Abstract
Malignant cells remodel their metabolism to meet the demands of uncontrolled cell proliferation. These demands lead to differential requirements in energy, biosynthetic precursors, and signaling intermediates. Both genetic programs arising from oncogenic events as well transcriptional programs and epigenomic events are important in providing the necessary metabolic network activity. Accumulating evidence has established that environment factors such has a major a role in shaping cancer cell metabolism. For metabolism, diet and nutrition are the major aspects of the environment and have emerged as being a key component in determining cancer cell metabolism. In this review, we will discuss these emerging concepts in cancer metabolism and how diet and nutrition influence cancer cell metabolism.
eTOC Blurb
Altered nutrient uptake results in changes to metabolic pathway activity and is a common feature of cancer metabolism. In this special issue of Molecular Cell, the authors review the emerging concept of how diet and nutrition exert influence over cancer cell metabolism.
Introduction
Cancer cells exhibit metabolic alterations to satisfy the demands the biosynthetic, bioenergetic, and signaling demands of uncontrolled proliferation. Altered metabolism at the molecular level has been described for nearly 100 years since Otto Warburg and colleagues reported that malignant cells took up more glucose than normal tissue counterparts and preferentially fermented glucose to make lactate in lieu of the more energetically efficient oxidation to CO2. It is now widely appreciated that alterations in the uptake and metabolism of carbohydrates, lipids, and amino acids occur in tumors. The particular nutrients which are more avidly taken up by cancer cells are highly variable, with subsets of cancers exhibiting altered glucose metabolism, altered one-carbon metabolism, and increased reliance on amino acid metabolism along with many other pathways. These observations are used clinically as imaging modalities and are the subjects of ongoing drug development efforts (Stuani, 2019; Vander Heiden, 2017). These concepts have been reviewed extensively over the years (Weinberg, 2015; Wolpaw, 2018).
Cancer metabolism, as with all processes in life, is comprised of both genetic and environmental components. Nearly all oncogene and tumor suppressor genes have the capacity to alter metabolism in some form (Vander Heiden, 2017). Mutations that engage signaling pathways and transcriptional programs hard-wire some elements of the metabolic network by altering gene expression and activity through the placement of post-translational modifications onto metabolic enzymes and transporters, for example. These cancer-associated processes are also engaged by environmental factors specific to the spatial environment within the tissue of origin such as the presence of growth factors and cytokines, cell-cell contacts. Beyond these factors that shape the activity of the internal cellular metabolic network, the availability of nutrients which is completely shaped by the environment has a dominant role in defining cancer cell metabolism (Palm, 2017; Zhu, 2019). Nutrient availability that any malignant cell might experience comes from the metabolites released from surrounding cells and metabolite composition of the plasma in the vasculature.
Plasma metabolite levels are set by a conglomeration of physiological processes involving interactions with the gut, liver, muscle, pancreas, and other tissues. Nutrient availability in the plasma begins with dietary intake, and concentrations of metabolites vary dramatically based on their intake from the diet (Mentch, 2015). Certain diets are known to be associated with some aspects of health. For example, Mediterranean diets has been associated with longer lifespans. Conversely, Western diets are associated with obesity, cancer, and coronary heart disease. Interestingly, diets such as formations of plant-based diets have long been investigated as cancer therapy (Gerson, 1978). While strong epidemiological evidence linking these dietary patterns to disease exists, an understanding of the molecular mechanisms that underlie these effects is not well developed. Even less understood is how diet shapes metabolic pathways in health—let alone cancer progression and treatment (Goncalves, 2019a; Kanarek et al., 2020; Lien, 2019). Ongoing research into central carbon metabolism which processes carbohydrates, lipids, and amino acids, provide insights into potential opportunities to modulate cancer cell metabolism. In this review, we will discuss emerging findings that provide a molecular link from diet to cancer and present considerations for therapeutic strategies to target these mechanisms.
Mechanistic principles that link diet to cellular cancer metabolism
As mentioned, cancer cell metabolism is highly influenced by environmental factors, including tumor acidity, stromal and immune cell populations, and mechanic properties of tumor architecture. These features have been reviewed elsewhere (Bi, 2020; Wolpaw, 2018). Of particular interest, the nutrients available in the microenvironmental milieu which surrounds the tumor are responsible for defining much of tumor cell metabolism (Chen, 2019; Tasdogan, 2020). Indeed, while tumoral metabolism is highly heterogeneous and driven by anatomical location, genetic profiles, and other factors, some tumor types have been shown to develop metabolic dependencies on certain nutrients, like glutamine and cysteine. The availability of nutrients is dictated by the flow of plasma nutrients from systemic circulation to tumor cells (Muir, 2018). As such, the determinants of plasma nutrient availability are of particular interest, as it is a major point of control over tumor metabolism which is also amenable to both pharmacological and lifestyle/environmental interventions.
Nutrient availability exerts control over cellular metabolism through multiple mechanisms. The cellular uptake of nutrients from the surrounding microenvironment is one such process, which is regulated tightly by the kinetic properties of active transporters, including their Michaelis constants. (Cox, 2000). Physiologically, cancer cells encounter concentrations of nutrients which are at or above those found in corresponding normal tissues. These increases in nutrient availability influence the rate of nutrient uptake which then propagate to changes in metabolic flux through the metabolic network and downstream functions (Palm, 2017). This paradigm is best illustrated by the ability of the liver to sense high glucose levels and respond with increased glycogen synthesis. This switch is accomplished, in part, via the high Km of hepatic glucose transport (~5 mM). When in a fasted state, glucose concentrations are low (~3 mM) allowing glucose to mostly bypass hepatic uptake and reenter circulation. However, in a fed state, plasma glucose concentrations increase (~8 mM), leading to a proportional increase in glucose uptake (Mueckler, 2013). This three-fold increase in glucose uptake from baseline prompts glycogen synthesis, and thus allows the liver to accomplish its key glucose homeostatic function.
The plasma metabolome reflects dietary intake in the context of individual metabolic heterogeneity which includes factors as such liver function, gut microbiome composition, and muscle/adipose metabolism (Zeevi, 2015). An emerging, but still undeveloped, field of research has considered the application of metabolite profiling in conjunction with dietary tracking to predict circulating plasma alterations that occur as a result of nutrient intake. While overall dietary patterns are reproducibly reflected in the plasma metabolome, further work is needed to better define more granular features of diet, including individual foods and their effects on metabolite concentrations (Wittenbecher, 2015).
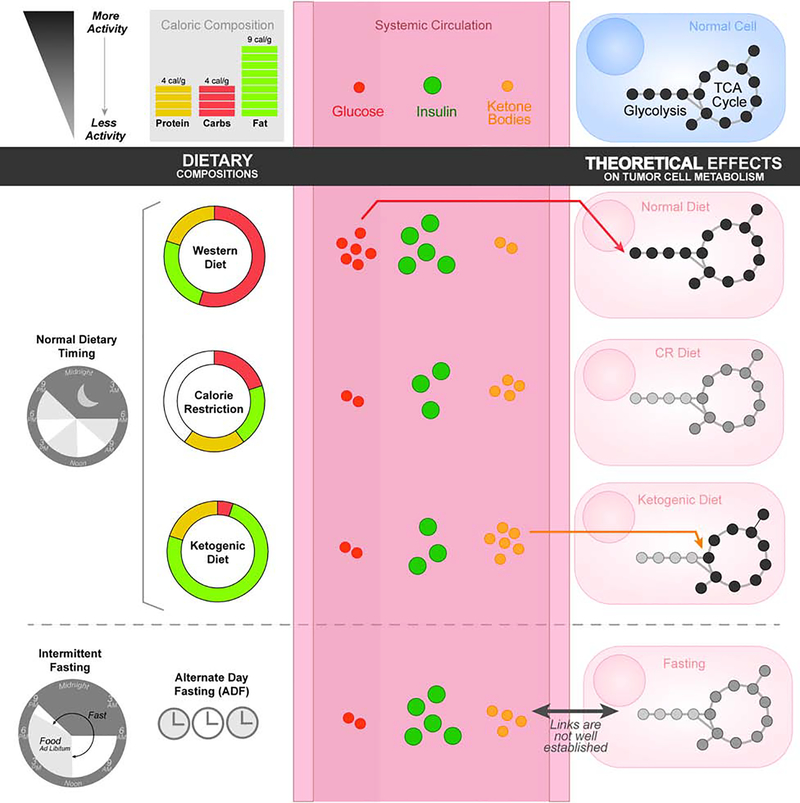
The clinical value of measuring dietary patterns and metabolic outcomes is evidenced by the established links between certain diets and dysregulations of whole body metabolism. The constellation of chronic conditions associated with metabolic syndrome—obesity, insulin resistance, and hyperglycemia—have also all been associated with higher cancer risk and poorer patient prognosis (Perry, 2020). The link from obesity to cancer has been attributed to a number of mechanisms including ER-stress, inflammation, hormonal signaling, and possibly altered metabolism due to changes in plasma metabolite levels (Iyengar, 2016; Ulrich, 2018). Hyperglycemia is also associated with greater cancer risk and progression. This protumorigenic effect may be due to both systemic effects of insulin/insulin-related growth factor-1 (IGF-1), other growth factors, and inflammatory signaling as well as direct uptake of glucose by tumor cells to drive epigenetic and biosynthetic changes (Goncalves, 2018). Thus, the role of diet-mediated changes to systemic metabolism and how they may affect tumor metabolism (Figure 1) warrants further study.
Figure 1:
Dietary compositions affect circulating metabolic factors and nutrient availability which, in turn, are thought to have effects on tumor cell metabolism. Dietary timing as well as caloric compositions are known to affect endocrine secretions of insulin which thereby affect cellular uptake of glucose. Limitations of glucose and an abundance of ketone bodies during the ketosis induced by the ketogenic diet has been linked to reduced glycolysis and enhanced oxidative processing. These suggest that, while these mechanisms are not well understood, diets may have important roles in driving cancer cell metabolism.
Macronutrient metabolism and molecular mechanisms of cancer
Caloric Restriction
Calorie restriction (Figure 1) without malnutrition (CR) has been shown to extend lifespan and delay the onset of age-related diseases, including cancer. This phenomenon has been widely studied in rodents and the anti-cancer and longevity-promoting effects of CR appear to be conserved across species ranging from worms to non-human primates (Fontana et al., 2010; Mattison et al., 2017). Numerous animal studies show that CR can prevent many cancers, as well as restrict progression and metastasis (Lv et al., 2014). These positive effects have been shown in cancers from diverse tissues including mammary, lung, prostate, brain, bladder, pancreatic, hepatic, skin, colorectal, and ovarian (Castejon et al., 2020; Lv et al., 2014). The molecular mechanisms believed to underlie these effects have been largely attributed to reduced circulating levels of several hormones such as growth factors and cytokines. Particularly, CR has been associated with lower levels of circulating IGF-1, which is known to engage signaling networks involving RAS/MAPK and PI3K/AKT/mTOR which are common dysregulated pathways in cancers (Goncalves, 2018). Thus, CR appears to exert a host of effects on signaling pathways that could have anti-cancer activity. In addition, CR has also been shown to have antiangiogenic and proapoptotic effects on tumors in mice, as well as systemic anti-inflammatory effects (O’Flanagan, 2017). The effects on metabolism are less understood but have been the subject of preliminary studies. CR slows basal metabolic rate, an effect which has been shown in humans (Redman et al., 2018). This is theorized to slow aging and protect from age-related disease due to a decrease in the production of mitochondrial reactive oxygen species and associated cellular oxidative damage (Sohal and Allen, 1985). Compared to ad libitum (AL) feeding, food intake in CR mice has been also shown to trigger an initial increase in FA synthesis followed by a compensatory increase in FA oxidation (Bruss et al., 2010). This cyclical pattern of adipose tissue metabolism reflects dynamic changes in expression of FA synthesis enzymes like FA synthase (FAS) and acetyl-CoA carboxylase (ACC1). In addition, CR has been linked to decreased glycolysis and alterations in FA membrane composition, with decreased levels of polyunsaturated FAs (PUFAs) and increased monounsaturated FAs (MUFAs) (Jove et al., 2014). Both increased fatty acid oxidation and decreased membrane polyunsaturation are thought to help protect cells from oxidative damage (Weinberg, 2015). CR has also been shown to alter other metabolic pathways including the carnitine shuttle pathway, sphingosine metabolism, and methionine metabolism (Green et al., 2017). In the future, targeting metabolic pathways to assess the extent that altered metabolism caused by CR can exert anti-cancer effects will be interesting.
In addition to the demonstrated effects metabolic rate, human studies have shown that CR is capable of altering certain molecular factors associated with cancer prevention from animal studies (Most et al., 2017). Results from the CALERIE-2 (Comprehensive Assessment of Long-term Effects of Reducing Intake of Energy) trials showed that an approximate 15% reduction in calories in non-obese adults over two years reduces thyroid hormone activity and reactive oxygen species production (Redman et al., 2018). Similar results along with a reduction in fasting insulin concentrations were obtained in a population of mostly overweight but non-obese participants achieving approximately 18% reduction in caloric intake over six months (Heilbronn et al., 2006; Most et al., 2017). However, these studies failed to find a reduction in levels of serum IGF-1. An observational study on members of the Calorie Restriction Society, a group of individuals who have been voluntarily restricting caloric intake while maintaining adequate nutrition, found that compared to matched controls, IGF-1 levels were only reduced in members if protein intake was also reduced (Most et al., 2017). Currently, clinical trials are on-going to more directly assess the impact of CR on cancer prevention and treatment (Castejon et al., 2020).
However, caution must be taken before universally applying CR as an anti-cancer strategy, as much remains to be understood about whether CR imposes differential effects on cancer based on underlying metabolic dependencies, tumor location/microenvironment, mutational status, and/or metastasis versus primary tumors (Garcia-Jimenez and Goding, 2019; Kalaany and Sabatini, 2009).
Fasting
Recently, fasting regimens such as alternate day-fasting or time-restricted feeding have been shown to have health-promoting effects (de Cabo, 2019; Longo, 2014; Mattson, 2019; Mitchell et al., 2019). Fasting (Figure 1), defined by periods of caloric deprivation ranging from hours to days, is an attractive alternative to calorie restriction as certain fasting regimens are easier to adhere to and may be better tolerated by cancer patients who are at risk of undesired weight loss and cachexia (Safdie et al., 2009). Interestingly, short-term fasts dramatically alter serum IGF-1 levels in humans, whereas long-term CR may not. Studies in yeast and mammalian cells have shown that periods of nutrient restriction protect non-oncogene-expressing yeast and normal cells against oxidative stress and chemotherapy, whereas nutrient restriction offers no protection or sensitizes oncogene-expressing yeast and cancer cells to these conditions (Lee et al., 2012; Raffaghello et al., 2008). In mice, 24–60 hours of fasting protected against extremely high doses of chemotherapy (Lee et al., 2012; Raffaghello et al., 2008), radiotoxicity (de la Cruz Bonilla, 2019), and increased the effectiveness of chemotherapies in mice grafted with pancreatic cancer, melanoma, breast cancer, glioma, and neuroblastoma cells (D’Aronzo et al., 2015; Lee et al., 2012), which in some cases resulted in long-term cancer-free survival (Lee et al., 2012).
Mechanistically, fasting is thought to be protective towards non-neoplastic cells because hormonal and metabolic changes during fasting direct normal cells towards a stress-resistance state characterized by a switch from growth processes to maintenance and repair (Di Biase, 2017; Lee et al., 2012; Nencioni et al., 2018). Cancer cells are unable to adopt this stress-resistance state and therefore not protected or sensitized to stress. Fasting and “fasting-mimicking diets” may also improve cancer outcomes by enhancing anti-cancer immune function through the promotion of T cell-mediated tumor cytotoxicity (Di Biase et al., 2016).
The effects of fasting and dietary timing (Figure 1) on human cancer risk and progression remains understudied (Marinac et al., 2016; Yokoyama et al., 2016). However, measures associated with cancer have been shown to be impacted by fasting in humans. A controlled trial of alternate-day-fasting, a dietary regimen where food is consumed ad-libitum every other day and followed by a day of complete caloric restriction, yielded reductions in plasma methionine and thyroid hormone T3 levels (Stekovic et al., 2019). Another recent study in humans has demonstrated significant changes in blood metabolite profiles after 58 hours of fasting (Teruya, 2019). These changes reflect alterations in antioxidant defense mechanisms, mitochondrial activity, and pentose phosphate pathway rewiring, among other changes to metabolism which are implicated in cancers. In cancer patients, 5–56 hours of fasting prior to and/or following chemotherapy was well-tolerated and reduced chemotherapy side effects, consistent with previously discussed results in yeast, mice, and mammalian cells (Safdie et al., 2009). As such, fasting appears to be an intriguing way to influence cancer cell metabolism which may synergize with chemotherapy and radiation.
Macronutrient Balance
Carbohydrate and Fat intake
Individual macronutrient intake may play as large of a role as caloric intake in dictating longevity, health, and disease. Diets high in saturated fats and carbohydrates, like Western diets, are linked to poorer health outcomes. The plasma metabolite signature reflects this burden of increased central carbon metabolism, with high levels of short-chain acylcarnitines and circulating amino acids (Bouchard-Mercier, 2013; Douris, 2015). Recent work has focused on how diets low in carbohydrates or protein influence these factors and the extent of their benefits in preventing or treating cancer. The ketogenic diet (KD) is a type of low-carbohydrate diet that has been most extensively studied in relation to cancer. The classical ketogenic diet is used to treat epilepsy and is defined as a “high-fat, moderate-protein, low-carbohydrate diet”, with a specified 4:1 or 3:1 weight ratio of fat to combined protein and carbohydrates (Huffman and Kossoff, 2006). Ketogenic diets result in lower blood glucose levels which leads to ketosis involving reduced glycolysis and increased β-oxidation (Puchalska, 2017). The production of ketone bodies from fatty acids in the liver provides energy-producing substrates for the brain and other organs. This causes a number of effects on central carbon metabolism including altered glucose and central carbon metabolism which tumors are known to require (Allen et al., 2014; Goncalves, 2019b; Liberti and Locasale, 2016). Ketogenic diets have also been hypothesized to work through mechanisms similar to those postulated in caloric restriction, including reducing anabolic hormones like IGF-1 and insulin and increasing oxidative stress in tumors (Allen et al., 2014; Xia, 2017). Interestingly, a recent study showed that the ketogenic diet synergizes with PI3K inhibitors, likely through suppression of insulin production (Hopkins, 2018).
Currently there is no rigorous evidence that low-carbohydrate or ketogenic diets decrease cancer incidence or improve outcomes in humans (Erickson et al., 2017) but much remains to be studied. Mouse studies on ketogenic diets as interventions in xenograft models have shown mixed results, with some reports of reduced tumor growth (Caso et al., 2013; Klement, 2016), others showing effects on tumor growth in combination with chemotherapy or radiotherapy (Allen et al., 2013), and others reporting no benefit of KD (Maurer et al., 2011). It should be noted that these studies have considerable differences in the macronutrient compositions of their control and ketogenic diets, and in most of these studies, protein intake was lower in KD groups. Because protein intake has been shown to influence cancer, this is a major confounding factor. However, two of these studies kept protein intake consistent between groups and still saw a beneficial effect of KD and low-carbohydrate diets in prostate cancer xenograft models (Caso et al., 2013), suggesting low-carbohydrate or ketogenic diets could be beneficial in some cancer settings. It is known that tumors can vary widely as to their dependence on glucose metabolism (Liberti et al., 2017). However, it is unknown whether a tumor’s preference for glucose metabolism predicts its responsiveness to diets that alter blood glucose levels.
Protein Intake
Although protein intake has a strong effect on health, less work has been done to assess how dietary protein affects cancer. One study found that middle-aged US men and women who reported moderate (10–19% calories from protein) or high (20% or more calories from protein) protein intake had a significantly increased risk of cancer mortality compared to those reporting low protein intake, however the trend was reversed for those over the age of 65 (Levine, 2014). Interestingly, the increased cancer risk among middle-aged subjects was somewhat diminished if their dietary protein came from plant rather than animal sources, which have markedly different amino acid content. This study also found that mice consuming a low-protein (4% calories from protein) diet had decreased tumor incidence and growth of implanted breast and melanoma cells compared to those consuming a high-protein (18% calories from protein) diet. Both human and mice in low-protein groups had lower IGF-1 levels, which has been previously reported in humans with decreased protein intake (Fontana et al., 2008). Another study found that mice consuming a low-carbohydrate, high protein (58% calories from protein) diet had slower growth of implanted squamous cell carcinoma and colorectal carcinoma tumors and decreased incidence of tumors in a spontaneous breast cancer model compared to mice consuming a Western diet (Ho et al., 2011). Mice consuming the low-carb, high protein diet did not show decreased levels of IGF-1 but did have lower blood glucose and insulin levels.
Different studies give contrasting views on whether protein or carbohydrate restriction is more beneficial for cancer treatment and prevention, which could be explained in several ways. It may be that different cancer types respond differently to macronutrient depletion as shown in Figure 1, with some extremely glycolytic cancers being more affected by carbohydrate-restricted diets than protein restricted ones, and cancers dependent on IGF-1 signaling responding best to protein restriction. Another possible explanation is that restricting any macronutrient in the diet is beneficial compared to a diet replete with all three macronutrients, as depleting at least one macronutrient triggers some of the same starvation responses that are seen in calorie restriction and thought to be anti-cancer. It is also unclear whether low-protein diets may reduce cancer cell growth through the deprivation of one or more amino acids, as cancers often show increased dependence on particular amino acids (discussed more below). Further research is needed to answer these questions and better determine how the macronutrient compositions of diets affect cancer.
Dietary Amino Acid Balance in Cancer
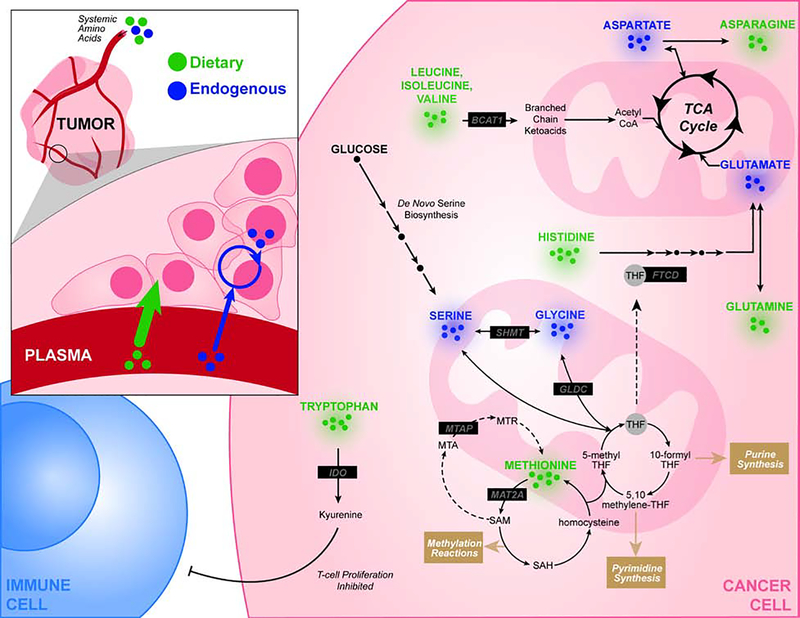
The increased metabolic demands of cancerous proliferation require altered amino acid metabolism, as illustrated in Figure 2. As such, tumor cells adapt to uptake and metabolize microenvironmental nutrients to meet these demands. Amino acids serve as both proteinogenic precursors and as metabolic intermediates are taken up in tumors. As metabolites, they serve as catabolic substrates for the TCA cycle, their oxidation and reduction maintains cellular redox balance, they serve as signaling intermediates that propagate nutrient status to growth signaling such as mTOR, and modifiers of chromatin and nucleic acids (Liu, 2020; Reid, 2017). They also participate in all aspects of anabolic metabolism, some of which are included in Figure 2.
Figure 2:
Circulating amino acids are metabolized in cancer cells to provide unique biosynthetic and energetic requirements of the tumor. Abbreviations: MTAP (S-methyl-5’-thioadenosine phosphorylase), MAT2A (Methionine Adenosyltransferase 2A), MTA (5’-methylthioadenosine), THF (tetrahydrofolate), BCAT1 (Branched chain amino acid transaminase 1), FTCD (Formimidoyltransferase cyclodeaminase)
Amino Acid Metabolism in Cancer
Methionine Metabolism
Methionine is a sulfur-containing amino acid involved in redox homeostasis, chromatin and nucleic acid methylation, polyamine synthesis, and other metabolic processes. As an essential amino acid, dietary methionine is converted to S-adenosyl-methionine (SAM), which serves as a universal methyl group donor for methylation reactions, and homocysteine, which interfaces with folate cycle and transsulfuration pathway. Methylthioadenosine (MTA) is also metabolized to methionine via activity of methylthioadenosine phosphorylase (MTAP) as a part of the methionine salvage pathway. Altered methionine metabolism is implicated in tumor biology (Sanderson, 2019a). Methionine may provide a useful clinical staging tool, with L-[methyl-11C] methionine (MET) uptake of considerable prognostic utility (Lückerath, 2015). Elevated protein expression and activity have been noted for enzymes involved in methionine metabolism, including methionine adenosyltransferase 2A (MAT2A) and methyltransferase nicotinamide N-methyltransferase (NNMT) in tumor cells (Eckert, 2019; Ulanovskaya, 2013; Wang, 2019).
Targeting of methionine metabolism in cancer has yielded results, with administration of recombinant methioninase (rMETase) showing success in preclinical models (Hoffman, 2019). Another avenue of methionine targeting of particular interest is dietary methionine restriction (MR), an intervention which has been shown to extend lifespan (Bárcena, 2018; Lee, 2014). The restriction of methionine availability can drive potent reductions in methionine metabolism, as illustrated by a recent study which showed that MTAP-deleted cells subjected to MR abrogated MTA accumulation to levels seen in MTAP-expressing cells (Sanderson, 2019b). In cancer, MR has produced therapeutic responses in preclinical models ranging from patient-derived xenografts to anatomically-restricted autochthonous models with genetically defined driver mutations. Synergies with both chemotherapy and radiation have been observed. In each of these cases the mechanisms underlying MR appear to be cell autonomous and coupled to altered demands for nucleotide biosynthesis and redox metabolism that result from alterations in methionine metabolism imposed by MR. Defining a clinically feasible regimen of dietary methionine restriction has been challenging, as methionine depletion is associated with toxicities. Recently, it has been demonstrated the reduced methionine levels can be well tolerated over a 3-week period with no significant side effects in healthy humans (Gao et al., 2019). Together with observed benefits as an antineoplastic agent, MR presents an enticing opportunity to selectively target a metabolic vulnerability of cancer through a dietary intervention.
Cysteine Metabolism
Cysteine, which can be synthesized from methionine via the transsulfuration pathway or taken up in its oxidized form cystine via the system xc- amino acid antiporter, is limiting for glutathione production and therefore critical for cellular redox homeostasis (Zhu et al., 2019). Certain cancers have been shown to upregulate the system xc- transporter under oxidative stress (Polewski et al., 2016), and intracellular cysteine depletion can lead to an oxidative, iron-dependent form of non-apoptotic cell death termed ferroptosis (Dixon et al., 2012). Cancer cells often have higher basal levels of reactive oxygen species generation and can display increased iron uptake, therefore it has been proposed that pharmacological induction of ferroptosis could be a selective strategy to treat cancer (Hassannia et al., 2019). Although interesting, it remains for future study to investigate whether dietary strategies such as cysteine/cystine limitation, alterations in antioxidant consumption, or iron supplementation could influence tumor responsiveness to ferroptosis and redox processes (Piskounova et al., 2015; Sayin et al., 2014).
Branched Chain Amino Acids (BCAAs)
Leucine, isoleucine, and valine make up the class of BCAAs which are known to function as both metabolic intermediates and in nutrient sensing signaling pathways implicated in cancer. BCAAs are catabolized by branched-chain aminotransferase 1 (BCAT1) in a number of tissues (Neinast, 2019). Using α-ketoglutarate as a cofactor, BCAT1 catalyzes the conversion of BCAAs to glutamate and their corresponding branched-chain ketoacids (BCKAs). BCKAs subsequently undergo decarboxylation in the mitochondria to form acyl-CoA esters which can be used to replenish the TCA cycle.
This pathway provides an alternative to glucose utilization in the TCA cycle, and accordingly, BCAT1 overexpression has been reported in many cancer types including glioblastomas (Tönjes, 2013), breast cancers, and several hematological malignancies (Raffel, 2017). In human and mouse models of chronic myeloid leukemia (CML), aberrant metabolic activity through BCAT1 was shown to be required for neoplastic growth. However, the mechanisms which dictate BCAA metabolism appear to be highly variable across tumor types. A study of mutant Kras-driven pancreatic ductal adenocarcinoma (PDAC) and non-small cell lung cancer (NSCLC) mouse models showed significant differences in BCAA utilization, with BCAA-derived nitrogen supporting nonessential amino acid and DNA synthesis in NSCLC tumors (Mayers, 2016). As such, both genetic and microenvironmental context shapes BCAA metabolism in different cancers and requires further characterization.
These observations suggest that restricting BCAA availability through dietary manipulation is of interest (Sivanand, 2020). While this remains to be characterized in cancer models, studies in mice have demonstrated that dietary valine serves a crucial role in maintaining hematopoietic stem cells (HSC) in the bone marrow niche, a requirement which may be recapitulated in cancer stem cells (CSCs) which give rise to hematological malignancies (Taya, 2016). BCAAs have been largely studied in the context of obesity and insulin resistance, and elevated levels have been associated with systemic metabolic disease states. Circulating BCAA levels in the plasma have been linked to skeletal muscle breakdown as a result of cancer-associated cachexia, a condition of muscle wasting observed in patients with advanced disease. Exogenous supplementation of BCAAs has shown limited benefits in alleviating cachectic muscle loss. Interestingly, a recent study has shown that this systemic elevation of BCAAs occurs prior to development of PDAC in human and mouse models, suggesting a model whereby liberated tissue BCAAs may function as nutrient sources for neoplastic cells (Mayers, 2014).
Tryptophan
The tumor microenvironment includes tumor cells, extracellular matrix, as well as the immune and stromal cells. While the complex interplay between different factors in this milieu is very complex, tryptophan catabolism has been implicated as a key process which drives the suppression of antitumor immune cell activity. More specifically, the metabolic processing of tryptophan to its immune modulatory metabolite, kynurenine, occurs via the enzymatic activity of indoleamine-2,3-dioxygenase (IDO) in tumor cells (Uyttenhove, 2003). This catabolic process is replicated by tryptophan-2,3-dioxygenase (TDO) in the liver and brain and affects systemic tryptophan levels. While IDO has been widely implicated in cancer, TDO has been shown to be overexpressed in malignant gliomas as well, providing evidence for the tumor-promoting role of tryptophan metabolism. The mechanisms that underlie these observations are poorly understood but may involve the engagement of aryl hydrocarbon receptors (Opitz, 2011). Such a coordinated response modulated via tryptophan metabolism may allow tumors to alter their interaction with the immune system, and has drawn significant interest as a possible target for dietary intervention or drug development(Platten, 2019).
Serine and Glycine
The intersection of folate and methionine cycles forms the core cellular processing for one-carbon units, molecular building blocks required for the biosynthesis of lipids, nucleotides and proteins as well as a major component of redox maintenance (Locasale, 2013; Yang, 2016). This one-carbon metabolism network integrates nutritional status via inputs of various amino acids, including serine and glycine, to generate functional outputs as shown in Figure 2. Accordingly, restriction of serine and derived glycine in the diet has been shown to attenuate tumor growth in a number of xenograft and autochthonous murine models (Labuschagne, 2014; LeBoeuf, 2020; Maddocks, 2017; Sullivan, 2019). While serine and glycine can both be synthesized de novo from glycolysis, increased levels of both serine uptake and biosynthesis suggest that one-carbon metabolism is often altered in cancer. The uses are multifaceted including nucleotide synthesis, sphingolipid synthesis, mitochondrial function, methylation metabolism and redox maintenance (Gao, 2018; Reid, 2018).
Arginine, Histidine, Aspartate, and Asparagine
Tumors often exhibit specific auxotrophy for other individual amino acids as well. The dependence of cancer cells on exogenous uptake of these nutrients appear to be cancer-specific metabolic vulnerabilities whose targeting may synergize with pharmacological therapies in the future.
In the case of asparagine, intracellular concentrations have been shown to regulate the uptake of other amino acids including serine, arginine, and histidine through its function as an exchange factor (Krall, 2016). Indeed, possibly through mTORC1 pathway activation and also effects on nucleotide synthesis, the maintenance of intracellular asparagine serves as an important regulator of cell proliferation seen in cancer (Pavlova, 2018; Zhang, 2014). Since cancer cells often exhibit asparagine auxotrophy, asparaginase administration reducing exogenous asparagine bioavailability has shown some success in limiting tumor proliferation and metastatic potential (Hettmer, 2015; Knott, 2018). Cancer cells are known to import arginine to be used in a myriad of roles including regulation of cell proliferation, protein modification, and immune modulation (Qiu, 2015). Abnormal expression and function of the enzymes involved in arginine catabolism, particularly arginosuccinate synthase (ASS), have been described in a number of cancers. Interventions targeting arginine auxotrophy and altered metabolism have shown success in preclinical xenograft models; however, further studies are needed to determine if these effects may extend further.
The amino acid histidine has also been implicated in modulating the efficacy and toxicity of methotrexate treatment, an antimetabolite therapy which is known to disrupt the generation of THF from dihydrofolate (DHF) as a part of the folate cycle. Given that THF is a crucial cofactor for the function of a number of enzymes involved cell proliferation, methotrexate can reduce tumor growth. The pool of available THF can be further reduced by increases in histidine metabolism which is performed via THF-dependent formimidoyltransferase cyclodeaminase (FTCD) activity. In fact, histidine rich diets have been shown to boost the effectiveness of methotrexate treatment and lower the toxicity noted in murine cancer models (Kanarek, 2018).
Microbiota, Diet, and Cancer
A discussion of diet remains incomplete without considering the gut microbiome, a consortium of trillions of microbes which contribute in meaningful but poorly understood ways to host metabolism. The microbial composition of the gut microbiome has likely undergone significant adaptations as human diets have evolved over both short and long term time scales, with Western diets high in fats and carbohydrates associated with the evolution of greater populations of polysaccharide-degrading microbiota to extract calories and provide needed food-derived energy and CR associated with increased probiotic microbes and suppressed proinflammatory strains (Turnbaugh, 2009; Zheng et al., 2018). The shifts in the species of microflora which colonize the gut are associated with metabolic phenotypes in the individual host (Perry, 2016; Turnbaugh, 2009).
While the precise role of the microbiome in cancer is not well defined, diet-driven changes in gut microbiota are relevant in shaping tumor development, progression, and therapy. Microbial imbalance, or dysbiosis, between colorectal cancer (CRC) tissue and adjacent mucosa has been described in the literature, with neoplastic enrichment of specific microbes like Fusobacterium nucleatum (Fn), Escherichia coli, and Bacteroides fragilis (Collins et al., 2011; Iida, 2013; Nakatsu et al., 2015). In addition, recent evidence has demonstrated co-migration of commensal microbiota within CRC metastases (Bullman, 2017) and microbial influence in modulating antitumor immune activation and therapy response (Jin, 2019; Routy et al., 2018; Viaud, 2013). Furthermore, recent emerging data is suggesting that many elements of cancer can be predicted by microbiome composition with signatures present in peripheral blood in the form of cell-free DNA(Poore et al., 2020). Mechanisms that link cross-talk from microbes to tumor cells are also emerging such as a recent study of NAD+ metabolism that shows tumor cells can satisfy requirements of NAD synthesis from the provision of nutrients derived from microbial sources (Shats et al., 2020). Given the microbiome is known to exert control over a multitude of cellular and whole-body functions, it is interesting to speculate that additional links between diet and tumor behavior exist(Cani, 2018).
Concluding Remarks
Our molecular understanding of diet and nutrition is still in its infancy, a lack of understanding which extends to human health as well. It is highly debated both in popular culture and among scientists as to what constitutes a healthy diet. The answer ranges from low carbohydrate “keto” diets to diets high in carbohydrates but low in saturated fat such as typical plant-based diets. Even less understood is how these diets affect metabolism at the cellular level. While studies which have utilized metabolomics technology and stable isotope tracing are emerging and have led to new developments on fundamental questions in diet, much remains to be understood. Indeed, better controlled dietary intake studies with varied populations are necessary to understand the breadth of inter-patient variability in normal metabolism. Without understanding this and the effect of diet, an understanding of tumor metabolism is difficult to develop.
Nevertheless, there are a few intriguing examples of specific diets being able to target specific aspects of tumor metabolism with profound consequences that can be molecularly interpreted. These effects can occur both systemically, as appears to be the case in the insulin-lowering anti-tumor effects of the ketogenic diet, or cell autonomously, which appears to be the case for restriction of serine/glycine or methionine. By affecting whole body nutrient uptake, the modulation of diet can have profound influences on tumor cell metabolism. This can occur alone or in combination with therapies that may interact with the diet modality in question. These observations, while promising as potential future therapeutic targets, require a deeper mechanistic understanding which we expect to develop in the coming years.
Acknowledgements
JWL thanks the Marc Lustgarten Foundation, the National Institutes of Health (R01CA193256) and the American Cancer Society (129832-RSG-16-214-01-TBE) for their generous support. We apologize to those whose work wasn’t cited due to space limitations.
Footnotes
Declaration of Interests
JWL advises Nanocare Technologies, Raphael Pharmaceuticals and Restoration Foodworks.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen BG, Bhatia SK, Anderson CM, Eichenberger-Gilmore JM, Sibenaller ZA, Mapuskar KA, Schoenfeld JD, Buatti JM, Spitz DR, and Fath MA (2014). Ketogenic diets as an adjuvant cancer therapy: History and potential mechanism. Redox Biol 2, 963–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen BG, Bhatia SK, Buatti JM, Brandt KE, Lindholm KE, Button AM, Szweda LI, Smith BJ, Spitz DR, and Fath MA (2013). Ketogenic diets enhance oxidative stress and radio-chemo-therapy responses in lung cancer xenografts. Clin Cancer Res 19, 3905–3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bárcena C, Quirós PM, Durand S, Mayoral P, Rodríguez F, Caravia XM, Mariño G, Garabaya C, Fernández-García MT, Kroemer G and Freije JM (2018). Methionine restriction extends lifespan in progeroid mice and alters lipid and bile acid metabolism. Cell reports 24, 2392–2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi J, Chowdhry S, Wu S, Zhang W, Masui K, and Mischel PS (2020). Altered cellular metabolism in gliomas — an emerging landscape of actionable co-dependency targets. Nat Rev Cancer 20, 57–70. [DOI] [PubMed] [Google Scholar]
- Bouchard-Mercier A, Rudkowska I, Lemieux S, Couture P, & Vohl MC (2013). The metabolic signature associated with the Western dietary pattern: a cross-sectional study. BMC Nutrition Journal 12, 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruss MD, Khambatta CF, Ruby MA, Aggarwal I, and Hellerstein MK (2010). Calorie restriction increases fatty acid synthesis and whole body fat oxidation rates. Am J Physiol Endocrinol Metab 298, E108–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullman S, Pedamallu CS, Sicinska E, Clancy TE, Zhang X, Cai D, Neuberg D, Huang K, Guevara F, Nelson T Chipashvili O, Hagan T, Walker M, Ramachandran A, Diosdado B, Serna G, Mulet N, Landolfi S, Ramon y Cajal S, Fasani R, Aguirre AJ, Ng K, Elez E, Ogino S, Tabernero J, Fuchs C, Hahn WC, Nuciforo P, Meyerson M (2017). Analysis of Fusobacterium persistence and antibiotic response in colorectal cancer. Science 358, 1443–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cani PD (2018). Human gut microbiome: hopes, threats and promises. Gut 67, 1716–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caso J, Masko EM Ii, J.A., Poulton SH, Dewhirst M, Pizzo SV, and Freedland SJ (2013). The effect of carbohydrate restriction on prostate cancer tumor growth in a castrate mouse xenograft model. Prostate 73, 449–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castejon M, Plaza A, Martinez-Romero J, Fernandez-Marcos PJ, Cabo R, and Diaz-Ruiz A (2020). Energy Restriction and Colorectal Cancer: A Call for Additional Research. Nutrients 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen PH, Cai L, Huffman K, Yang C, Kim J, Faubert B, Boroughs L, Ko B, Sudderth J, McMillan EA and Girard L (2019). Metabolic diversity in human non-small cell lung cancer cells. Molecular Cell 76, 838–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins D, Hogan AM, and Winter DC (2011). Microbial and viral pathogens in colorectal cancer. The lancet oncology 12, 504–512. [DOI] [PubMed] [Google Scholar]
- Cox (2000). Lehninger Principles of Biochemistry. (New York: Worth Publishers; ). [Google Scholar]
- D’Aronzo M, Vinciguerra M, Mazza T, Panebianco C, Saracino C, Pereira SP, Graziano P, and Pazienza V (2015). Fasting cycles potentiate the efficacy of gemcitabine treatment in in vitro and in vivo pancreatic cancer models. Oncotarget 6, 18545–18557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Cabo R.a.M., M.P. (2019). Effects of Intermittent Fasting on Health, Aging, and Disease. New England Journal of Medicine 381, 2541–2551. [DOI] [PubMed] [Google Scholar]
- de la Cruz Bonilla M, Stemler KM, Jeter-Jones S, Fujimoto TN, Molkentine J, Torres GMA, Zhang X, Broaddus RR, Taniguchi CM and Piwnica-Worms H (2019). Fasting reduces intestinal radiotoxicity, enabling dose-escalated radiation therapy for pancreatic cancer. International Journal of Radiation Oncology, Biology, Physics 105, 537–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Biase S, Lee C, Brandhorst S, Manes B, Buono R, Cheng CW, Cacciottolo M, Martin-Montalvo A, de Cabo R, Wei M, et al. (2016). Fasting-Mimicking Diet Reduces HO-1 to Promote T Cell-Mediated Tumor Cytotoxicity. Cancer Cell 30, 136–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Biase S, Shim HS, Kim KH, Vinciguerra M, Rappa F, Wei M, Brandhorst S, Cappello F, Mirzaei H, Lee C and Longo VD (2017). Fasting regulates EGR1 and protects from glucose-and dexamethasone-dependent sensitization to chemotherapy. PLoS biology 15, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, et al. (2012). Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149, 1060–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douris N, Melman T, Pecherer JM, Pissios P, Flier JS, Cantley LC, Locasale JW and Maratos-Flier E (2015). Adaptive changes in amino acid metabolism permit normal longevity in mice consuming a low-carbohydrate ketogenic diet. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease 1852, 2056–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert MA, Coscia F, Chryplewicz A, Chang JW, Hernandez KM, Pan S, Tienda SM, Nahotko DA, Li G, Blaženović I and Lastra RR (2019). Proteomics reveals NNMT as a master metabolic regulator of cancer-associated fibroblasts. Nature 569, 723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson N, Boscheri A, Linke B, and Huebner J (2017). Systematic review: isocaloric ketogenic dietary regimes for cancer patients. Med Oncol 34, 72. [DOI] [PubMed] [Google Scholar]
- Fontana L, Partridge L, and Longo VD (2010). Extending healthy life span--from yeast to humans. Science 328, 321–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Weiss EP, Villareal DT, Klein S, and Holloszy JO (2008). Long-term effects of calorie or protein restriction on serum IGF-1 and IGFBP-3 concentration in humans. Aging Cell 7, 681–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Lee K, Reid MA, Sanderson SM, Qiu C, Li S, Liu J and Locasale JW (2018). Serine availability influences mitochondrial dynamics and function through lipid metabolism. Cell reports 22, 3507–3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Sanderson SM, Dai Z, Reid MA, Cooper DE, Lu M, Richie JP, Ciccarella A, Calcagnotto A, Mikhael PG, et al. (2019). Dietary methionine influences therapy in mouse cancer models and alters human metabolism. Nature 572, 397–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Jimenez C, and Goding CR (2019). Starvation and Pseudo-Starvation as Drivers of Cancer Metastasis through Translation Reprogramming. Cell Metab 29, 254–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerson M (1978). The cure of advanced cancer by diet therapy: a summary of 30 years of clinical experimentation. Physiol Chem Phys 10, 449–464. [PubMed] [Google Scholar]
- Goncalves MD, Hopkins BD and Cantley LC (2018). Phosphatidylinositol 3-kinase, growth disorders, and cancer. New England Journal of Medicine 379, 2052–2062. [DOI] [PubMed] [Google Scholar]
- Goncalves MD, Hopkins BD and Cantley LC (2019a). Dietary fat and sugar in promoting cancer development and progression. Annual Review of Cancer Biology 3, 255–273. [Google Scholar]
- Goncalves MD, Lu C, Tutnauer J, Hartman TE, Hwang SK, Murphy CJ, Pauli C, Morris R, Taylor S, Bosch K and Yang S (2019b). High-fructose corn syrup enhances intestinal tumor growth in mice. Science 363, 1345–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green CL, Mitchell SE, Derous D, Wang Y, Chen L, Han JJ, Promislow DEL, Lusseau D, Douglas A, and Speakman JR (2017). The effects of graded levels of calorie restriction: IX. Global metabolomic screen reveals modulation of carnitines, sphingolipids and bile acids in the liver of C57BL/6 mice. Aging Cell 16, 529–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassannia B, Vandenabeele P, and Vanden Berghe T (2019). Targeting Ferroptosis to Iron Out Cancer. Cancer Cell 35, 830–849. [DOI] [PubMed] [Google Scholar]
- Heilbronn LK, de Jonge L, Frisard MI, DeLany JP, Larson-Meyer DE, Rood J, Nguyen T, Martin CK, Volaufova J, Most MM, et al. (2006). Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. JAMA 295, 1539–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hettmer S, Schinzel AC, Tchessalova D, Schneider M, Parker CL, Bronson RT, Richards NG, Hahn WC and Wagers AJ (2015). Functional genomic screening reveals asparagine dependence as a metabolic vulnerability in sarcoma. Elife 4, e09436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho VW, Leung K, Hsu A, Luk B, Lai J, Shen SY, Minchinton AI, Waterhouse D, Bally MB, Lin W, et al. (2011). A low carbohydrate, high protein diet slows tumor growth and prevents cancer initiation. Cancer Res 71, 4484–4493. [DOI] [PubMed] [Google Scholar]
- Hoffman RM, Tan Y, Li S, Han Q and Zavala J (2019). Pilot Phase I Clinical Trial of Methioninase on High-Stage Cancer Patients: Rapid Depletion of Circulating Methionine.. Methods Mol Biol. 1866, 231–242. [DOI] [PubMed] [Google Scholar]
- Hopkins BD, Pauli C, Du X, Wang DG, Li X, Wu D, Amadiume SC, Goncalves MD, Hodakoski C, Lundquist MR and Bareja R, Mukherjee SD, and Cantley LC (2018). Suppression of insulin feedback enhances the efficacy of PI3K inhibitors. Nature 560, 499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffman J, and Kossoff EH (2006). State of the ketogenic diet(s) in epilepsy. Curr Neurol Neurosci Rep 6, 332–340. [DOI] [PubMed] [Google Scholar]
- Iida N, Dzutsev A, Stewart CA, Smith L, Bouladoux N, Weingarten RA, Molina DA, Salcedo R, Back T, Cramer S and Dai RM (2013). Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science 342, 967–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyengar NM, Gucalp A, Dannenberg AJ and Hudis CA (2016). Obesity and cancer mechanisms: tumor microenvironment and inflammation. Journal of clinical oncology 34, 4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin C, Lagoudas GK, Zhao C, Bullman S, Bhutkar A, Hu B, Ameh S, Sandel D, Liang XS, Mazzilli S and Whary MT, Meyerson M, Germain R, Blainey PC, Fox JG, and Jacks T (2019). Commensal microbiota promote lung cancer development via γδ T cells. Cell 176, 998–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jove M, Naudi A, Ramirez-Nunez O, Portero-Otin M, Selman C, Withers DJ, and Pamplona R (2014). Caloric restriction reveals a metabolomic and lipidomic signature in liver of male mice. Aging Cell 13, 828–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaany NY, and Sabatini DM (2009). Tumours with PI3K activation are resistant to dietary restriction. Nature 458, 725–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanarek N, Keys HR, Cantor JR, Lewis CA, Chan SH, Kunchok T, Abu-Remaileh M, Freinkman E, Schweitzer LD and Sabatini DM (2018). Histidine catabolism is a major determinant of methotrexate sensitivity. Nature 559, 632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanarek N, Petrova B, and Sabatini DM (2020). Dietary modifications for enhanced cancer therapy. Nature 579, 507–517. [DOI] [PubMed] [Google Scholar]
- Klement RJ, Champ CE, Otto C and Kämmerer U (2016). Anti-tumor effects of ketogenic diets in mice: a meta-analysis. PLoS One 11, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott SR, Wagenblast E, Khan S, Kim SY, Soto M, Wagner M, Turgeon MO, Fish L, Erard N, Gable AL and Maceli AR, Steffen Dickopf, Papachristou Evangelia K., D’Santos Clive S., Carey Lisa A., Wilkinson John E., Chuck Harrell J, Perou CM, Goodarzi H, Poulogiannis G & Hannon GJ (2018). Asparagine bioavailability governs metastasis in a model of breast cancer. Nature 554, 378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krall AS, Xu S, Graeber TG, Braas D and Christofk HR (2016). Asparagine promotes cancer cell proliferation through use as an amino acid exchange factor. Nature communications 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labuschagne CF, Van Den Broek NJ, Mackay GM, Vousden KH and Maddocks OD (2014). Serine, but not glycine, supports one-carbon metabolism and proliferation of cancer cells. Cell reports 7, 1248–1258. [DOI] [PubMed] [Google Scholar]
- LeBoeuf SE, Wu WL, Karakousi TR, Karadal B, Jackson SR, Davidson SM, Wong KK, Koralov SB, Sayin VI and Papagiannakopoulos T (2020). Activation of Oxidative Stress Response in Cancer Generates a Druggable Dependency on Exogenous Non-essential Amino Acids. Cell Metabolism 31, 339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BC, Kaya A, Ma S, Kim G, Gerashchenko MV, Yim SH, Hu Z, Harshman LG and Gladyshev VN (2014). Methionine restriction extends lifespan of Drosophila melanogaster under conditions of low amino-acid status. Nature communications 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C, Raffaghello L, Brandhorst S, Safdie FM, Bianchi G, Martin-Montalvo A, Pistoia V, Wei M, Hwang S, Merlino A, et al. (2012). Fasting cycles retard growth of tumors and sensitize a range of cancer cell types to chemotherapy. Sci Transl Med 4, 124ra127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine ME, Suarez JA, Brandhorst S, Balasubramanian P, Cheng CW, Madia F, Fontana L, Mirisola MG, Guevara-Aguirre J, Wan J and Passarino G (2014). Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell metabolism 19, 407–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberti MV, Dai Z, Wardell SE, Baccile JA, Liu X, Gao X, Baldi R, Mehrmohamadi M, Johnson MO, Madhukar NS, et al. (2017). A Predictive Model for Selective Targeting of the Warburg Effect through GAPDH Inhibition with a Natural Product. Cell Metab 26, 648–659 e648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberti MV, and Locasale JW (2016). The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem Sci 41, 211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien E.C.a.V.H., M.G. (2019). A framework for examining how diet impacts tumour metabolism. Nature Reviews Cancer 19, 651–661. [DOI] [PubMed] [Google Scholar]
- Liu G.Y.a.S., D.M. (2020). mTOR at the nexus of nutrition, growth, ageing and disease. Nature Reviews Molecular Cell Biology, 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locasale JW (2013). Serine, glycine and one-carbon units: cancer metabolism in full circle. Nature Reviews Cancer 13, 572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo V.D.a.M., M.P. (2014). Fasting: molecular mechanisms and clinical applications. Cell metabolism 19, 181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lückerath K, Lapa C, Albert C, Herrmann K, Jörg G, Samnick S, Einsele H, Knop S and Buck AK (2015). 11C-Methionine-PET: a novel and sensitive tool for monitoring of early response to treatment in multiple myeloma. Oncotarget 6, 8418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv M, Zhu X, Wang H, Wang F, and Guan W (2014). Roles of caloric restriction, ketogenic diet and intermittent fasting during initiation, progression and metastasis of cancer in animal models: a systematic review and meta-analysis. PLoS One 9, e115147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddocks OD, Athineos D, Cheung EC, Lee P, Zhang T, van den Broek NJ, Mackay GM, Labuschagne CF, Gay D, Kruiswijk F and Blagih J (2017). Modulating the therapeutic response of tumours to dietary serine and glycine starvation. Nature 544, 372. [DOI] [PubMed] [Google Scholar]
- Marinac CR, Nelson SH, Breen CI, Hartman SJ, Natarajan L, Pierce JP, Flatt SW, Sears DD, and Patterson RE (2016). Prolonged Nightly Fasting and Breast Cancer Prognosis. JAMA Oncol 2, 1049–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattison JA, Colman RJ, Beasley TM, Allison DB, Kemnitz JW, Roth GS, Ingram DK, Weindruch R, de Cabo R, and Anderson RM (2017). Caloric restriction improves health and survival of rhesus monkeys. Nat Commun 8, 14063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson R.d.C.a.M.P. (2019). Effects of Intermittent Fasting on Health, Aging, and Disease. New England Journal of Medicine 381, 2541–2551. [DOI] [PubMed] [Google Scholar]
- Maurer GD, Brucker DP, Bahr O, Harter PN, Hattingen E, Walenta S, Mueller-Klieser W, Steinbach JP, and Rieger J (2011). Differential utilization of ketone bodies by neurons and glioma cell lines: a rationale for ketogenic diet as experimental glioma therapy. BMC Cancer 11, 315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayers JR, Torrence ME, Danai LV, Papagiannakopoulos T, Davidson SM, Bauer MR, Lau AN, Ji BW, Dixit PD, Hosios AM and Muir A (2016). Tissue of origin dictates branched-chain amino acid metabolism in mutant Kras-driven cancers. Science 353, 1161–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayers JR, Wu C, Clish CB, Kraft P, Torrence ME, Fiske BP, Yuan C, Bao Y, Townsend MK, Tworoger SS and Davidson SM (2014). Elevation of circulating branched-chain amino acids is an early event in human pancreatic adenocarcinoma development. Nature medicine 20, 1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentch SJ, Mehrmohamadi M, Huang L, Liu X, Gupta D, Mattocks D, Padilla PG, Ables G, Bamman MM, Thalacker-Mercer AE, Nichenametla SN, and Locasale JW (2015). Histone methylation dynamics and gene regulation occur through the sensing of one-carbon metabolism. Cell metabolism 22, 861–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SJ, Bernier M, Mattison JA, Aon MA, Kaiser TA, Anson RM, Ikeno Y, Anderson RM, Ingram DK, and de Cabo R (2019). Daily Fasting Improves Health and Survival in Male Mice Independent of Diet Composition and Calories. Cell Metab 29, 221–228 e223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Most J, Tosti V, Redman LM, and Fontana L (2017). Calorie restriction in humans: An update. Ageing Res Rev 39, 36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueckler M.a.T., B. (2013). The SLC2 (GLUT) family of membrane transporters. Molecular aspects of medicine 34, 121–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir A, & Vander Heiden MG (2018). The nutrient environment affects therapy. Science 360, 962–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsu G, Li X, Zhou H, Sheng J, Wong SH, Wu WKK, Ng SC, Tsoi H, Dong Y, and Zhang N (2015). Gut mucosal microbiome across stages of colorectal carcinogenesis. Nature communications 6, 8727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neinast MD, Jang C, Hui S, Murashige DS, Chu Q, Morscher RJ, Li X, Zhan L, White E, Anthony TG and Rabinowitz JD (2019). Quantitative analysis of the whole-body metabolic fate of branched-chain amino acids. Cell metabolism 29, 417–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nencioni A, Caffa I, Cortellino S, and Longo VD (2018). Fasting and cancer: molecular mechanisms and clinical application. Nat Rev Cancer 18, 707–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Flanagan CH, Smith LA, McDonell SB and Hursting SD (2017). When less may be more: calorie restriction and response to cancer therapy. BMC medicine 15, 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opitz CA, Litzenburger UM, Sahm F, Ott M, Tritschler I, Trump S, Schumacher T, Jestaedt L, Schrenk D, Weller M and Jugold M (2011). An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature 478, 197. [DOI] [PubMed] [Google Scholar]
- Palm W.a.T., C.B. (2017). Nutrient acquisition strategies of mammalian cells. Nature 546, 234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlova NN, Hui S, Ghergurovich JM, Fan J, Intlekofer AM, White RM, Rabinowitz JD, Thompson CB and Zhang J (2018). As extracellular glutamine levels decline, asparagine becomes an essential amino acid. Cell metabolism 27, 428–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry RJ, Peng L, Barry NA, Cline GW, Zhang D, Cardone RL, … & Shulman GI (2016). Acetate mediates a microbiome–brain–β-cell axis to promote metabolic syndrome. Nature 534, 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R.J.a.S., G.I.(2020). Mechanistic Links between Obesity, Insulin, and Cancer. Trends in Cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piskounova E, Agathocleous M, Murphy MM, Hu Z, Huddlestun SE, Zhao Z, Leitch AM, Johnson TM, DeBerardinis RJ, and Morrison SJ (2015). Oxidative stress inhibits distant metastasis by human melanoma cells. Nature 527, 186–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platten M, Nollen EA, Röhrig UF, Fallarino F and Opitz CA (2019). Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nature Reviews Drug Discovery 18, 379–401. [DOI] [PubMed] [Google Scholar]
- Polewski MD, Reveron-Thornton RF, Cherryholmes GA, Marinov GK, Cassady K, and Aboody KS (2016). Increased Expression of System xc- in Glioblastoma Confers an Altered Metabolic State and Temozolomide Resistance. Mol Cancer Res 14, 1229–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poore GD, Kopylova E, Zhu Q, Carpenter C, Fraraccio S, Wandro S, Kosciolek T, Janssen S, Metcalf J, Song SJ, et al. (2020). Microbiome analyses of blood and tissues suggest cancer diagnostic approach. Nature 579, 567–574. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Puchalska P.a.C., P.A. (2017). Multi-dimensional roles of ketone bodies in fuel metabolism, signaling, and therapeutics. Cell metabolism 25, 262–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu F, Huang J and Sui M (2015). Targeting arginine metabolism pathway to treat arginine-dependent cancers. Cancer Letters 364, 1–7. [DOI] [PubMed] [Google Scholar]
- Raffaghello L, Lee C, Safdie FM, Wei M, Madia F, Bianchi G, and Longo VD (2008). Starvation-dependent differential stress resistance protects normal but not cancer cells against high-dose chemotherapy. Proc Natl Acad Sci U S A 105, 8215–8220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffel S, Falcone M, Kneisel N, Hansson J, Wang W, Lutz C, Bullinger L, Poschet G, Nonnenmacher Y, Barnert A and Bahr C (2017). BCAT1 restricts αKG levels in AML stem cells leading to IDH mut-like DNA hypermethylation. Nature 551, 384. [DOI] [PubMed] [Google Scholar]
- Redman LM, Smith SR, Burton JH, Martin CK, Il’yasova D, and Ravussin E (2018). Metabolic Slowing and Reduced Oxidative Damage with Sustained Caloric Restriction Support the Rate of Living and Oxidative Damage Theories of Aging. Cell Metab 27, 805–815 e804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid MA, Allen AE, Liu S, Liberti MV, Liu P, Liu X, Dai Z, Gao X, Wang Q, Liu Y and Lai L (2018). Serine synthesis through PHGDH coordinates nucleotide levels by maintaining central carbon metabolism. Nature communications 9, 5442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid MA, Dai Z and Locasale JW (2017). The impact of cellular metabolism on chromatin dynamics and epigenetics. Nature cell biology 19, 1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routy B, Gopalakrishnan V, Daillère R, Zitvogel L, Wargo JA, and Kroemer G (2018). The gut microbiota influences anticancer immunosurveillance and general health. Nature Reviews Clinical Oncology 15, 382–396. [DOI] [PubMed] [Google Scholar]
- Safdie FM, Dorff T, Quinn D, Fontana L, Wei M, Lee C, Cohen P, and Longo VD (2009). Fasting and cancer treatment in humans: A case series report. Aging (Albany NY) 1, 988–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson SM, Gao X, Dai Z and Locasale JW (2019a). Methionine metabolism in health and cancer: a nexus of diet and precision medicine. Nature Reviews Cancer 19, 625–637. [DOI] [PubMed] [Google Scholar]
- Sanderson SM, Mikhael PG, Ramesh V, Dai Z and Locasale JW (2019b). Nutrient availability shapes methionine metabolism in p16/MTAP-deleted cells. Science Advances 5, 7769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayin VI, Ibrahim MX, Larsson E, Nilsson JA, Lindahl P, and Bergo MO (2014). Antioxidants accelerate lung cancer progression in mice. Sci Transl Med 6, 221ra215. [DOI] [PubMed] [Google Scholar]
- Shats I, Williams JG, Liu J, Makarov MV, Wu X, Lih FB, Deterding LJ, Lim C, Xu X, Randall TA, et al. (2020). Bacteria Boost Mammalian Host NAD Metabolism by Engaging the Deamidated Biosynthesis Pathway. Cell Metab 31, 564–579 e567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivanand S.a.V.H., M.G. (2020). Emerging Roles for Branched-Chain Amino Acid Metabolism in Cancer. Cancer Cell 37, 147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal RS, and Allen RG (1985). Relationship between metabolic rate, free radicals, differentiation and aging: a unified theory. Basic Life Sci 35, 75–104. [DOI] [PubMed] [Google Scholar]
- Stekovic S, Hofer SJ, Tripolt N, Aon MA, Royer P, Pein L, Stadler JT, Pendl T, Prietl B, Url J, et al. (2019). Alternate Day Fasting Improves Physiological and Molecular Markers of Aging in Healthy, Non-obese Humans. Cell Metab 30, 462–476 e465. [DOI] [PubMed] [Google Scholar]
- Stuani L, Sabatier M and Sarry JE (2019). Exploiting metabolic vulnerabilities for personalized therapy in acute myeloid leukemia. BMC biology 17, 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan MR, Mattaini KR, Dennstedt EA, Nguyen AA, Sivanand S, Reilly MF, Meeth K, Muir A, Darnell AM, Bosenberg MW and Lewis CA (2019). Increased serine synthesis provides an advantage for tumors arising in tissues where serine levels are limiting. Cell metabolism 29, 1410–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasdogan A, Faubert B, Ramesh V, Ubellacker JM, Shen B, Solmonson A, Murphy MM, Gu Z, Gu W, Martin M, Kasitinon SY, Vandergriff T, Mathews TP, Zhao Z, Schadendorf D, DeBerardinis RJ and Morrison SJ (2020). Metabolic heterogeneity confers differences in melanoma metastatic potential. Nature 577, 115–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taya Y, Ota Y, Wilkinson AC, Kanazawa A, Watarai H, Kasai M, Nakauchi H and Yamazaki S (2016). Depleting dietary valine permits nonmyeloablative mouse hematopoietic stem cell transplantation. Science 354, 1152–1155. [DOI] [PubMed] [Google Scholar]
- Teruya T, Chaleckis R, Takada J, Yanagida M and Kondoh H (2019). Diverse metabolic reactions activated during 58-hr fasting are revealed by non-targeted metabolomic analysis of human blood. Scientific reports 9, 854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tönjes M, Barbus S, Park YJ, Wang W, Schlotter M, Lindroth AM, Pleier SV, Bai AH, Karra D, Piro RM and Felsberg J (2013). BCAT1 promotes cell proliferation through amino acid catabolism in gliomas carrying wild-type IDH1. Nature medicine 19, 901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, Jones WJ, Roe BA, Affourtit JP and Egholm M (2009). A core gut microbiome in obese and lean twins. Nature 457, 480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulanovskaya OA, Zuhl AM and Cravatt BF (2013). NNMT promotes epigenetic remodeling in cancer by creating a metabolic methylation sink. Nature chemical biology 9, 300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich CM, Himbert C, Holowatyj AN and Hursting SD (2018). Energy balance and gastrointestinal cancer: risk, interventions, outcomes and mechanisms. Nature reviews Gastroenterology & hepatology 15, 683–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyttenhove C, Pilotte L, Théate I, Stroobant V, Colau D, Parmentier N, Boon T and Van den Eynde BJ (2003). Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2, 3-dioxygenase. Nature medicine 9, 1269. [DOI] [PubMed] [Google Scholar]
- Vander Heiden MG, & DeBerardinis RJ (2017). Understanding the intersections between metabolism and cancer biology. Cell 168, 657–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viaud S, Saccheri F, Mignot G, Yamazaki T, Daillère R, Hannani D, Enot DP, Pfirschke C, Engblom C, Pittet MJ and Schlitzer A (2013). The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 342, 971–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Yip LY, Lee JHJ, Wu Z, Chew HY, Chong PKW, Teo CC, Ang HYK, Peh KLE, Yuan J and Ma S (2019). Methionine is a metabolic dependency of tumor-initiating cells. Nature medicine 25, 825. [DOI] [PubMed] [Google Scholar]
- Weinberg SE, & Chandel NS (2015). Targeting mitochondria metabolism for cancer therapy. Nature chemical biology 11, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenbecher C, Mühlenbruch K, Kröger J, Jacobs S, Kuxhaus O, Floegel A, Fritsche A, Pischon T, Prehn C, Adamski J and Joost HG (2015). Amino acids, lipid metabolites, and ferritin as potential mediators linking red meat consumption to type 2 diabetes. The American journal of clinical nutrition 101, 1241–1250. [DOI] [PubMed] [Google Scholar]
- Wolpaw AJ, & Dang CV (2018). Exploiting metabolic vulnerabilities of cancer with precision and accuracy. Trends in cell biology 28, 201–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia S, Lin R, Jin L, Zhao L, Kang HB, Pan Y, Liu S, Qian G, Qian Z, Konstantakou E and Zhang B (2017). Prevention of dietary-fat-fueled ketogenesis attenuates BRAF V600E tumor growth. Cell metabolism 25, 358–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Licastro D, Cava E, Veronese N, Spelta F, Rizza W, Bertozzi B, Villareal DT, Hotamisligil GS, Holloszy JO and Fontana L (2016). Long-term calorie restriction enhances cellular quality-control processes in human skeletal muscle. Cell reports 14, 422–428. [DOI] [PubMed] [Google Scholar]
- Yokoyama Y, Onishi K, Hosoda T, Amano H, Otani S, Kurozawa Y, and Tamakoshi A (2016). Skipping Breakfast and Risk of Mortality from Cancer, Circulatory Diseases and All Causes: Findings from the Japan Collaborative Cohort Study. Yonago Acta Med 59, 55–60. [PMC free article] [PubMed] [Google Scholar]
- Zeevi D, Korem T, Zmora N, Israeli D, Rothschild D, Weinberger A, Ben-Yacov O, Lador D, Avnit-Sagi T, Lotan-Pompan M and Suez J (2015). Personalized nutrition by prediction of glycemic responses. Cell 163, 1079–1094. [DOI] [PubMed] [Google Scholar]
- Zhang J, Fan J, Venneti S, Cross JR, Takagi T, Bhinder B, Djaballah H, Kanai M, Cheng EH, Judkins AR and Pawel B (2014). Asparagine plays a critical role in regulating cellular adaptation to glutamine depletion. Molecular cell 56, 205–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X, Wang S, and Jia W (2018). Calorie restriction and its impact on gut microbial composition and global metabolism. Frontiers of Medicine 12, 634–644. [DOI] [PubMed] [Google Scholar]
- Zhu J, Berisa M, Schworer S, Qin W, Cross JR, and Thompson CB (2019). Transsulfuration Activity Can Support Cell Growth upon Extracellular Cysteine Limitation. Cell Metab 30, 865–876 e865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J.a.T., C.B. (2019). Metabolic regulation of cell growth and proliferation. Nature Reviews Molecular Cell Biology 20, 436–450. [DOI] [PMC free article] [PubMed] [Google Scholar]