripheral sensory nerves. It was demonstrated that clorodonate, a first-generation bisphosphonate was able to attenuate hyperalgesia in rodents models of carrageenan- and complete Freund’s adjuvant (CFA)-evoked inflammatory pain via inhibition of vesicular ATP release, as well as in a partial sciatic nerve injury model of chronic neuropathic pain [98]. Furthermore, the acidification that accompanies both inflammation and the hypoxic tumor microenvironment can promote activation of the P2XRs by known phenomenon of pH modulation of the receptor protein [40].
P2X3R antagonist antinociceptive effects:
Both pharma and academic labs have maintained a long-term effort to produce a P2X3R antagonist that might be effective in controlling pain [25,76,165,172]. However, there are currently no P2X3 antagonists in clinical trials for pain, but one is in trials for chronic cough [134]. Various P2X3 antagonists have been reported [65], including A-317491 9, which is potent and selective but not orally bioavailable. This first-reported selective P2X3R antagonist reduces neuropathic, inflammatory and chemogenic pain following intrathecal or intraplantar administration [129]. P2X3R antagonists have been shown to be efficacious in reducing chronic pain in animal models of cancer [55,94] and in several animal models of neuropathic pain such as chronic constriction injury (CCI) of sciatic nerve [152], spinal nerve injury [67], partial ligation of the unilateral infraorbital nerve [154], chronic pancreatitis [189], maternal separation [198] and inflammatory pain [88,91]. Recently, nucleotide derivatives with the ribose 2′,3′-hydroxyl groups in a chemically protected state were reported to be P2X3R antagonists, e.g. 10 [44]. Upregulation of various purine receptors in pain pathways is a consequence of the chronic pain state [123]. Peripheral inflammation is associated with plasticity of purinergic signaling within sensory ganglia. The P2X3R in the DRG and trigeminal ganglia (TG, responsible for sensation in the face and cranium and motor functions in the mouth) are located on the neurons, at the pre- or post-synaptic terminals [27,135,139,157,186]. Neuronal plasticity leading to upregulation of the P2X3R on these ganglia has been observed in conditions of inflammation [49]. Novel P2X3R antagonists such as 11 were studied in models of chronic pain [93]. A methyl ester prodrug derivative appears to be cleaved in vivo to form the active carboxylic acid.
P2X4R antagonist antinociceptive effects:
The P2X4R, P2X7R and P2Y12R are upregulated in chronic pain states leading to hypersensitivity of the microglia and associated neurons [64]. This is a function of peripheral nerve injury (PNI), but not peripheral tissue inflammation. P2X4R activation has an additional consequence of the release of brain-derived neurotrophic factor (BDNF) from microglial cells, which acts upon neighboring neurons to interfere with the GABAergic anti-nociceptive system. Fibronectin acts upon pain-activated microglia to upregulate the expression of P2X4 [14,128]. BDNF binds to neuronal tropomyosin receptor kinase B (TrkB) in spinal lamina I to interfere with K+-Cl−co-transporter (KCC2), which normally maintains a low level of intracellular chloride in the SC neurons [43,111]. Consistent with a lack of microglial role in the development and maintenance of neuropathic pain in females, this mechanism was found to be essential only in male mice [156] since the P2rx4 gene was upregulated only in dorsal horn of male, but not female mice, after PNI [47,95,147,156]. Despite qualitative sex differences in neuroimmune signaling after nerve injury, neurons in males and females ultimately experience a similar reduction in KCC2 function, leading to comparable levels of pain hypersensitivity [127]. The rise in intracellular chloride ions as a result of macrophage P2X4R activation diminishes the ability of drugs that directly or indirectly activate GABAA ion channels, to reduce chronic pain. Therefore, if a clinical P2X4R antagonist were to be made available, it might make the existing pain medicines more effective as well as having its own antinociceptive activity. Furthermore, a peripheral action of P2X4R antagonists on macrophages reduces the release of prostaglandin E2 (PGE-2) by a p38-mitogen-activated protein kinase (MAPK)-dependent mechanism, which acts as a pro-nociceptive signal [179]. Therefore, both spinal and peripheral sites of action of such antagonists could contribute to an analgesic effect [28].
Thus, P2X4R and P2X7R antagonists have been considered for pain control through their action on spinal microglial and other cells. Pharmaceutical companies and academic labs have developed numerous compounds to antagonize these receptors that are drug-like molecules and well tolerated in the body (Figure 2) [35,45,138,168]. PSB-12062 12 is a P2X4R antagonist of μM affinity (human 1.38 μM, rat 0.928 μM, mouse 1.76 μM) [161], but it has low aqueous solubility. P2X4R knockout (KO) mice displayed normal acute pain responses and tissue damaged-induced pain, but reduced chronic pain, especially tactile allodynia [181]. P2X4R antagonists have been shown to be efficacious in reducing chronic pain in diabetes-induced neuropathic mechanical hyperalgesia [170] and in a model of trigeminal allodynia [117]. IgG#191 is a potent and selective antibody binding the head domain of P2X4 and able to reverse mechanical hyperalgesia in a mouse model of neuropathic pain induced by the Seltzer partial sciatic nerve ligation. IgG#191 does not cross the blood-brain barrier (BBB) but a chimeric construct containing a domain which can be transported across the BBB into the central nervous system (CNS) showed dose-dependent reversal of mechanical hyperalgesia, indicating that with appropriate molecular modification, IgG#191 could access the SC from the periphery [190]. The selective P2X4R antagonist NP-1815-PX 13 displayed anti-allodynic effects in traumatic nerve damage and herpetic mouse models without affecting acute pain, however it does not readily cross the BBB [128]. NP-1815-PX was discovered as a result of a screen of chemical libraries and displays high aqueous solubility. NC-2600 (structure not disclosed) was developed in a collaboration between Prof. K. Inoue at Kyushu Univ. and academic and pharma collaborators (Nippon Chemipharma) and entered a Phase 1 clinical trial for chronic neuropathic pain in Japan, in June 2016.
P2X7R antagonist antinociceptive effects:
Various P2X7R antagonists have been reported to be active against pain and inflammation [31]. P2X7R antagonists A-438079 14 and A-740003 15 have been shown to be efficacious in reducing chronic neuropathic and inflammatory pain [71,137]. Furthermore, the more potent antagonist A-740003 is highly P2X7R-selective in both human and rodent species. Microglial P2X7R activation contributes to maintaining advanced bone cancer pain by releasing proinflammatory cytokine interleukin-18 (IL-18) [197]. However, there have been clinical trials for P2X7R antagonists in inflammatory diseases, cancer, and depression. JNJ 47965567 16 is a brain penetrant P2X7R (human, mouse and rat) antagonist of nearly nM affinity. Several positron-emission tomography (PET) ligands (17, 18) for brain imaging of the P2X7R have been reported. P2X7R antagonist GSK1482160 19 developed to target peripheral pain and has been in a clinical trial for inflammatory pain (Table 2). It was also radiolabeled with 11C for PET imaging [57,191]. AZ 11645373 20 is a highly selective human P2X7R antagonist but does not bind to rodent P2X7Rs.
Table 2.
Representative recent clinical trials of AR agonists, an A1AR PAM and P2R antagonists in pain conditions (data from clinicaltrials.gov, accessed 5-8-2019). The only ongoing clinical development is P2X4R antagonist NC-2600 (unpublished).
| Receptor | Condition | Compound (route) |
Years | Phase, NCT# | References |
|---|---|---|---|---|---|
| A1 | Neuropathic pain | adenosine 1 (i.t.) | 2014-2018 | 2, 00349921 | [208] |
| Perioperative pain | adenosine 1 (i.v.) | 2006 | 2, 00298636 | [92] | |
| Neuropathic pain | GW493838 5 | 2002-2003 | 2, 00376454 | [48] | |
| Postherpetic neuralgia | T-62 18 | 2008-2012 | 2, 00809679 | [130] | |
| A2A | Diabetic nerve pain | BVT.115929 7a | 2007-2014 | 2, 00452777 | [101] |
| Diabetic foot ulcers | Sonedenoson 7b | 2006-2012 | 2, 00318214 | [132] | |
| A2B | none | - | - | [164] | |
| A3 | none | - | - | [85] | |
| P2X4 | neuropathic pain | NC-2600 | 2016-2017 | 1 | www.chemiphar.co.jp |
| P2X7 | inflammatory pain | GSK1482160 50 | 2009-2017 | 1, 00849134 | [57] |
Pannexin-1 (Panx1) hemichannels are permeable to small molecules (<1 KD) and are the source of much extracellular ATP in the SC subsequent to morphine treatment, which can activate P2XRs [26]. ATP originating in spinal microglia in the dorsal horn contributes to long-term synaptic facilitation that is characteristic of opioid withdrawal. Pharmacological inhibition of ATP breakdown exacerbates opioid withdrawal, suggesting its detrimental action occurs through P2Rs, although a potential beneficial effect of adenosine produced from ATP hydrolysis was not addressed.
P2YR ligands and their use in relieving chronic pain
Certain P2Rs present on the DRG and on the associated SGCs (satellite glial cells) are upregulated in pain states [123]. The receptors that are upregulated or activated in sensory neurons, such as the DRG and sciatic nerve include: neuronal and glial P2Y1R, P2Y2R, P2Y6R, P2Y11R (all Gq-coupled) as well as P2Y12R and P2Y14R (Gi-coupled) [11,133,207]. Adenosine-5’-diphosphate (ADP) is the principal endogenous agonist of P2Y1R, P2Y12R and P2Y13R. UDP is the principal endogenous agonist of P2Y6R. UDP-glucose is the principal endogenous agonist of P2Y14R.
P2Y1R antagonist antinociceptive effects:
P2Y1R antagonists have been shown to be efficacious in reducing chronic and acute pain in several animal models such as neuropathic pain model of spinal nerve ligation (SNL), CCI, spared nerve injury (SNI) [12], formalin and carrageenan-induced inflammatory pain [11,109], visceral hypersensitivity in rats with experimental irritable bowel syndrome [192], cancer-induced bone pain (CIBP) [38] and migraine [124]. The different cell types involved include: dorsal root, trigeminal, nodose sensory neurons, SGCs [145] and in the SC, astrocyte [59] and some of the dorsal horn neurons [102]. Potent and selective P2Y1R antagonist MRS2500 2 reduces peripheral interleukin-1β (IL-1β)-mediated thermal hypersensitivity [109]. P2Y1R activation stimulates visceral nociceptors, an effect blocked by MRS2500 [68]. The upregulation of neuronal purine receptors in pain states can be interrupted by receptor antagonists. For example, MRS2500 reduced upregulation of P2Y1R in DRG induced by nerve injury [33]. Also, the P2Y1R intensifies the thermal hyperalgesia mediated by transient receptor potential cation channel subfamily V member 1 (TRPV1) in peripheral nerves, through its protein kinase C-dependent phosphorylation, leading to a pro-nociceptive TRPV1 as shown in an ischemic model [110]. This process was blocked by P2Y1R antagonist MRS2179 1.
P2Y12R antagonist antinociceptive effects:
The Gi-coupled P2Y subfamily consists of three subtypes: P2Y12R, P2Y13R and P2Y14R. P2Y12R already has antagonists that are used clinically for the prevention of thrombosis. This receptor is considered a primary sensor of ATP and ADP in vivo, is present on neurons of peripheral sensory ganglia, and its presence in SGCs is induced in pain states [97]. A neuronal P2Y12R is also involved in excitability. 3 days of successive administration of uncharged P2Y12R antagonist MRS2395 8 reversed glial cell activation in rat SGCs of the TG and alleviated mechanical and heat sensitivity of the tongue [125]. Also, PSB-0739 5, a competitive, polyanionic antagonist of P2Y12R has proven useful in studies of pain [72]. The microglial cells are the resident macrophage-like immune cells in the brain and SC, accounting for roughly 10-15% of the total cell numbers. Naturally, purine receptors have prominent roles in the function of microglial cells. In the SC, the microglial cells tend to amplify the pain signals by producing proinflammatory cytokines and other nociceptive modulators. Potential mechanisms have been described for purine receptors on activated microglial cells to induce hyperexcitability in the spinal dorsal horn, to increase neuropathic pain [146,180].
P2Y12R antagonists have been shown to be efficacious in reducing chronic and acute pain in the CFA-induced inflammatory pain model, in some models of neuropathic pain induced by partial sciatic nerve ligation (PSNL) [72], SNI [103] and spinal nerve transection (SNT) [64], in CIBP [118] and in some orofacial pain models (tongue cancer pain [167] and neuropathic tongue pain [97,163]). This likely involves action at multiple cell types: neurons, microglia and satellite cells of the dorsal and TG [163,177,178,188]. P2Y12R serves as a find-me signal for microglial cells, i.e. it induces motility [70]. Because P2Y12R antagonists are already in widespread use in the clinic, there are opportunities for testing purine-based hypotheses in humans that are not yet feasible for most other purine receptors. However, a liability of P2Y12R antagonists would be the potential for increased bleeding. Currently there is no basis to separate the two-mechanism based actions of P2Y12R antagonists, i.e. analgesic and antithrombotic effects. Numerous P2Y12R antagonists and prodrug inhibitors have been developed as antithrombotics. Among the clinically accepted antagonists of the P2Y12R, cangrelor (ARC-69931MX 6) is a competitive P2Y12R antagonist of sub-nM affinity. However, as a nucleotide analogue it is not orally bioavailable, but it is active upon injection in vivo. Ticagrelor 7 is also a competitive P2Y12R antagonist, but it additionally inhibits ENT1, thus raising the levels of endogenous adenosine. Other P2Y12R antagonists, e.g. thienopyridines clopidogrel and prasugrel (structures not shown), used widely in the clinic act non-competitively by binding to the receptor after preactivation in the body; thus, they are prodrugs. MRS2395 8 is a weak, uncharged P2Y12R antagonist that has been used in pain studies [97,118,167].
P2Y6R antagonist antinociceptive effects:
Other microglial P2YRs that have a role in pain are P2Y4R, P2Y6R and P2Y13R [123]. P2Y6R serves to induce phagocytosis in microglial cells [105]. SGCs express P2Y14R, which is associated with inflammatory cytokine release [114]. There are few known antagonists of the P2Y6R, but agonist structure-activity relationships (SAR) has been extensively explored. MRS2578 3 is a potent, but hydrophobic and likely noncompetitive P2Y6R antagonist, and the more recently reported TIM-38 4 is a weak antagonist [78]. P2Y6R activation suppresses the P2X4R-induced current amplitude, activation and channel permeability in microglia, suggesting that a P2Y6R agonist might provide therapeutic benefit [16]. However, a recent paper reports the opposite effect based on a protective effect elicited by P2Y6 antagonist MRS2578 but not P2Y6 agonist UDP in a model of neuropathic pain. In particular, intraperitoneal administration of MRS2578 alleviated CCI-induced hyperalgesia. Conversely, treatment of UDP on CCI rats increased pain intensity [18].
P2Y14R antagonist antinociceptive effects:
P2Y14R is widely expressed throughout the body [34], and it is found in multiple parts of the nervous system [133]. Its mRNA is expressed in various immune cells as well as glia and neuronal cells [34,133], therefore P2Y14R is speculated to play a role in neuroimmune responses. Evidence supporting this was observed after acute challenge of rats with lipopolysaccharide (LPS) where an increase in P2Y14R mRNA expression across various regions of the brain occurred [36,133]. In addition, P2Y14R seem to be involved in chronic pain. One study observed increase in P2Y14R mRNA levels in the dorsal horn of SC from days 3 to 14 after a spared nerve injury [103]. Moreover, the in-situ hybridization of dorsal horn sections showed that expression of P2Y14R mRNA was elevated selectively on the ipsilateral side and that it co-localized with Iba1, which indicates that the upregulation happened in microglia [103]. The same study found that the administration of antisense-locked nucleic acid (AS-LNA) to inhibit P2Y14R for 7 days reversed SNI-induced mechanical allodynia on days 5 and 7 [103]. The P2Y14R is expressed on both neurons and SGCs in the rat TG [114]. Its upregulation in TG was observed in an inflammatory pain model (CFA-induced pain) and that was associated with increased release of anti-inflammatory cytokines and the activation of MAP kinases [115]. In addition, using PPTN, which is a selective P2Y14R antagonist, attenuated mechanical hyperalgesia in this inflammatory pain model [115].
Functional modal shifts of purine receptors occur upon microglial activation: P2X4R and A2AAR are upregulated, and P2Y12R, A1AR and A3AR are downregulated [104]. Thus, when considering the application of purinergic modulation to chronic neuropathic pain it is important to consider the relationships in the pathological state, as distinct from the normal state of a tissue.
AR ligands and their use in relieving chronic pain
The ARs are activated by endogenous adenosine, the ubiquitous agonist produced by all cells. AR activity can also be modulated by exogenous agonists and antagonists or by inhibition of proteins that control adenosine transport, formation and degradation [5,33]. AR agonists and antagonists can be competitive with adenosine by binding to the same common site conserved among the four ARs (the orthosteric binding site), or they can interact non-competitively by binding to a separate, allosteric site (Figure 3). Each of the four ARs has numerous selective orthosteric agonists and antagonists, and their ligand binding SAR have been reviewed elsewhere [89]. Extensive molecular modeling of the ARs has also been performed based on X-ray structures of the A2AAR and more recently of the A1AR [61]. This modeling is informative in interpreting the SAR at any given subtype and guiding the discovery of new AR ligands [89]. Allosteric enhancers, i.e. positive allosteric modulators (PAMs), for the A1AR and the A3AR have been well characterized pharmacologically [82].
A1AR antinociceptive effects:
A number of clinical trials of potential analgesic agents acting as agonists at the Gi/o-coupled A1AR (e.g. GW493838 24) and the Gs-coupled A2AAR (e.g. BVT.115959 27 and sonedenoson 28) have been performed in recent years (Table 2, Figure 3) [208]. However, all of the trials were discontinued due to lack of efficacy or the presence of side effects. The reason for the lack of efficacy is unclear, because the same compound tested clinically, i.e. A1AR PAM T-62 40, produced significant benefit in extensive preclinical tests of efficacy in pain and related conditions. The side effects, in general, may or may not be AR mechanism-related. Therefore, it may not be appropriate to rule out testing any new potential analgesic agents acting at the A1AR or the A2AAR, simply based on the unsuccessful trials so far. However, it must be noted that A1AR or A2AAR agonists would likely display cardiovascular side effects that would limit the dose range and utility of these agents in the general population. Only two AR agonists have been approved for clinical use, but not for pain: adenosine itself and regadenoson 31, but both lack oral bioavailability. Several non-selective AR antagonists are used clinically, but the only selective AR antagonist currently approved for human use is istradefylline 51 [21]. Nonselective AR antagonist caffeine 47 is sometimes present in over-the-counter pain medications.
A1AR KO mice (A1AR−/−) were used to evaluate the role of the A1AR in nociception. Under normal conditions, as well as during inflammatory or neuropathic pain, A1AR−/− animals showed hypersensitivity to heat in comparison to the wild-type (WT) mice. No significant differences were found in terms of mechanical withdrawal threshold and cold response. A1AR−/− mice also showed reduced antinociceptive effect of morphine given intrathecally, but not systemically [193]. The basis for the analgesic actions of A1AR activation are multifold, as reviewed by Sawynok [151]. A1AR is highly expressed in the nervous system, including on the central terminals of primary afferent neurons of the SC and cell bodies in the dorsal horn. A1AR activation produces presynaptic inhibition of primary afferent neurotransmission onto dorsal horn neurons by decreasing release of glutamate, substance P and other transmitters from primary afferents. A1AR also hyperpolarizes dorsal horn neurons by increasing K+ conductance and potentiates the inhibitory postsynaptic transmission mediated by glycine receptors [9]. A1AR activation on nociceptive neurons by adenosine generated from locally-released ATP has also been suggested as a mechanism of action of acupuncture [62]. Consistently, it is reported that caffeine, a non-selective AR antagonist, and 8-cyclopentyl-1,3-dipropylxanthine (DPCPX 49), a selective A1AR antagonist, reduce the antinociceptive effect of acupuncture [56,201]. Central or peripheral administration of A1AR agonist CPA 21 in the mouse improved mechanical allodynia resulting from diabetic neuropathy [99]. Data on A1AR antagonists or A1AR KO mice in diabetic neuropathy models has not been reported. CPA showed the ability to attenuate the mechanical hyperalgesia induced by PGE2, but when injected repeatedly it causes tolerance, dependence and changes in nociceptor function producing mechanical hyperalgesia similar to the effect of μ-opioid receptor agonists [6]. Downregulation of A1AR contributes to neuropathic pain in a mouse model of neuropathy induced by resiniferatoxin, a capsaicin analogue that acts on TRPV1 [96]. Luongo et al. [121] demonstrated the analgesic properties of an A1AR agonist in a mouse model of neuropathic pain (SNI) that were antagonized by the A1AR-selective antagonist DPCPX. The agonist used was Cl-(±)-ENBA 3, which is particularly potent and selective (>3000-fold selective in binding to the mouse A1AR) [30]. However, Cl-(±)-ENBA is a mixture of two diastereoisomers, each of which can have distinct pharmacokinetic and pharmacodynamic properties. An A1AR agonist of similar affinity, selectivity and in vivo activity is MRS7469 26, which is a pure isomer [176]. A caveat in the use of A1AR agonists in vivo is that even compounds that have only 100s of fold selectivity in binding to the A1AR compared to A2AAR and A3AR can display nonselective activity at the latter subtypes. For example, Reitman and coworkers found that A1AR agonist CPA 1 activates mast cell A3AR to produce hypothermia in mice at the same modest doses (e.g. 0.3 mg/kg, intraperitoneal (i.p.)) at which a central A1AR activation was seen [30]. Thus, it is essential to use antagonists to probe the receptor subtype selectivity of an assumed selective agonist in a given model. The use of AR subtype KO mice also can strengthen the conclusion that a particular AR subtype is involved.
Paeoniflorin 45, a natural phytochemical and a component of Chinese traditional medicine, protected against mechanical and thermal pain in the PSNL mouse model at high doses. This protection was blocked by moderately selective A1AR antagonist CPT 48 and in A1AR KO mice [199]. However, the mechanism of A1AR activation by paeoniflorin was not established. Neuropeptide S injected intracerebroventricularly significantly reduced formalin-induced nociception during both phases of the formalin test by activating both A1AR and A2AAR during phase 1, but only the A2AAR during phase 2 [69]. UNC32A 25 is an A1AR agonist structurally related to adenosine 5′-monophosphate (5′-AMP), a nucleotide able to activate A1AR. UNC32A did not show cardiovascular side effects and caused dose-dependent antinociceptive effects in WT mice when administered orally [106].
Allosteric modulation of the ARs is one means of potentially avoiding their cardiovascular side effects [77,184]. A1AR PAM TRR469 42, an aminothiophene, was anti-allodynic in a chronic neuropathic pain model without locomotor or cataleptic side effects. It attenuated nociceptive responses in the formalin and writhing tests, similarly to morphine’s effects. Coadministration of TRR469 and an A1AR agonist CCPA 2 produced additive protection in a pain model. The effects of coadministered CCPA and TRR469, or each agent alone were antagonized by PSB36 50, a highly A1AR-selective antagonist. The anti-nociceptive effect of TRR469 was additive with those of morphine. VCP171 41, an A1AR PAM of the same chemotype as 40 and 42, reduced the evoked excitatory postsynaptic current (eEPSC) amplitude in the SC dorsal horn to a larger degree in the nerve injured state than in control rats, suggesting that an elevated adenosinergic tone in that tissue would enable the use of A1AR PAMs in pain therapy [77].
The role of the A2AAR in pain is somewhat controversial in that pro- and anti-nociceptive have been documented.
A2AAR antinociceptive effects:
A2AAR activation as well as A1AR activation can reduce the release of excitatory neurotransmitters such as glutamate [63]. Watkins and colleagues showed that selective A2AAR agonist ATL-313 30 infused in the spinal column decreased chronic pain in a manner that was long lasting, and repeated administrations in four-week intervals remained efficacious [120]. They also demonstrated that a single intrathecal injection of A2AR agonists CGS21680 29 or ATL313 administered between 1 and 7 weeks after SC injury reversed spinal neuropathic avulsion pain (SNAP) for up to 6 weeks [108]. A single i.p. injection of CGS21680 was also able to significantly decrease the formalin-induced licking behavior in both the early (0-15 min) and late (15-60 min) phase in a mouse model of formalin-induced inflammatory pain [136]. Furthermore, a single peripheral injection of the potent but nonselective A2A-agonists, 5’-(N-ethyl)-carboxamido-adenosine and 2-phenylaminoadenosine induced a decrease in mechanical nociceptive threshold in the hindpaw of the rat that was antagonized by an A2AAR antagonist, PD 081360-0002 (HTQZ, 52) [166]. Intracerebroventricular injection of adonis, an agonist-like monoclonal antibody with high specificity for the A2AAR, led to a significant dose-dependent increase in hot-plate and tail-flick latencies in mice. This effect was prevented by caffeine and ZM241385 53, a specific A2AAR antagonist [29]. In another study, LASSBio-1359 32, an A2A AR agonist of atypical structure, inhibits the hyperalgesic response caused by acute and chronic inflammation induced by formalin, carrageenan or CFA injection [131].
A2AAR pronociceptive effects:
A2AAR activation might enhance the release and stimulatory effects of excitatory neurotransmitters such as glutamate [153]. It has been demonstrated that KO mice lacking the A2AAR are less sensitive to nociceptive stimuli; this is due to a large reduction in the density of NMDA glutamate receptors [74] as well as a reduced neuronal activity in the SC [75]. In A2AAR KO mice, carrageenan-induced hyperalgesia was significantly reduced compared to WT controls; furthermore, a selective A2AAR antagonist ZM241385, when injected into the hindpaw, reduced the mechanical hyperalgesia in female mice, but not in males [113]. A2AAR KO mice also showed a significant decrease of mechanical allodynia in a model of neuropathic pain induced by sciatic nerve injury when compared to the WT group [24].
In acetic acid-induced writhing and tail immersion tests in the mouse, a series of novel A2AAR-selective antagonists administered i.p. decreased acute pain [183]. These antagonists were described as promising for the treatment of chronic pain, as well. In another study, the A2AAR agonist CGS21680 induced mechanical sensitization of esophageal C fibers that was abolished by pretreatment with selective A2A antagonist SCH58261 54 [23]. Moreover, thermal and chemical stimuli, induced respectively by hot plate and acetic acid showed that an i.p. injection of the agonist CGS21680 produces pro-nociceptive effects [13]. Therefore, although most studies showed analgesic effects, it seems that A2AAR activation might in some cases intensify response to nociceptive stimuli. The role of A2AAR in pain transmission needs to be explored further.
A few studies have examined the involvement of the A2BAR in pain; however it is known that activation of the A2BAR has both pro-inflammatory and anti-inflammatory effects [164]. The lack of studies in pain is partly a result of the fact that selective agonists for this subtype are not as plentiful or well-characterized as agonists of the other ARs. Nevertheless, a study of ADA KO mice showed that prolonged increase in plasma adenosine activates A2BAR on myeloid cells that results in increased sensitivity and chronic pain [73]. Furthermore, the A2BAR-selective adenosine receptor antagonists PSB-1115 56, PSB-50, PSB-53, PSB-55 and enprofylline (structures not shown) displayed a dose-dependent analgesic effect in the hot-plate test in mice, an acute animal pain model [4,73]. The selective A2BAR antagonist PSB-603 57 has also proven useful as a pharmacological probe of this AR subtype. Although limited, such data suggests a pronociceptive role of this receptor.
A3AR antinociceptive effects:
Until recently, the dogma was that the pain relieving effects of adenosine were mediated predominantly by actions at the A1AR and perhaps at the A2AAR [208]. These conclusions were made without examining the contributions of the Gi-coupled A3AR [85]. A focus on the A1AR and A2AAR failed to harness the potent analgesic effects of adenosine [20,208]. Thus, despite demonstrated preclinical efficacy in several pain models, agonists of A1AR and A2AAR have not been the focus of clinical trials due to significant cardiovascular side effects [208]. A3AR mRNA is predominantly expressed in human and rodent testis, lung, kidney, placenta, heart, brain, spleen, liver, uterus, bladder, jejunum, proximal colon, eye, DRG and SC (Table 1) [22,146]. Early in the study of the A3AR, soon after its cloning in 1992-3 [150,206], it was thought that the A3AR was absent in the brain [148]. However, subsequent studies established its neuronal expression in the thalamus, hypothalamus, cortex, cerebellum and other brain regions in rodents [195]. Based on exonal RNA sequencing, in human cadaver-derived tissue, A3AR mRNA is highly expressed in testes, SC, substantia nigra, adrenal gland, spleen, small intestine, amygdala, hypothalamus, tibial nerve, tibial nerve, hippocampus, bladder, lung, adipose tissue, whole blood, transverse colon and coronary arteries [79]. When compared to A1R and A2AAR, A3AR is expressed at much lower levels in the CNS [21]. However, A3AR has higher expression on many immune cell types, including glial cells [3,140,144]. In rodents, it is expressed in astrocytes, neurons, oligodendrocyte precursor cells, newly formed oligodendrocyte, myelinating oligodendrocyte, microglia/macrophage and endothelial cells [204]. In humans, it was found in fetal and mature astrocytes, oligodendrocyte, microglia/macrophage and endothelial cells [205].
A3AR can be found on both peripheral [149] and central neurons [60,81,119,202] of the brain and SC [22,66]. In pain processing centers in rodents, A3AR transcript and protein have been identified in the lumbar SC and rostral ventromedial medulla (RVM) [116]. The A3AR is significantly upregulated in settings of inflammation and cancer in numerous cell types and both in rodents and humans [22,53,79,140]; noteworthy activation of phosphoinositide 3-kinases (PI3K), cAMP response element-binding protein (CREB) and nuclear factor-κB (NFκB) plays critical roles in A3AR transcription [41]. The intracellular signaling pathways associated with the A3AR include the inhibition of adenylate cyclase through the Gi protein and also activation or deactivation of a wide range of kinases, such as MAPK, extracellular signal-regulated kinase (ERK), p38 mitogen-activated protein kinase (p38), jun N-terminal kinases (JNK), PI3K, protein kinase B (AKT), and glycogen synthase kinase 3β (GSK3β) [79]. These kinases and the transcription factors that they modulate are relevant to cancer, cell survival and proliferation, as well as pain [90,126,143,155,194,203]. In general, A3AR agonists tend to restore a balance in signaling that is subject to pathological deviation in disease states, such as cancer and autoimmune inflammatory diseases [79]. This generality applies to chronic pain states, as well [86].
The development of highly selective A3AR agonists such as MRS5698 35 and MRS5980 37 [173,174] provide exquisite tools to now probe the mechanistic roles of the A3AR axis in pain; their selectivity for A3AR is >1000-fold when compared to A1AR and A2AAR [173,174]. The high affinity and selectivity of MRS5698 was observed for several species, with Ki values of 3 nM at both human and mouse A3ARs [175]. MRS5841 16, another member of the 2-arylethynyl series [141], was selective at both human and mouse A3ARs and was excluded from diffusing across biological membranes. Thus, MRS5841 was a useful tool in separating effects of A3AR activation in the peripheral and the CNS because of its permanently charged sulfonate group. Another means of distinguishing classes of GPCR agonists is according to their preferred signaling pathway, which is potentially a means of reducing side effects. Several studies have addressed the question of biased A3AR agonists. Although a high degree of bias has not yet been found, some agonists tend to favor the Gi-dependent pathway over the β-arrestin pathway [58,162]. These ligand tools, some of which are now commercially available, are anticipated to further our understanding of the role of the A3AR axis in pain states.
Between 2004 and 2006, the early literature examining the role of A3AR in pain was confounded by results from three contradictory papers gathered from A3AR-targeted compounds with poor specificity, e.g. N6-benzyl–NECA (structure not shown) or from a single study performed in A3AR−/− mice [85]. In 2007, we revisited the A3AR hypothesis and demonstrated in a series of studies using pharmacological tools and genetic A3AR KO mice (A3AR−/−) that activation of A3AR exerts potent antinociceptive effects in models of traumatic nerve-injury induced neuropathic and chemotherapy-induced neuropathic pain and bone cancer pain [39,54,116,160,175,187] validating the observations in models of non-neuropathic pain states [200]. Pharmacological probes used included moderately selective agonists IB-MECA 33, Cl-IB-MECA 34 and highly selective A3AR agonists such as MRS5698 and MRS5980, as well as an antagonist for the mouse A3AR, MRS1523 60. It is to be emphasized that human A3AR antagonist MRS1220 59 is not selective when applied in rodent species. Noteworthy, A3AR agonists do not interfere with anti-tumor effects of widely used chemotherapeutics but instead are of themselves antitumor agents [8,41,50,51,53,182]. Cl-IB-MECA is currently in phase II clinical trials for hepatocellular carcinoma as an anti-cancer agent [1,2,159]. Therefore, the use of A3AR agonists may provide dual benefits in the treatment of a variety of cancer-related pain states.
In addition to traumatic-nerve injury and chemotherapy-induced neuropathic pain [39,54,116,160,171,175,187], A3AR agonists are effective in formalin-induced inflammatory pain [142], breast cancer bone metastasis [182] and diabetic neuropathy [196].
The beneficial effects of A3AR agonists are exerted in the periphery and in the central nervous system (SC and RVM); we have proposed that their high degree of potency may be the outcome of their potential synergy at multiple pain transmission sites [85], just like opioids [19]. We are currently examining this possibility. Moreover, A3AR agonists exert their effects through multiple molecular mechanisms; we postulate that such polypharmacology may also account for their potency and efficacy. In neuropathic pain models where nerve damage is induced by CCI or chemotherapy treatment (oxaliplatin), A3AR agonists exert their beneficial effects by suppressing spinal microglial and astrocytic activation, respectively [86,171,187]. In animal models of chemotherapy-induced neuropathic pain, A3AR appear to be disease-modifying, i.e. achieving not only symptomatic relief, but also reversing some of the pathological processes in pain signaling pathways that contribute to vicious cycles [39,50,86,116,187]. A3AR signaling inhibits neuropathic pain by attenuating the production of proinflammatory cytokines such as tumor necrosis factor (TNF) and IL-1β, and increasing formation of the anti-inflammatory interleukin-10 (IL-10) and interleukin-4 (IL-4) in the SC [86,187]. The functional role of IL-10 in the beneficial effects exerted by A3AR has been established [10,187]. A3AR agonists can also inhibit activation of MAPKs (p38 kinase) and of NFκB in SC in response to nerve injury [84,100,171]. Noteworthy, A3AR agonists have been reported to inhibit MAPKs, NFκB activation and release of inflammatory cytokines in models of inflammation (i.e. rheumatoid arthritis) and in cells and tissues harvested from patients with rheumatoid arthritis, psoriasis and Crohn’s disease [41].
A3AR agonists modulate redox-dependent events in the SC, reducing the activation of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase and avoid the posttranslational modification and inactivation of glutamate transporter glutamate transporter-1 (GLT-1) and glutamine synthetase (GS) which regulate synaptic glutamate homeostasis [84]. Also, inhibitory neurotransmission can be triggered by A3AR agonists. In a model of CCI both IB-MECA and MRS5698 were able to reverse neuropathic pain via a spinal mechanism of action that modulates GABA activity directly or through KCC2 function [54]. An in vivo study conducted on sensory DRG neurons isolated from rats revealed A3AR expression in DRG neurons and showed that A3AR agonists Cl-IB-MECA, and the highly selective MRS5980 are able to inhibit Ca2+ currents evoked by a voltage-ramp protocol [42]. The pain-relieving effects observed following A3AR activation in the rat could be mediated by DRG neurons, which underscores the potential of A3AR agonists as effective therapies to relieve pain in different pathologies.
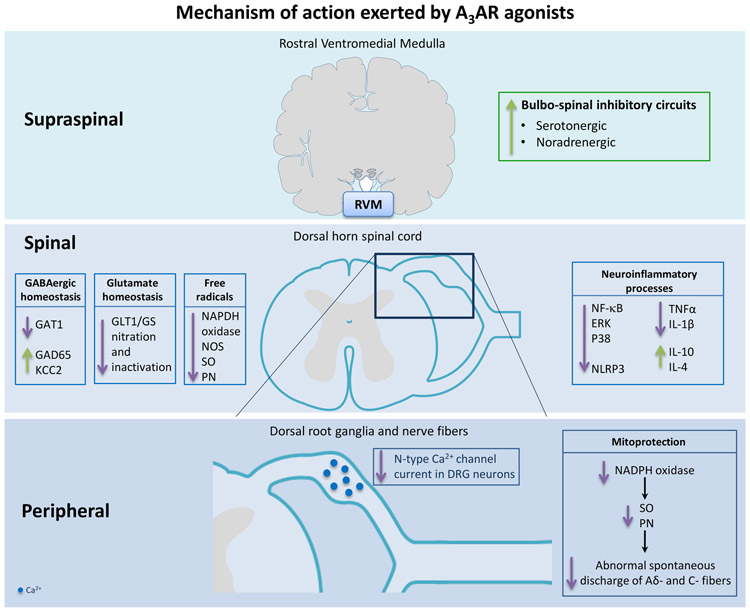
A3AR agonists are also mitoprotective as they preserve the activity of the major mitochondrial enzyme, manganese superoxide dismutase, preventing the sustained formation of oxygen and nitrogen-derived free radicals (superoxide and peroxynitrite); here mitoprection in peripheral sensory afferents may contribute to their ability to attenuate increased abnormal spontaneous discharge observed in models of chemotherapy-induced neuropathic pain [15,83,84]. Some of the mechanisms of action depicted so far for A3AR are captured in Figure 5. The development of highly selective A3AR agonists such as MRS5698 and MRS5980 [173,174] provide exquisite tools to now probe the mechanistic roles of the A3AR axis in pain; their selectivity for A3AR is >1000-fold when compared to A1AR and A2AAR [173,174]. These tools which are now commercially available are anticipated to further our understanding of the role of the A3AR axis in pain states.
Figure 5.
Schematic representation of the molecular signaling pathways of A3AR agonists. Ca2+, calcium ions; ERK, extracellular signal-regulated kinase; GABA, gamma-aminobutyric Acid; GAD65, glutamic acid decarboxylase 65; GAT1, GABA transporter type 1; GLT-1, glutamate transporter 1; GS, glutamine synthetase; IL-1β, interleukin-1β; IL-4, interleukin-4; IL-10, interleukin-10; KCC2, potassium-chloride cotransporter protein; NADPH oxidase, nicotinamide adenine dinucleotide phosphate oxidase; NF-kB, nuclear factor-κB; NLRP3, nucleotide-binding oligomerization domain-like receptor protein 3; NOS, nitric oxide synthase; p38, p38 mitogen-activated protein kinase; PN, peroxynitrite; RVM, rostral ventromedial medulla; TNFα, tumor necrosis factor alpha; SO, superoxide.
The antinociceptive effects of A3AR agonists are not dependent upon endogenous opioid or endocannabinoid pathways [116,205]. Moreover, A3AR agonists produce conditioned place preference (CPP) only in nerve injured rodents, with no effects in sham animals, suggesting that A3AR agonists provide relief of spontaneous pain [116]. Therefore, A3AR agonists have the potential to selectively modify pathological but not protective pain, while avoiding the tolerance and abuse potential associated with opioid therapy [85]. To this end, antinociceptive effects of A3AR agonists persists even with prolonged treatment (at least up to 2 weeks of treatment) [116,160,187] suggesting that tolerance does not develop to A3AR agonism. Similar findings have been observed in animal models of autoimmune disorders and cancer, where chronic administration of A3AR agonists maintains anti-inflammatory/anti-cancer effects even during A3AR down-regulation [122]. Fishman postulated that the functionality of A3AR agonists in inflammation/tumor-growth may be dependent on the down-regulation of A3AR to inhibit downstream regulatory proteins [52].
In addition to their potential use as stand-alone analgesics, A3AR agonists may be able to be given in conjunction with currently used drugs as these can increase the analgesic potency of morphine, gabapentin, and amitriptyline [39]. Recently, Kim and co-workers demonstrated that the anti-allodynic effects of amitriptyline and its inhibitory effects on the activation of ERK and CREB and inflammatory cytokines in the SC in a rat model of spinal nerve ligation induced neuropathic pain was abrogated by an A3AR antagonist MRS1191 58 [100]. This suggests that part of the action mediated by amitriptyline is mediated via activation of the A3AR [100]. The link between amitriptyline and the adenosine/A3AR axis remains to be elucidated.
Conclusions
Modulation of purinergic signaling can be used to reduce pain in various models, generally through AR activation or P2YR or P2XR inhibition. Among AR ligands, both A1AR agonists and an A1AR PAM have been tested in the clinic without success so far, but optimism remains based on mechanistic considerations. The efficacy of A2AAR ligands in pain depends on the route of administration: antagonists in the periphery and agonists in the spinal column. A3AR agonists would be relatively free of serious cardiovascular side effects and are also efficacious in diverse rodent models of chronic pain. Benefit in pain models has been observed with various P2XR antagonists: P2X3R, P2X4R and P2X7R, although P2X4R is the more promising target because of its functional presence in microglial cells. There is conflicting data concerning the role of various P2YRs, but P2Y1, P2Y12R and P2Y14R antagonists appear to be consistently antinociceptive. Thus, the prospects are encouraging for the eventual use of purine receptor modulators as non-addictive treatment of chronic neuropathic pain. A3AR agonists (IB-MECA and Cl-IB-MECA) are currently in clinical trials for chronic inflammatory diseases, liver diseases and cancer with a good safety profile [1,2,46,158,159]. Significant effects are across rodent models with pharmacological tool compounds, such as A3AR agonists, which provide the impetus to discover new drugs acting through purinergic pathways. Highly selective A3AR agonists are anticipated to provide an advantage by providing a wide therapeutic index for chronic administrationThe A3AR has been identified as an exciting target for AR agonists for pain management, and highly selective A3AR agonists are moving through preclinical and clinical development for the treatment of neuropathic pain states.
Acknowledgements
KAJ acknowledges support from the NIDDK Intramural Res. Program (ZIADK31117).
Footnotes
Author Disclosure Statements:
Dr. Salvemini is founder of BioIntervene, Inc. a company developing A3AR agonists as analgesics for chronic pain. All other authors declare no conflict of interest.
References
- [1].A Phase 1-2 Study of CF102 in Patients With Advanced Hepatocellular Carcinoma. https://ClinicalTrials.gov/show/NCT00790218.
- [2].Phase 2, Randomized, Double-Blind, Placebo-Controlled of the Efficacy and Safety of CF102 in Hepatocellular Carcinoma (HCC). https://ClinicalTrials.gov/show/NCT02128958.
- [3].Abbracchio MP, Rainaldi G, Giammarioli AM, Ceruti S, Brambilla R, Cattabeni F, Barbieri D, Franceschi C, Jacobson KA,Malorni W. The A3 adenosine receptor mediates cell spreading, reorganization of actin cytoskeleton, and distribution of Bcl-XL: studies in human astroglioma cells. Biochem Biophys Res Commun, 1997. 241(2): p. 297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Abo-Salem OM, Hayallah AM, Bilkei-Gorzo A, Filipek B, Zimmer A,Muller CE. Antinociceptive effects of novel A2B adenosine receptor antagonists. J Pharmacol Exp Ther, 2004. 308(1): p. 358–66. [DOI] [PubMed] [Google Scholar]
- [5].Antonioli L, Fornai M, Blandizzi C, Pacher P,Hasko G. Adenosine signaling and the immune system: When a lot could be too much. Immunol Lett, 2019. 205: p. 9–15. [DOI] [PubMed] [Google Scholar]
- [6].Araldi D, Ferrari LF,Levine JD. Adenosine-A1 receptor agonist induced hyperalgesic priming type II. Pain, 2016. 157(3): p. 698–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Arribas-Blazquez M, Olivos-Ore LA, Barahona MV, Sanchez de la Muela M,Solar V,Jimenez E,Gualix J,McIntosh JM,Ferrer-Montiel A,Miras-Portugal MT,Artalejo AR. Overexpression of P2X3 and P2X7 Receptors and TRPV1 Channels in Adrenomedullary Chromaffin Cells in a Rat Model of Neuropathic Pain. Int J Mol Sci, 2019. 20(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Baharav E, Bar-Yehuda S, Madi L, Silberman D, Rath-Wolfson L, Halpren M, Ochaion A, Weinberger A,Fishman P. Antiinflammatory effect of A3 adenosine receptor agonists in murine autoimmune arthritis models. J Rheumatol, 2005. 32(3): p. 469–76. [PubMed] [Google Scholar]
- [9].Bai HH, Liu JP, Yang L, Zhao JY, Suo ZW, Yang X,Hu XD. Adenosine A1 receptor potentiated glycinergic transmission in spinal cord dorsal horn of rats after peripheral inflammation. Neuropharmacology, 2017. 126: p. 158–167. [DOI] [PubMed] [Google Scholar]
- [10].Bar-Yehuda S, Luger D, Ochaion A, Cohen S, Patokaa R, Zozulya G, Silver PB, de Morales JM, Caspi RR,Fishman P. Inhibition of experimental auto-immune uveitis by the A3 adenosine receptor agonist CF101. Int J Mol Med, 2011. 28(5): p. 727–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Barragan-Iglesias P, Mendoza-Garces L, Pineda-Farias JB, Solano-Olivares V, Rodriguez-Silverio J, Flores-Murrieta FJ, Granados-Soto V,Rocha-Gonzalez HI. Participation of peripheral P2Y1, P2Y6 and P2Y11 receptors in formalin-induced inflammatory pain in rats. Pharmacol Biochem Behav, 2015. 128: p. 23–32. [DOI] [PubMed] [Google Scholar]
- [12].Barragan-Iglesias P, Pineda-Farias JB, Bravo-Hernandez M, Cervantes-Duran C, Price TJ, Murbartian J,Granados-Soto V. Predominant role of spinal P2Y1 receptors in the development of neuropathic pain in rats. Brain Res, 2016. 1636: p. 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bastia E, Varani K, Monopoli A,Bertorelli R. Effects of A(1) and A(2A) adenosine receptor ligands in mouse acute models of pain. Neurosci Lett, 2002. 328(3): p. 241–4. [DOI] [PubMed] [Google Scholar]
- [14].Beggs S Salter MW. Microglia-neuronal signalling in neuropathic pain hypersensitivity 2.0. Curr Opin Neurobiol, 2010. 20(4): p. 474–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bennett GJ, Doyle T,Salvemini D. Mitotoxicity in distal symmetrical sensory peripheral neuropathies. Nat Rev Neurol, 2014. 10(6): p. 326–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bernier LP, Ase AR, Boue-Grabot E,Seguela P. Inhibition of P2X4 function by P2Y6 UDP receptors in microglia. Glia, 2013. 61(12): p. 2038–49. [DOI] [PubMed] [Google Scholar]
- [17].Bernier LP, Ase AR,Seguela P. P2X receptor channels in chronic pain pathways. Br J Pharmacol, 2018. 175(12): p. 2219–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bian J, Zhang Y, Liu Y, Li Q, Tang H-b,Liu Q. P2Y6 Receptor-Mediated Spinal Microglial Activation in Neuropathic Pain. Pain Research and Management, 2019. 2019: p. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Bodnar RJ. Supraspinal circuitry mediating opioid antinociception: antagonist and synergy studies in multiple sites. J Biomed Sci, 2000. 7(3): p. 181–94. [DOI] [PubMed] [Google Scholar]
- [20].Boison D Adenosine kinase: exploitation for therapeutic gain. Pharmacol Rev, 2013. 65(3): p. 906–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Borea PA, Gessi S, Merighi S, Vincenzi F,Varani K. Pharmacology of Adenosine Receptors: The State of the Art. Physiol Rev, 2018. 98(3): p. 1591–1625. [DOI] [PubMed] [Google Scholar]
- [22].Borea PA, Varani K, Vincenzi F, Baraldi PG, Tabrizi MA, Merighi S,Gessi S. The A3 adenosine receptor: history and perspectives. Pharmacol Rev, 2015. 67(1): p. 74–102. [DOI] [PubMed] [Google Scholar]
- [23].Brozmanova M, Mazurova L, Ru F, Tatar M, Hu Y, Yu S,Kollarik M. Mechanisms of the adenosine A2A receptor-induced sensitization of esophageal C fibers. Am J Physiol Gastrointest Liver Physiol, 2016. 310(3): p. G215–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bura SA, Nadal X, Ledent C, Maldonado R,Valverde O. A 2A adenosine receptor regulates glia proliferation and pain after peripheral nerve injury. Pain, 2008. 140(1): p. 95–103. [DOI] [PubMed] [Google Scholar]
- [25].Burgey CSP, PA, US), Nguyen Diem (Eagleville, PA, US), Deng Zhengwu (Harleysville, PA, US), Paone Daniel V. (Lansdale, PA, US), Potteiger Craig M. (Reading, PA, US), Vacca Joseph P. (Telford, PA, US), P2X3 receptor antagonists for treatment of pain. 2012, Merck Sharp & Dohme Corp; (Rahway, NJ, US): United States. [Google Scholar]
- [26].Burma NE, Bonin RP, Leduc-Pessah H, Baimel C, Cairncross ZF, Mousseau M, Shankara JV, Stemkowski PL, Baimoukhametova D, Bains JS, Antle MC, Zamponi GW, Cahill CM, Borgland SL, De Koninck Y,Trang T. Blocking microglial pannexin-1 channels alleviates morphine withdrawal in rodents. Nat Med, 2017. 23(3): p. 355–360. [DOI] [PubMed] [Google Scholar]
- [27].Burnstock G P2X receptors in sensory neurones. Br J Anaesth, 2000. 84(4): p. 476–88. [DOI] [PubMed] [Google Scholar]
- [28].Burnstock G Purinergic Mechanisms and Pain. Adv Pharmacol, 2016. 75: p. 91–137. [DOI] [PubMed] [Google Scholar]
- [29].By Y, Condo J, Durand-Gorde JM, Lejeune PJ, Mallet B, Guieu R,Ruf J. Intracerebroventricular injection of an agonist-like monoclonal antibody to adenosine A(2A) receptor has antinociceptive effects in mice. J Neuroimmunol, 2011. 230(1-2): p. 178–82. [DOI] [PubMed] [Google Scholar]
- [30].Carlin JL, Jain S, Gizewski E, Wan TC, Tosh DK, Xiao C, Auchampach JA, Jacobson KA, Gavrilova O,Reitman ML. Hypothermia in mouse is caused by adenosine A1 and A3 receptor agonists and AMP via three distinct mechanisms. Neuropharmacology, 2017. 114: p. 101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Carroll WA, Donnelly-Roberts D,Jarvis MF. Selective P2X(7) receptor antagonists for chronic inflammation and pain. Purinergic Signal, 2009. 5(1): p. 63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Cavalli E, Mammana S, Nicoletti F, Bramanti P,Mazzon E. The neuropathic pain: An overview of the current treatment and future therapeutic approaches. Int J Immunopathol Pharmacol, 2019. 33: p. 2058738419838383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Cekic CLinden J Purinergic regulation of the immune system. Nat Rev Immunol, 2016. 16(3): p. 177–92. [DOI] [PubMed] [Google Scholar]
- [34].Chambers JK, Macdonald LE, Sarau HM, Ames RS, Freeman K, Foley JJ, Zhu Y, McLaughlin MM, Murdock P, McMillan L, Trill J, Swift A, Aiyar N, Taylor P, Vawter L, Naheed S, Szekeres P, Hervieu G, Scott C, Watson JM, Murphy AJ, Duzic E, Klein C, Bergsma DJ, Wilson S,Livi GP. A G protein-coupled receptor for UDP-glucose. J Biol Chem, 2000. 275(15): p. 10767–71. [DOI] [PubMed] [Google Scholar]
- [35].CHAMBERS L,J (GlaxoSmithKline, New Frontiers Science Park SouthThird Avenue, Harlow Essex CM19 5AW, GB), GLEAVE Robert (GlaxoSmithKline, New Frontiers Science Park SouthThird Avenue, Harlow Essex CM19 5AW, GB), SENGER Stefan (GlaxoSmithKline, Gunnels Wood Road, Stevenage Hertfordshire SG1 2NY, GB), WALTER Daryl, Simon (GlaxoSmithKline, New Frontiers Science Park SouthThird Avenue, Harlow Essex CM19 5AW, GB), SUBSTITUTED N-PHENYLMETHYL −5-OXO-PROLINE-2-AMIDES AS P2X7-RECEPTOR ANTAGONISTS AND THEIR METHODS OF USE. 2008, GLAXO GROUP LIMITED; (Glaxo Wellcome House, Berkeley Avenue, Greenford Middlesex UB6 0NN, GB),CHAMBERS, Laura, J (GlaxoSmithKline, New Frontiers Science Park SouthThird Avenue, Harlow Essex CM19 5AW, GB),GLEAVE, Robert (GlaxoSmithKline, New Frontiers Science Park SouthThird Avenue, Harlow Essex CM19 5AW, GB),SENGER, Stefan (GlaxoSmithKline, Gunnels Wood Road, Stevenage Hertfordshire SG1 2NY, GB),WALTER, Daryl, Simon (GlaxoSmithKline, New Frontiers Science Park SouthThird Avenue, Harlow Essex CM19 5AW, GB). [Google Scholar]
- [36].Charlton ME, Williams AS, Fogliano M, Sweetnam PM,Duman RS. The isolation and characterization of a novel G protein-coupled receptor regulated by immunologic challenge. Brain Res, 1997. 764(1-2): p. 141–8. [DOI] [PubMed] [Google Scholar]
- [37].Chen CC, Akopian AN, Sivilotti L, Colquhoun D, Burnstock G,Wood JN. A P2X purinoceptor expressed by a subset of sensory neurons. Nature, 1995. 377(6548): p. 428–31. [DOI] [PubMed] [Google Scholar]
- [38].Chen J, Wang L, Zhang Y,Yang J. P2Y1 purinoceptor inhibition reduces extracellular signal-regulated protein kinase 1/2 phosphorylation in spinal cord and dorsal root ganglia: implications for cancer-induced bone pain. Acta Biochim Biophys Sin (Shanghai), 2012. 44(4): p. 367–72. [DOI] [PubMed] [Google Scholar]
- [39].Chen Z, Janes K, Chen C, Doyle T, Bryant L, Tosh DK, Jacobson KA,Salvemini D. Controlling murine and rat chronic pain through A3 adenosine receptor activation. FASEB J, 2012. 26(5): p. 1855–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Coddou C, Stojilkovic SS,Huidobro-Toro JP. Allosteric modulation of ATP-gated P2X receptor channels. Rev Neurosci, 2011. 22(3): p. 335–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Cohen SFishman P Targeting the A3 adenosine receptor to treat cytokine release syndrome in cancer immunotherapy. Drug Des Devel Ther, 2019. 13: p. 491–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Coppi E, Cherchi F, Fusco I, Failli P, Vona A, Dettori I, Gaviano L, Lucarini E, Jacobson KA, Tosh DK, Salvemini D, Ghelardini C, Pedata F, Di Cesare Mannelli L,Pugliese AM. Adenosine A3 receptor activation inhibits pronociceptive N-type Ca2+ currents and cell excitability in dorsal root ganglion neurons. Pain, 2019. 160(5): p. 1103–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW,De Koninck Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature, 2005. 438(7070): p. 1017–21. [DOI] [PubMed] [Google Scholar]
- [44].Dal Ben D, Marchenkova A, Thomas A, Lambertucci C, Spinaci A, Marucci G, Nistri A,Volpini R. 2’,3’-O-Substituted ATP derivatives as potent antagonists of purinergic P2X3 receptors and potential analgesic agents. Purinergic Signal, 2017. 13(1): p. 61–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Dalgarno R, Leduc-Pessah H, Pilapil A, Kwok CH,Trang T. Intrathecal delivery of a palmitoylated peptide targeting Y382-384 within the P2X7 receptor alleviates neuropathic pain. Mol Pain, 2018. 14: p. 1744806918795793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].David M, Akerman L, Ziv M, Kadurina M, Gospodinov D, Pavlotsky F, Yankova R, Kouzeva V, Ramon M, Silverman MH,Fishman P. Treatment of plaque-type psoriasis with oral CF101: data from an exploratory randomized phase 2 clinical trial. J Eur Acad Dermatol Venereol, 2012. 26(3): p. 361–7. [DOI] [PubMed] [Google Scholar]
- [47].Doyon N, Vinay L, Prescott SA,De Koninck Y. Chloride Regulation: A Dynamic Equilibrium Crucial for Synaptic Inhibition. Neuron, 2016. 89(6): p. 1157–1172. [DOI] [PubMed] [Google Scholar]
- [48].Elzein EZablocki J A1 adenosine receptor agonists and their potential therapeutic applications. Expert Opin Investig Drugs, 2008. 17(12): p. 1901–10. [DOI] [PubMed] [Google Scholar]
- [49].Fabbretti E ATP P2X3 receptors and neuronal sensitization. Front Cell Neurosci, 2013. 7: p. 236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Fishman P, Bar-Yehuda S, Liang BT,Jacobson KA. Pharmacological and therapeutic effects of A3 adenosine receptor agonists. Drug Discov Today, 2012. 17(7-8): p. 359–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Fishman P, Bar-Yehuda S, Madi L,Cohn I. A3 adenosine receptor as a target for cancer therapy. Anticancer Drugs, 2002. 13(5): p. 437–43. [DOI] [PubMed] [Google Scholar]
- [52].Fishman P, Bar-Yehuda S, Madi L, Rath-Wolfson L, Ochaion A, Cohen S,Baharav E. The PI3K-NF-kappaB signal transduction pathway is involved in mediating the anti-inflammatory effect of IB-MECA in adjuvant-induced arthritis. Arthritis Res Ther, 2006. 8(1): p. R33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Fishman P, Bar-Yehuda S, Synowitz M, Powell JD, Klotz KN, Gessi S,Borea PA. Adenosine receptors and cancer. Handb Exp Pharmacol, 2009(193): p. 399–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Ford A, Castonguay A, Cottet M, Little JW, Chen Z, Symons-Liguori AM, Doyle T, Egan TM, Vanderah TW, De Koninck Y, Tosh DK, Jacobson KA,Salvemini D. Engagement of the GABA to KCC2 signaling pathway contributes to the analgesic effects of A3AR agonists in neuropathic pain. J Neurosci, 2015. 35(15): p. 6057–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Fujita M, Andoh T, Sasaki A, Saiki I,Kuraishi Y. Involvement of peripheral adenosine 5’-triphosphate and P2X purinoceptor in pain-related behavior produced by orthotopic melanoma inoculation in mice. Eur J Neurosci, 2010. 31(9): p. 1629–36. [DOI] [PubMed] [Google Scholar]
- [56].Fujita T, Feng C,Takano T. Presence of caffeine reversibly interferes with efficacy of acupuncture-induced analgesia. Sci Rep, 2017. 7(1): p. 3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Gao M, Wang M, Meyer JA, Territo PR, Hutchins GD, Zarrinmayeh H,Zheng QH. Synthesis and in vitro biological evaluation of new P2X7R radioligands [(11)C]halo-GSK1482160 analogs. Bioorg Med Chem Lett, 2019. 29(12): p. 1476–1480. [DOI] [PubMed] [Google Scholar]
- [58].Gao ZGJacobson KA. Translocation of arrestin induced by human A(3) adenosine receptor ligands in an engineered cell line: comparison with G protein-dependent pathways. Pharmacol Res, 2008. 57(4): p. 303–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Gerevich ZIlles P P2Y receptors and pain transmission. Purinergic Signal, 2004. 1(1): p. 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Giannaccini G, Betti L, Palego L, Fabbrini L, Schmid L, Castagna M, Giusti L, Mascia G,Lucacchini A. Species comparison of adenosine receptor subtypes in brain and testis. Neurochem Res, 2008. 33(5): p. 852–60. [DOI] [PubMed] [Google Scholar]
- [61].Glukhova A, Thal DM, Nguyen AT, Vecchio EA, Jorg M, Scammells PJ, May LT, Sexton PM,Christopoulos A. Structure of the Adenosine A1 Receptor Reveals the Basis for Subtype Selectivity. Cell, 2017. 168(5): p. 867–877 e13. [DOI] [PubMed] [Google Scholar]
- [62].Goldman N, Chen M, Fujita T, Xu Q, Peng W, Liu W, Jensen TK, Pei Y, Wang F, Han X, Chen JF, Schnermann J, Takano T, Bekar L, Tieu K,Nedergaard M. Adenosine A1 receptors mediate local anti-nociceptive effects of acupuncture. Nat Neurosci, 2010. 13(7): p. 883–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Gomes CV, Kaster MP, Tome AR, Agostinho PM,Cunha RA. Adenosine receptors and brain diseases: neuroprotection and neurodegeneration. Biochim Biophys Acta, 2011. 1808(5): p. 1380–99. [DOI] [PubMed] [Google Scholar]
- [64].Gu N, Eyo UB, Murugan M, Peng J, Matta S, Dong H,Wu LJ. Microglial P2Y12 receptors regulate microglial activation and surveillance during neuropathic pain. Brain Behav Immun, 2016. 55: p. 82–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Gum RJ, Wakefield B,Jarvis MF. P2X receptor antagonists for pain management: examination of binding and physicochemical properties. Purinergic Signal, 2012. 8(Suppl 1): p. 41–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Haeusler D, Grassinger L, Fuchshuber F, Horleinsberger WJ, Hoftberger R, Leisser I, Girschele F, Shanab K, Spreitzer H, Gerdenitsch W, Hacker M, Wadsak W,Mitterhauser M. Hide and seek: a comparative autoradiographic in vitro investigation of the adenosine A3 receptor. Eur J Nucl Med Mol Imaging, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Hasegawa S, Kohro Y, Tsuda M,Inoue K. Activation of cytosolic phospholipase A2 in dorsal root ganglion neurons by Ca2+/calmodulin-dependent protein kinase II after peripheral nerve injury. Mol Pain, 2009. 5: p. 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Hockley JR, Tranter MM, McGuire C, Boundouki G, Cibert-Goton V, Thaha MA, Blackshaw LA, Michael GJ, Baker MD, Knowles CH, Winchester WJ,Bulmer DC. P2Y Receptors Sensitize Mouse and Human Colonic Nociceptors. J Neurosci, 2016. 36(8): p. 2364–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Holanda AD, Asth L, Santos AR, Guerrini R, de PS-RV, Calo G, Andre E,Gavioli EC. Central adenosine A1 and A2A receptors mediate the antinociceptive effects of neuropeptide S in the mouse formalin test. Life Sci, 2015. 120: p. 8–12. [DOI] [PubMed] [Google Scholar]
- [70].Honda S, Sasaki Y, Ohsawa K, Imai Y, Nakamura Y, Inoue K,Kohsaka S. Extracellular ATP or ADP induce chemotaxis of cultured microglia through Gi/o-coupled P2Y receptors. J Neurosci, 2001. 21(6): p. 1975–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Honore P, Donnelly-Roberts D, Namovic MT, Hsieh G, Zhu CZ, Mikusa JP, Hernandez G, Zhong C, Gauvin DM, Chandran P, Harris R, Medrano AP, Carroll W, Marsh K, Sullivan JP, Faltynek CR,Jarvis MF. A-740003 [N-(1-{[(cyanoimino)(5-quinolinylamino) methyl]amino}−2,2-dimethylpropyl)-2-(3,4-dimethoxyphenyl)acetamide], a novel and selective P2X7 receptor antagonist, dose-dependently reduces neuropathic pain in the rat. J Pharmacol Exp Ther, 2006. 319(3): p. 1376–85. [DOI] [PubMed] [Google Scholar]
- [72].Horvath G, Goloncser F, Csolle C, Kiraly K, Ando RD, Baranyi M, Kovanyi B, Mate Z, Hoffmann K, Algaier I, Baqi Y, Muller CE, Von Kugelgen I,Sperlagh B. Central P2Y12 receptor blockade alleviates inflammatory and neuropathic pain and cytokine production in rodents. Neurobiol Dis, 2014. 70: p. 162–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Hu X, Adebiyi MG, Luo J, Sun K, Le TT, Zhang Y, Wu H, Zhao S, Karmouty-Quintana H, Liu H, Huang A, Wen YE, Zaika OL, Mamenko M, Pochynyuk OM, Kellems RE, Eltzschig HK, Blackburn MR, Walters ET, Huang D, Hu H,Xia Y. Sustained Elevated Adenosine via ADORA2B Promotes Chronic Pain through Neuro-immune Interaction. Cell Rep, 2016. 16(1): p. 106–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Hussey MJ, Clarke GD, Ledent C, Hourani SM,Kitchen I. Reduced response to the formalin test and lowered spinal NMDA glutamate receptor binding in adenosine A2A receptor knockout mice. Pain, 2007. 129(3): p. 287–94. [DOI] [PubMed] [Google Scholar]
- [75].Hussey MJ, Clarke GD, Ledent C, Kitchen I,Hourani SM. Genetic deletion of the adenosine A(2A) receptor in mice reduces the changes in spinal cord NMDA receptor binding and glucose uptake caused by a nociceptive stimulus. Neurosci Lett, 2010. 479(3): p. 297–301. [DOI] [PubMed] [Google Scholar]
- [76].ICHIHASHI Y-, Futabacho 3-chome, Toyonaka-sh, Osaka 25, ?5610825, JP),, PURINE DERIVATIVE AND PHARMACEUTICAL COMPOSITION THEREOF. 2016, SHIONOGI & CO., LTD. (1-8 Doshomachi 3-chome, Chuo-ku Osaka-sh, Osaka 45, ?5410045, JP),. [Google Scholar]
- [77].Imlach WL, Bhola RF, May LT, Christopoulos A,Christie MJ. A Positive Allosteric Modulator of the Adenosine A1 Receptor Selectively Inhibits Primary Afferent Synaptic Transmission in a Neuropathic Pain Model. Mol Pharmacol, 2015. 88(3): p. 460–8. [DOI] [PubMed] [Google Scholar]
- [78].Ito M, Egashira SI, Yoshida K, Mineno T, Kumagai K, Kojima H, Okabe T, Nagano T, Ui M,Matsuoka I. Identification of novel selective P2Y6 receptor antagonists by high-throughput screening assay. Life Sci, 2017. 180: p. 137–142. [DOI] [PubMed] [Google Scholar]
- [79].Jacobson KA, Merighi S, Varani K, Borea PA, Baraldi S, Aghazadeh Tabrizi M, Romagnoli R, Baraldi PG, Ciancetta A, Tosh DK, Gao ZG,Gessi S. A3 Adenosine Receptors as Modulators of Inflammation: From Medicinal Chemistry to Therapy. Med Res Rev, 2018. 38(4): p. 1031–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Jacobson KAMuller CE. Medicinal chemistry of adenosine, P2Y and P2X receptors. Neuropharmacology, 2016. 104: p. 31–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Jacobson KA, Nikodijevic O, Shi D, Gallo-Rodriguez C, Olah ME, Stiles GL,Daly JW. A role for central A3-adenosine receptors. Mediation of behavioral depressant effects. FEBS Lett, 1993. 336(1): p. 57–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Jacobson KA, Tosh DK, Jain S,Gao ZG. Historical and Current Adenosine Receptor Agonists in Preclinical and Clinical Development. Front Cell Neurosci, 2019. 13: p. 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Janes K, Doyle T, Bryant L, Esposito E, Cuzzocrea S, Ryerse J, Bennett GJ,Salvemini D. Bioenergetic deficits in peripheral nerve sensory axons during chemotherapy-induced neuropathic pain resulting from peroxynitrite-mediated post-translational nitration of mitochondrial superoxide dismutase. Pain, 2013. 154(11): p. 2432–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Janes K, Esposito E, Doyle T, Cuzzocrea S, Tosh DK, Jacobson KA,Salvemini D. A3 adenosine receptor agonist prevents the development of paclitaxel-induced neuropathic pain by modulating spinal glial-restricted redox-dependent signaling pathways. Pain, 2014. 155(12): p. 2560–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Janes K, Symons-Liguori AM, Jacobson KA,Salvemini D. Identification of A3 adenosine receptor agonists as novel non-narcotic analgesics. Br J Pharmacol, 2016. 173(8): p. 1253–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Janes K, Wahlman C, Little JW, Doyle T, Tosh DK, Jacobson KA,Salvemini D. Spinal neuroimmune activation is independent of T-cell infiltration and attenuated by A3 adenosine receptor agonists in a model of oxaliplatin-induced peripheral neuropathy. Brain Behav Immun, 2015. 44: p. 91–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Jarvis MF. Therapeutic potential of adenosine kinase inhibition-Revisited. Pharmacol Res Perspect, 2019. 7(4): p. e00506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Jarvis MF, Burgard EC, McGaraughty S, Honore P, Lynch K, Brennan TJ, Subieta A, Van Biesen T, Cartmell J, Bianchi B, Niforatos W, Kage K, Yu H, Mikusa J, Wismer CT, Zhu CZ, Chu K, Lee CH, Stewart AO, Polakowski J, Cox BF, Kowaluk E, Williams M, Sullivan J,Faltynek C. A-317491, a novel potent and selective non-nucleotide antagonist of P2X3 and P2X2/3 receptors, reduces chronic inflammatory and neuropathic pain in the rat. Proc Natl Acad Sci U S A, 2002. 99(26): p. 17179–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Jespers W, Oliveira A, Prieto-Diaz R, Majellaro M, Aqvist J, Sotelo E,Gutierrez-de-Teran H. Structure-Based Design of Potent and Selective Ligands at the Four Adenosine Receptors. Molecules, 2017. 22(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Ji RR, Gereau RWt, Malcangio M,Strichartz GR. MAP kinase and pain. Brain Res Rev, 2009. 60(1): p. 135–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Jiang Q, Li WX, Sun JR, Zhu TT, Fan J, Yu LH, Burnstock G, Yang H,Ma B. Inhibitory effect of estrogen receptor beta on P2X3 receptors during inflammation in rats. Purinergic Signal, 2017. 13(1): p. 105–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Jin XMi W Adenosine for postoperative analgesia: A systematic review and meta-analysis. PLoS One, 2017. 12(3): p. e0173518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Jung YH, Kim YO, Lin H, Cho JH, Park JH, Lee SD, Bae J, Kang KM, Kim YG, Pae AN, Ko H, Park CS, Yoon MH,Kim YC. Discovery of Potent Antiallodynic Agents for Neuropathic Pain Targeting P2X3 Receptors. ACS Chem Neurosci, 2017. 8(7): p. 1465–1478. [DOI] [PubMed] [Google Scholar]
- [94].Kaan TK, Yip PK, Patel S, Davies M, Marchand F, Cockayne DA, Nunn PA, Dickenson AH, Ford AP, Zhong Y, Malcangio M,McMahon SB. Systemic blockade of P2X3 and P2X2/3 receptors attenuates bone cancer pain behaviour in rats. Brain, 2010. 133(9): p. 2549–64. [DOI] [PubMed] [Google Scholar]
- [95].Kahle KT, Deeb TZ, Puskarjov M, Silayeva L, Liang B, Kaila K,Moss SJ. Modulation of neuronal activity by phosphorylation of the K-Cl cotransporter KCC2. Trends Neurosci, 2013. 36(12): p. 726–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Kan HW, Chang CH, Lin CL, Lee YC, Hsieh ST,Hsieh YL. Downregulation of adenosine and adenosine A1 receptor contributes to neuropathic pain in resiniferatoxin neuropathy. Pain, 2018. 159(8): p. 1580–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Katagiri A, Shinoda M, Honda K, Toyofuku A, Sessle BJ,Iwata K. Satellite glial cell P2Y12 receptor in the trigeminal ganglion is involved in lingual neuropathic pain mechanisms in rats. Mol Pain, 2012. 8: p. 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Kato Y, Hiasa M, Ichikawa R, Hasuzawa N, Kadowaki A, Iwatsuki K, Shima K, Endo Y, Kitahara Y, Inoue T, Nomura M, Omote H, Moriyama Y,Miyaji T. Identification of a vesicular ATP release inhibitor for the treatment of neuropathic and inflammatory pain. Proc Natl Acad Sci U S A, 2017. 114(31): p. E6297–E6305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Katz NK, Ryals JM,Wright DE. Central or peripheral delivery of an adenosine A1 receptor agonist improves mechanical allodynia in a mouse model of painful diabetic neuropathy. Neuroscience, 2015. 285: p. 312–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Kim Y, Kwon SY, Jung HS, Park YJ, Kim YS, In JH, Choi JW, Kim JA,Joo JD. Amitriptyline inhibits the MAPK/ERK and CREB pathways and proinflammatory cytokines through A3AR activation in rat neuropathic pain models. Korean J Anesthesiol, 2019. 72(1): p. 60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Knezevic NN, Cicmil N, Knezevic I,Candido KD. Discontinued neuropathic pain therapy between 2009-2015. Expert Opin Investig Drugs, 2015. 24(12): p. 1631–46. [DOI] [PubMed] [Google Scholar]
- [102].Kobayashi K, Fukuoka T, Yamanaka H, Dai Y, Obata K, Tokunaga A,Noguchi K. Neurons and glial cells differentially express P2Y receptor mRNAs in the rat dorsal root ganglion and spinal cord. J Comp Neurol, 2006. 498(4): p. 443–54. [DOI] [PubMed] [Google Scholar]
- [103].Kobayashi K, Yamanaka H, Yanamoto F, Okubo M,Noguchi K. Multiple P2Y subtypes in spinal microglia are involved in neuropathic pain after peripheral nerve injury. Glia, 2012. 60(10): p. 1529–39. [DOI] [PubMed] [Google Scholar]
- [104].Koizumi S, Ohsawa K, Inoue K,Kohsaka S. Purinergic receptors in microglia: functional modal shifts of microglia mediated by P2 and P1 receptors. Glia, 2013. 61(1): p. 47–54. [DOI] [PubMed] [Google Scholar]
- [105].Koizumi S, Shigemoto-Mogami Y, Nasu-Tada K, Shinozaki Y, Ohsawa K, Tsuda M, Joshi BV, Jacobson KA, Kohsaka S,Inoue K. UDP acting at P2Y6 receptors is a mediator of microglial phagocytosis. Nature, 2007. 446(7139): p. 1091–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Korboukh I, Hull-Ryde EA, Rittiner JE, Randhawa AS, Coleman J, Fitzpatrick BJ, Setola V, Janzen WP, Frye SV, Zylka MJ,Jin J. Orally active adenosine A(1) receptor agonists with antinociceptive effects in mice. J Med Chem, 2012. 55(14): p. 6467–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Kuan YHShyu BC. Nociceptive transmission and modulation via P2X receptors in central pain syndrome. Mol Brain, 2016. 9(1): p. 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Kwilasz AJ, Ellis A, Wieseler J, Loram L, Favret J, McFadden A, Springer K, Falci S, Rieger J, Maier SF,Watkins LR. Sustained reversal of central neuropathic pain induced by a single intrathecal injection of adenosine A2A receptor agonists. Brain Behav Immun, 2018. 69: p. 470–479. [DOI] [PubMed] [Google Scholar]
- [109].Kwon SG, Roh DH, Yoon SY, Choi SR, Choi HS, Moon JY, Kang SY, Beitz AJ,Lee JH. Involvement of peripheral P2Y1 receptors and potential interaction with IL-1 receptors in IL-1beta-induced thermal hypersensitivity in rats. Brain Res Bull, 2017. 130: p. 165–172. [DOI] [PubMed] [Google Scholar]
- [110].Kwon SG, Roh DH, Yoon SY, Moon JY, Choi SR, Choi HS, Kang SY, Han HJ, Beitz AJ, Oh SB,Lee JH. Acid evoked thermal hyperalgesia involves peripheral P2Y1 receptor mediated TRPV1 phosphorylation in a rodent model of thrombus induced ischemic pain. Mol Pain, 2014. 10: p. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Lee-Hotta S, Uchiyama Y,Kametaka S. Role of the BDNF-TrkB pathway in KCC2 regulation and rehabilitation following neuronal injury: A mini review. Neurochem Int, 2019. 128: p. 32–38. [DOI] [PubMed] [Google Scholar]
- [112].Lewis C, Neidhart S, Holy C, North RA, Buell G,Surprenant A. Coexpression of P2X2 and P2X3 receptor subunits can account for ATP-gated currents in sensory neurons. Nature, 1995. 377(6548): p. 432–5. [DOI] [PubMed] [Google Scholar]
- [113].Li L, Hao JX, Fredholm BB, Schulte G, Wiesenfeld-Hallin Z,Xu XJ. Peripheral adenosine A2A receptors are involved in carrageenan-induced mechanical hyperalgesia in mice. Neuroscience, 2010. 170(3): p. 923–8. [DOI] [PubMed] [Google Scholar]
- [114].Lin J, Liu F, Zhang YY, Song N, Liu MK, Fang XY, Liao DQ, Zhou C, Wang H,Shen JF. P2Y14 receptor is functionally expressed in satellite glial cells and mediates interleukin-1beta and chemokine CCL2 secretion. J Cell Physiol, 2019. 234(11): p. 21199–21210. [DOI] [PubMed] [Google Scholar]
- [115].Lin J, Zhang YY, Liu F, Fang XY, Liu MK, Huang CL, Wang H, Liao DQ, Zhou C,Shen JF. The P2Y14 receptor in the trigeminal ganglion contributes to the maintenance of inflammatory pain. Neurochem Int, 2019. 131: p. 104567. [DOI] [PubMed] [Google Scholar]
- [116].Little JW, Ford A, Symons-Liguori AM, Chen Z, Janes K, Doyle T, Xie J, Luongo L, Tosh DK, Maione S, Bannister K, Dickenson AH, Vanderah TW, Porreca F, Jacobson KA,Salvemini D. Endogenous adenosine A3 receptor activation selectively alleviates persistent pain states. Brain, 2015. 138(Pt 1): p. 28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Liu C, Zhang Y, Liu Q, Jiang L, Li M, Wang S, Long T, He W, Kong X, Qin G, Chen L, Zhang Y,Zhou J. P2X4-receptor participates in EAAT3 regulation via BDNF-TrkB signaling in a model of trigeminal allodynia. Mol Pain, 2018. 14: p. 1744806918795930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Liu M, Yao M, Wang H, Xu L, Zheng Y, Huang B, Ni H, Xu S, Zhou X,Lian Q. P2Y12 receptor-mediated activation of spinal microglia and p38MAPK pathway contribute to cancer-induced bone pain. J Pain Res, 2017. 10: p. 417–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Lopes LV, Rebola N, Pinheiro PC, Richardson PJ, Oliveira CR,Cunha RA. Adenosine A3 receptors are located in neurons of the rat hippocampus. Neuroreport, 2003. 14(12): p. 1645–8. [DOI] [PubMed] [Google Scholar]
- [120].Loram LC, Harrison JA, Sloane EM, Hutchinson MR, Sholar P, Taylor FR, Berkelhammer D, Coats BD, Poole S, Milligan ED, Maier SF, Rieger J,Watkins LR. Enduring reversal of neuropathic pain by a single intrathecal injection of adenosine 2A receptor agonists: a novel therapy for neuropathic pain. J Neurosci, 2009. 29(44): p. 14015–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Luongo L, Petrelli R, Gatta L, Giordano C, Guida F, Vita P, Franchetti P, Grifantini M, de Novellis V, Cappellacci L,Maione S. 5’-Chloro-5’-deoxy-(+/−)-ENBA, a potent and selective adenosine A(1) receptor agonist, alleviates neuropathic pain in mice through functional glial and microglial changes without affecting motor or cardiovascular functions. Molecules, 2012. 17(12): p. 13712–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Madi L, Bar-Yehuda S, Barer F, Ardon E, Ochaion A,Fishman P. A3 adenosine receptor activation in melanoma cells: association between receptor fate and tumor growth inhibition. J Biol Chem, 2003. 278(43): p. 42121–30. [DOI] [PubMed] [Google Scholar]
- [123].Magni GCeruti S P2Y purinergic receptors: new targets for analgesic and antimigraine drugs. Biochem Pharmacol, 2013. 85(4): p. 466–77. [DOI] [PubMed] [Google Scholar]
- [124].Magni G, Merli D, Verderio C, Abbracchio MP,Ceruti S. P2Y2 receptor antagonists as anti-allodynic agents in acute and sub-chronic trigeminal sensitization: role of satellite glial cells. Glia, 2015. 63(7): p. 1256–69. [DOI] [PubMed] [Google Scholar]
- [125].Magni G, Riccio D,Ceruti S. Tackling Chronic Pain and Inflammation through the Purinergic System. Curr Med Chem, 2018. 25(32): p. 3830–3865. [DOI] [PubMed] [Google Scholar]
- [126].Manning BDToker A AKT/PKB Signaling: Navigating the Network. Cell, 2017. 169(3): p. 381–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Mapplebeck JCS, Lorenzo LE, Lee KY, Gauthier C, Muley MM, De Koninck Y, Prescott SA,Salter MW. Chloride Dysregulation through Downregulation of KCC2 Mediates Neuropathic Pain in Both Sexes. Cell Rep, 2019. 28(3): p. 590–596 e4. [DOI] [PubMed] [Google Scholar]
- [128].Matsumura Y, Yamashita T, Sasaki A, Nakata E, Kohno K, Masuda T, Tozaki-Saitoh H, Imai T, Kuraishi Y, Tsuda M,Inoue K. A novel P2X4 receptor-selective antagonist produces anti-allodynic effect in a mouse model of herpetic pain. Sci Rep, 2016. 6: p. 32461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].McGaraughty S, Wismer CT, Zhu CZ, Mikusa J, Honore P, Chu KL, Lee CH, Faltynek CR,Jarvis MF. Effects of A-317491, a novel and selective P2X3/P2X2/3 receptor antagonist, on neuropathic, inflammatory and chemogenic nociception following intrathecal and intraplantar administration. Br J Pharmacol, 2003. 140(8): p. 1381–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Miao Y, Bhattarai A, Nguyen ATN, Christopoulos A,May LT. Structural Basis for Binding of Allosteric Drug Leads in the Adenosine A1 Receptor. Sci Rep, 2018. 8(1): p. 16836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Montes GC, Hammes N, da Rocha MD, Montagnoli TL, Fraga CA, Barreiro EJ, Sudo RT,Zapata-Sudo G. Treatment with Adenosine Receptor Agonist Ameliorates Pain Induced by Acute and Chronic Inflammation. J Pharmacol Exp Ther, 2016. 358(2): p. 315–23. [DOI] [PubMed] [Google Scholar]
- [132].Montesinos MC, Desai-Merchant A,Cronstein BN. Promotion of Wound Healing by an Agonist of Adenosine A2A Receptor Is Dependent on Tissue Plasminogen Activator. Inflammation, 2015. 38(6): p. 2036–41. [DOI] [PubMed] [Google Scholar]
- [133].Moore DJ, Murdock PR, Watson JM, Faull RL, Waldvogel HJ, Szekeres PG, Wilson S, Freeman KB,Emson PC. GPR105, a novel Gi/o-coupled UDP-glucose receptor expressed on brain glia and peripheral immune cells, is regulated by immunologic challenge: possible role in neuroimmune function. Brain Res Mol Brain Res, 2003. 118(1-2): p. 10–23. [DOI] [PubMed] [Google Scholar]
- [134].Muccino D Green S Update on the clinical development of gefapixant, a P2X3 receptor antagonist for the treatment of refractory chronic cough. Pulm Pharmacol Ther, 2019. 56: p. 75–78. [DOI] [PubMed] [Google Scholar]
- [135].Nakatsuka TGu JG. P2X purinoceptors and sensory transmission. Pflugers Arch, 2006. 452(5): p. 598–607. [DOI] [PubMed] [Google Scholar]
- [136].Nalepa I, Vetulani J, Borghi V, Kowalska M, Przewlocka B, Roman A,Pavone F. Changes induced by formalin pain in central alpha1-adrenoceptor density are modulated by adenosine receptor agonists. J Neural Transm (Vienna), 2010. 117(5): p. 549–58. [DOI] [PubMed] [Google Scholar]
- [137].Nelson DW, Gregg RJ, Kort ME, Perez-Medrano A, Voight EA, Wang Y, Grayson G, Namovic MT, Donnelly-Roberts DL, Niforatos W, Honore P, Jarvis MF, Faltynek CR,Carroll WA. Structure-activity relationship studies on a series of novel, substituted 1-benzyl-5-phenyltetrazole P2X7 antagonists. J Med Chem, 2006. 49(12): p. 3659–66. [DOI] [PubMed] [Google Scholar]
- [138].NEWCOM J,S (81 Myrtle Street, Norfolk, MA, 02056, US), SPEAR Kerry, L. (601 Lexington Road, Concord, MA, 01742, US), P2X4 RECEPTOR MODULATING COMPOUNDS. 2015, SUNOVION PHARMACEUTICALS INC. (84 Westford Drive, Marlborough, MA, 01752, US). [Google Scholar]
- [139].Novakovic SD, Kassotakis LC, Oglesby IB, Smith JA, Eglen RM, Ford AP,Hunter JC. Immunocytochemical localization of P2X3 purinoceptors in sensory neurons in naive rats and following neuropathic injury. Pain, 1999. 80(1-2): p. 273–82. [DOI] [PubMed] [Google Scholar]
- [140].Ochaion A, Bar-Yehuda S, Cohen S, Barer F, Patoka R, Amital H, Reitblat T, Reitblat A, Ophir J, Konfino I, Chowers Y, Ben-Horin S,Fishman P. The anti-inflammatory target A(3) adenosine receptor is over-expressed in rheumatoid arthritis, psoriasis and Crohn’s disease. Cell Immunol, 2009. 258(2): p. 115–22. [DOI] [PubMed] [Google Scholar]
- [141].Paoletta S, Tosh DK, Finley A, Gizewski ET, Moss SM, Gao ZG, Auchampach JA, Salvemini D,Jacobson KA. Rational design of sulfonated A3 adenosine receptor-selective nucleosides as pharmacological tools to study chronic neuropathic pain. J Med Chem, 2013. 56(14): p. 5949–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Petrelli R, Scortichini M, Kachler S, Boccella S, Cerchia C, Torquati I, Del Bello F, Salvemini D, Novellino E, Luongo L, Maione S, Jacobson KA, Lavecchia A, Klotz KN,Cappellacci L. Exploring the Role of N(6)-Substituents in Potent Dual Acting 5’-C-Ethyltetrazolyladenosine Derivatives: Synthesis, Binding, Functional Assays, and Antinociceptive Effects in Mice nabla. J Med Chem, 2017. 60(10): p. 4327–4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Popiolek-Barczyk K Mika J Targeting the Microglial Signaling Pathways: New Insights in the Modulation of Neuropathic Pain. Curr Med Chem, 2016. 23(26): p. 2908–2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Poulsen SA Quinn RJ. Adenosine receptors: new opportunities for future drugs. Bioorg Med Chem, 1998. 6(6): p. 619–41. [DOI] [PubMed] [Google Scholar]
- [145].Rajasekhar P, Poole DP, Liedtke W, Bunnett NW,Veldhuis NA. P2Y1 Receptor Activation of the TRPV4 Ion Channel Enhances Purinergic Signaling in Satellite Glial Cells. J Biol Chem, 2015. 290(48): p. 29051–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Ray P, Torck A, Quigley L, Wangzhou A, Neiman M, Rao C, Lam T, Kim JY, Kim TH, Zhang MQ, Dussor G,Price TJ. Comparative transcriptome profiling of the human and mouse dorsal root ganglia: an RNA-seq-based resource for pain and sensory neuroscience research. Pain, 2018. 159(7): p. 1325–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Rivera C, Voipio J, Thomas-Crusells J, Li H, Emri Z, Sipilä S, Payne JA, Minichiello L, Saarma M,Kaila K. Mechanism of activity-dependent downregulation of the neuron-specific K-Cl cotransporter KCC2. The Journal of neuroscience : the official journal of the Society for Neuroscience, 2004. 24(19): p. 4683–4691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Rivkees SA, Thevananther S,Hao H. Are A3 adenosine receptors expressed in the brain? Neuroreport, 2000. 11(5): p. 1025–30. [DOI] [PubMed] [Google Scholar]
- [149].Ru F, Surdenikova L, Brozmanova M,Kollarik M. Adenosine-induced activation of esophageal nociceptors. Am J Physiol Gastrointest Liver Physiol, 2011. 300(3): p. G485–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Salvatore CA, Jacobson MA, Taylor HE, Linden J,Johnson RG. Molecular cloning and characterization of the human A3 adenosine receptor. Proc Natl Acad Sci U S A, 1993. 90(21): p. 10365–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Sawynok J Adenosine receptor targets for pain. Neuroscience, 2016. 338: p. 1–18. [DOI] [PubMed] [Google Scholar]
- [152].Sharp CJ, Reeve AJ, Collins SD, Martindale JC, Summerfield SG, Sargent BS, Bate ST,Chessell IP. Investigation into the role of P2X(3)/P2X(2/3) receptors in neuropathic pain following chronic constriction injury in the rat: an electrophysiological study. Br J Pharmacol, 2006. 148(6): p. 845–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Sheth S, Brito R, Mukherjea D, Rybak LP,Ramkumar V. Adenosine receptors: expression, function and regulation. Int J Mol Sci, 2014. 15(2): p. 2024–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Shinoda M, Kawashima K, Ozaki N, Asai H, Nagamine K,Sugiura Y. P2X3 receptor mediates heat hyperalgesia in a rat model of trigeminal neuropathic pain. J Pain, 2007. 8(7): p. 588–97. [DOI] [PubMed] [Google Scholar]
- [155].Song G, Ouyang G,Bao S. The activation of Akt/PKB signaling pathway and cell survival. J Cell Mol Med, 2005. 9(1): p. 59–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Sorge RE, Mapplebeck JC, Rosen S, Beggs S, Taves S, Alexander JK, Martin LJ, Austin JS, Sotocinal SG, Chen D, Yang M, Shi XQ, Huang H, Pillon NJ, Bilan PJ, Tu Y, Klip A, Ji RR, Zhang J, Salter MW,Mogil JS. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat Neurosci, 2015. 18(8): p. 1081–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Staikopoulos V, Sessle BJ, Furness JB,Jennings EA. Localization of P2X2 and P2X3 receptors in rat trigeminal ganglion neurons. Neuroscience, 2007. 144(1): p. 208–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Stemmer SM, Benjaminov O, Medalia G, Ciuraru NB, Silverman MH, Bar-Yehuda S, Fishman S, Harpaz Z, Farbstein M, Cohen S, Patoka R, Singer B, Kerns WD,Fishman P. CF102 for the treatment of hepatocellular carcinoma: a phase I/II, open-label, dose-escalation study. Oncologist, 2013. 18(1): p. 25–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Stemmer SM, Manojlovic NS, Marinca MV, Petrov P, Cherciu N, Ganea D, Ciuleanu T-E, Puscas IA, Beg MS, Purcell WT, Croitoru A-E, Ilieva RN, Natošević S, Nita AL, Kalev DN, Harpaz Z, Farbstein M, Silverman MH, Fishman P,Llovet JM. A phase II, randomized, double-blind, placebo-controlled trial evaluating efficacy and safety of namodenoson (CF102), an A3 adenosine receptor agonist (A3AR), as a second-line treatment in patients with Child-Pugh B (CPB) advanced hepatocellular carcinoma (HCC). Journal of Clinical Oncology, 2019. 37(15_suppl): p. 2503–2503. [Google Scholar]
- [160].Stockstill K, Wahlman C, Braden K, Chen Z, Yosten GL, Tosh DK, Jacobson KA, Doyle TM, Samson WK,Salvemini D. Sexually dimorphic therapeutic response in bortezomib-induced neuropathic pain reveals altered pain physiology in female rodents. Pain, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Stokes L, Layhadi JA, Bibic L, Dhuna K,Fountain SJ. P2X4 Receptor Function in the Nervous System and Current Breakthroughs in Pharmacology. Front Pharmacol, 2017. 8: p. 291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Storme J, Tosh DK, Gao ZG, Jacobson KA,Stove CP. Probing structure-activity relationship in beta-arrestin2 recruitment of diversely substituted adenosine derivatives. Biochem Pharmacol, 2018. 158: p. 103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Sugawara S, Okada S, Katagiri A, Saito H, Suzuki T, Komiya H, Kanno K, Ohara K, Iinuma T, Toyofuku A,Iwata K. Interaction between calcitonin gene-related peptide-immunoreactive neurons and satellite cells via P2Y12 R in the trigeminal ganglion is involved in neuropathic tongue pain in rats. Eur J Oral Sci, 2017. 125(6): p. 444–452. [DOI] [PubMed] [Google Scholar]
- [164].Sun Y Huang P Adenosine A2B Receptor: From Cell Biology to Human Diseases. Front Chem, 2016. 4: p. 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Szanto G, Mako A, Vago I, Hergert T, Bata I, Farkas B, Kolok S,Vastag M. New P2X3 receptor antagonists. Part 2: Identification and SAR of quinazolinones. Bioorg Med Chem Lett, 2016. 26(16): p. 3905–12. [DOI] [PubMed] [Google Scholar]
- [166].Taiwo YO Levine JD. Direct cutaneous hyperalgesia induced by adenosine. Neuroscience, 1990. 38(3): p. 757–62. [DOI] [PubMed] [Google Scholar]
- [167].Tamagawa T, Shinoda M, Honda K, Furukawa A, Kaji K, Nagashima H, Akasaka R, Chen J, Sessle BJ, Yonehara Y,Iwata K. Involvement of Microglial P2Y12 Signaling in Tongue Cancer Pain. J Dent Res, 2016. 95(10): p. 1176–82. [DOI] [PubMed] [Google Scholar]
- [168].TANAKA S-, Futabacho 3-chome, Toyonaka-sh, Osaka 25, ?5610825, JP), OGAWA Tomoyuki (1-1, Futabacho 3-chome, Toyonaka-sh, Osaka 25, ?5610825, JP), KAI Hiroyuki (1-, Futabacho 3-chome, Toyonaka-sh, Osaka 25, ?5610825, JP), OGATA Yuki (1-1, Futabacho 3-chome, Toyonaka-sh, Osaka 25, ?5610825, JP), HIRAI Keiichiro (1-1, Futabacho 3-chome, Toyonaka-sh, Osaka 25, ?5610825, JP), KUROSE Noriyuki (1-1, Futabacho 3-chome, Toyonaka-sh, Osaka 25, ?5610825, JP), FUJII Yasuhiko (1-1, Futabacho 3-chome, Toyonaka-sh, Osaka 25, ?5610825, JP),, 6-MEMBERED HETEROCYCLIC DERIVATIVE AND PHARMACEUTICAL COMPOSITION COMPRISING SAME. 2016, SHIONOGI & CO., LTD. (1-8 Doshomachi 3-chome, Chuo-ku Osaka-sh, Osaka 45, ?5410045, JP),. [Google Scholar]
- [169].Tang Y, Yin HY, Liu J, Rubini P,Illes P. P2X receptors and acupuncture analgesia. Brain Res Bull, 2018. [DOI] [PubMed] [Google Scholar]
- [170].Teixeira JM, Dos Santos GG, Neves AF, Athie MCP, Bonet IJM, Nishijima CM, Farias FH, Figueiredo JG, Hernandez-Olmos V, Alshaibani S, Tambeli CH, Muller CE,Parada CA. Diabetes-induced Neuropathic Mechanical Hyperalgesia Depends on P2X4 Receptor Activation in Dorsal Root Ganglia. Neuroscience, 2019. 398: p. 158–170. [DOI] [PubMed] [Google Scholar]
- [171].Terayama R, Tabata M, Maruhama K,Iida S. A3 adenosine receptor agonist attenuates neuropathic pain by suppressing activation of microglia and convergence of nociceptive inputs in the spinal dorsal horn. Exp Brain Res, 2018. 236(12): p. 3203–3213. [DOI] [PubMed] [Google Scholar]
- [172].THOMPSON SGC, Phoenixville, PA, 19460, US), VENKATESAN Aranapakam (7100 Johnson Farm Lane, Unit# 105Chadds Ford, PA, 19317, US), PRIESTLEY Tony (336 Old Bailey Lane, West Chester, PA, 19382, US), KUNDU Mrinal (CK 106, Sector II Salt Lake, Kolkata 1, 091, IN), SAHA Ashis (25 Crescent Street, Apartment 227Waltham, MA, 02453, US), P2X3 AND/OR P2X2/3 COMPOUNDS AND METHODS. 2015, ASANA BIOSCIENCES, LLC; (400 Crossing Boulevard, Third FloorBridgewater, NJ, 08807-2863, US). [Google Scholar]
- [173].Tosh DK, Deflorian F, Phan K, Gao ZG, Wan TC, Gizewski E, Auchampach JA,Jacobson KA. Structure-guided design of A(3) adenosine receptor-selective nucleosides: combination of 2-arylethynyl and bicyclo[3.1.0]hexane substitutions. J Med Chem, 2012. 55(10): p. 4847–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Tosh DK, Finley A, Paoletta S, Moss SM, Gao ZG, Gizewski ET, Auchampach JA, Salvemini D,Jacobson KA. In vivo phenotypic screening for treating chronic neuropathic pain: modification of C2-arylethynyl group of conformationally constrained A3 adenosine receptor agonists. J Med Chem, 2014. 57(23): p. 9901–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Tosh DK, Padia J, Salvemini D,Jacobson KA. Efficient, large-scale synthesis and preclinical studies of MRS5698, a highly selective A3 adenosine receptor agonist that protects against chronic neuropathic pain. Purinergic Signal, 2015. 11(3): p. 371–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Tosh DK, Rao H, Bitant A, Salmaso V, Mannes P, Lieberman DI, Vaughan KL, Mattison JA, Rothwell AC, Auchampach JA, Ciancetta A, Liu N, Cui Z, Gao ZG, Reitman ML, Gavrilova O,Jacobson KA. Design and in Vivo Characterization of A1 Adenosine Receptor Agonists in the Native Ribose and Conformationally Constrained (N)-Methanocarba Series. J Med Chem, 2019. 62(3): p. 1502–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Tozaki-Saitoh H, Miyata H, Yamashita T, Matsushita K, Tsuda M,Inoue K. P2Y12 receptors in primary microglia activate nuclear factor of activated T-cell signaling to induce C-C chemokine 3 expression. J Neurochem, 2017. 141(1): p. 100–110. [DOI] [PubMed] [Google Scholar]
- [178].Tozaki-Saitoh H, Tsuda M, Miyata H, Ueda K, Kohsaka S,Inoue K. P2Y12 receptors in spinal microglia are required for neuropathic pain after peripheral nerve injury. J Neurosci, 2008. 28(19): p. 4949–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Trang T Salter MW. P2X4 purinoceptor signaling in chronic pain. Purinergic Signal, 2012. 8(3): p. 621–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Tsuda M Inoue K Neuron-microglia interaction by purinergic signaling in neuropathic pain following neurodegeneration. Neuropharmacology, 2016. 104: p. 76–81. [DOI] [PubMed] [Google Scholar]
- [181].Tsuda M, Kuboyama K, Inoue T, Nagata K, Tozaki-Saitoh H,Inoue K. Behavioral phenotypes of mice lacking purinergic P2X4 receptors in acute and chronic pain assays. Mol Pain, 2009. 5: p. 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Varani K, Vincenzi F, Targa M, Paradiso B, Parrilli A, Fini M, Lanza G,Borea PA. The stimulation of A(3) adenosine receptors reduces bone-residing breast cancer in a rat preclinical model. Eur J Cancer, 2013. 49(2): p. 482–91. [DOI] [PubMed] [Google Scholar]
- [183].Varano F, Catarzi D, Vincenzi F, Betti M, Falsini M, Ravani A, Borea PA, Colotta V,Varani K. Design, Synthesis, and Pharmacological Characterization of 2-(2-Furanyl)thiazolo[5,4-d]pyrimidine-5,7-diamine Derivatives: New Highly Potent A2A Adenosine Receptor Inverse Agonists with Antinociceptive Activity. J Med Chem, 2016. 59(23): p. 10564–10576. [DOI] [PubMed] [Google Scholar]
- [184].Vincenzi F, Targa M, Romagnoli R, Merighi S, Gessi S, Baraldi PG, Borea PA,Varani K. TRR469, a potent A(1) adenosine receptor allosteric modulator, exhibits anti-nociceptive properties in acute and neuropathic pain models in mice. Neuropharmacology, 2014. 81: p. 6–14. [DOI] [PubMed] [Google Scholar]
- [185].von Kugelgen I Hoffmann K Pharmacology and structure of P2Y receptors. Neuropharmacology, 2016. 104: p. 50–61. [DOI] [PubMed] [Google Scholar]
- [186].Vulchanova L, Riedl MS, Shuster SJ, Stone LS, Hargreaves KM, Buell G, Surprenant A, North RA,Elde R. P2X3 is expressed by DRG neurons that terminate in inner lamina II. Eur J Neurosci, 1998. 10(11): p. 3470–8. [DOI] [PubMed] [Google Scholar]
- [187].Wahlman C, Doyle TM, Little JW, Luongo L, Janes K, Chen Z, Esposito E, Tosh DK, Cuzzocrea S, Jacobson KA,Salvemini D. Chemotherapy-induced pain is promoted by enhanced spinal adenosine kinase levels through astrocyte-dependent mechanisms. Pain, 2018. 159(6): p. 1025–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Wang S, Wang Z, Li L, Zou L, Gong Y, Jia T, Zhao S, Yuan H, Shi L, Liu S, Wu B, Yi Z, Liu H, Gao Y, Li G, Deussing JM, Li M, Zhang C,Liang S. P2Y12 shRNA treatment decreases SGC activation to relieve diabetic neuropathic pain in type 2 diabetes mellitus rats. J Cell Physiol, 2018. 233(12): p. 9620–9628. [DOI] [PubMed] [Google Scholar]
- [189].Wang S, Zhu HY, Jin Y, Zhou Y, Hu S, Liu T, Jiang X,Xu GY. Adrenergic signaling mediates mechanical hyperalgesia through activation of P2X3 receptors in primary sensory neurons of rats with chronic pancreatitis. Am J Physiol Gastrointest Liver Physiol, 2015. 308(8): p. G710–9. [DOI] [PubMed] [Google Scholar]
- [190].Williams WA, Linley JE, Jones CA, Shibata Y, Snijder A, Button J, Hatcher JP, Huang L, Taddese B, Thornton P, Schofield DJ, Thom G, Popovic B, Dosanjh B, Wilkinson T, Hughes J, Dobson CL, Groves MA, Webster CI, Billinton A, Vaughan TJ,Chessell I. Antibodies binding the head domain of P2X4 inhibit channel function and reverse neuropathic pain. Pain, 2019. 160(9): p. 1989–2003. [DOI] [PubMed] [Google Scholar]
- [191].Wissmann CL, Wang M, Gao M, Zheng QH,Green MA. Development, validation and implementation of radio-HPLC methods for the P2X7-receptor-targeted [(11)C]GSK1482160 radiopharmaceutical. Appl Radiat Isot, 2018. 142: p. 8–11. [DOI] [PubMed] [Google Scholar]
- [192].Wu J, Cheng Y, Zhang R, Liu D, Luo YM, Chen KL, Ren S,Zhang J. P2Y1R is involved in visceral hypersensitivity in rats with experimental irritable bowel syndrome. World J Gastroenterol, 2017. 23(34): p. 6339–6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Wu WP, Hao JX, Halldner L, Lovdahl C, DeLander GE, Wiesenfeld-Hallin Z, Fredholm BB,Xu XJ. Increased nociceptive response in mice lacking the adenosine A1 receptor. Pain, 2005. 113(3): p. 395–404. [DOI] [PubMed] [Google Scholar]
- [194].Xu Y, Li N, Xiang R,Sun P. Emerging roles of the p38 MAPK and PI3K/AKT/mTOR pathways in oncogene-induced senescence. Trends Biochem Sci, 2014. 39(6): p. 268–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Yaar R, Lamperti ED, Toselli PA,Ravid K. Activity of the A3 adenosine receptor gene promoter in transgenic mice: characterization of previously unidentified sites of expression. FEBS Lett, 2002. 532(3): p. 267–72. [DOI] [PubMed] [Google Scholar]
- [196].Yan H, Zhang E, Feng C,Zhao X. Role of A3 adenosine receptor in diabetic neuropathy. J Neurosci Res, 2016. 94(10): p. 936–46. [DOI] [PubMed] [Google Scholar]
- [197].Yang Y, Li H, Li TT, Luo H, Gu XY, Lu N, Ji RR,Zhang YQ. Delayed activation of spinal microglia contributes to the maintenance of bone cancer pain in female Wistar rats via P2X7 receptor and IL-18. J Neurosci, 2015. 35(20): p. 7950–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Yasuda M, Shinoda M, Honda K, Fujita M, Kawata A, Nagashima H, Watanabe M, Shoji N, Takahashi O, Kimoto S,Iwata K. Maternal Separation Induces Orofacial Mechanical Allodynia in Adulthood. J Dent Res, 2016. 95(10): p. 1191–7. [DOI] [PubMed] [Google Scholar]
- [199].Yin D, Liu YY, Wang TX, Hu ZZ, Qu WM, Chen JF, Cheng NN,Huang ZL. Paeoniflorin exerts analgesic and hypnotic effects via adenosine A1 receptors in a mouse neuropathic pain model. Psychopharmacology (Berl), 2016. 233(2): p. 281–93. [DOI] [PubMed] [Google Scholar]
- [200].Yoon MH, Bae HB, Choi JI, Kim SJ, Chung ST,Kim CM. Roles of adenosine receptor subtypes in the antinociceptive effect of intrathecal adenosine in a rat formalin test. Pharmacology, 2006. 78(1): p. 21–6. [DOI] [PubMed] [Google Scholar]
- [201].Zhang M, Dai Q, Liang D, Li D, Chen S, Chen S, Han K, Huang L,Wang J. Involvement of adenosine A1 receptor in electroacupuncture-mediated inhibition of astrocyte activation during neuropathic pain. Arq Neuropsiquiatr, 2018. 76(11): p. 736–742. [DOI] [PubMed] [Google Scholar]
- [202].Zhang M, Hu H, Zhang X, Lu W, Lim J, Eysteinsson T, Jacobson KA, Laties AM,Mitchell CH. The A3 adenosine receptor attenuates the calcium rise triggered by NMDA receptors in retinal ganglion cells. Neurochem Int, 2010. 56(1): p. 35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Zhang W Liu HT. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res, 2002. 12(1): p. 9–18. [DOI] [PubMed] [Google Scholar]
- [204].Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O’Keeffe S, Phatnani HP, Guarnieri P, Caneda C, Ruderisch N, Deng S, Liddelow SA, Zhang C, Daneman R, Maniatis T, Barres BA,Wu JQ. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci, 2014. 34(36): p. 11929–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Zhang Y, Sloan SA, Clarke LE, Caneda C, Plaza CA, Blumenthal PD, Vogel H, Steinberg GK, Edwards MS, Li G, Duncan JA 3rd, Cheshier SH, Shuer LM, Chang EF, Grant GA, Gephart MG,Barres BA. Purification and Characterization of Progenitor and Mature Human Astrocytes Reveals Transcriptional and Functional Differences with Mouse. Neuron, 2016. 89(1): p. 37–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Zhou QY, Li C, Olah ME, Johnson RA, Stiles GL,Civelli O. Molecular cloning and characterization of an adenosine receptor: the A3 adenosine receptor. Proc Natl Acad Sci U S A, 1992. 89(16): p. 7432–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Zhu H, Yu Y, Zheng L, Wang L, Li C, Yu J, Wei J, Wang C, Zhang J, Xu S, Wei X, Cui W, Wang Q,Chen X. Chronic inflammatory pain upregulates expression of P2Y2 receptor in small-diameter sensory neurons. Metab Brain Dis, 2015. 30(6): p. 1349–58. [DOI] [PubMed] [Google Scholar]
- [208].Zylka MJ. Pain-relieving prospects for adenosine receptors and ectonucleotidases. Trends Mol Med, 2011. 17(4): p. 188–96. [DOI] [PMC free article] [PubMed] [Google Scholar]