Abstract
Introduction:
We have suggested that major pathological response (MPR) could serve as a surrogate endpoint for survival and provide rapid means of comparing different neoadjuvant treatment regimens. In here, we confirm that MPR is predictive of long-term overall survival (OS) in patients with NSCLC who underwent neoadjuvant chemotherapy and surgical resection, to assess agreement on MPR between two observers, and to determine the minimum number of slides needed to obtain an accurate determination of MPR.
Patients and Methods:
We identified 151 NSCLC patients who had been treated with neoadjuvant chemotherapy followed by complete surgical resection from 2008 to 2012. Tissue specimens were retrospectively evaluated by two pathologists who had been blinded to patients’ treatment and outcome. We assessed the relationships between MPR and OS, the levels of agreement between the pathologists, and determined the number of slides needed to obtain an accurate determination of MPR.
Results:
Our results reveal that MPR either examined by observer 1(experienced) or by observer 2 (trained) was significantly predictive of long-term OS after neoadjuvant chemotherapy. MPR was associated with long-term OS in NSCLC patients undergoing neoadjuvant chemotherapy on multivariable analysis (HR = 2.68, p = 0.01). The levels of agreement between two pathologists were high after direct in person training by one pathologist of the other (R2 = 0.994). Our data suggest that at least three slides should be read to accurately determine MPR.
Conclusions:
MPR is significantly predictive of long-term OS in neoadjuvant chemotherapy-treated NSCLC patients. MPR may serve as a surrogate endpoint for evaluating novel chemotherapies and immunotherapy response in biomarker-driven translational clinical trials.
Keywords: Lung cancer, neoadjuvant chemotherapy, MPR
Micro Abstract
The assessment of MPR was reproducible between observers. We confirmed that MPR is associated with OS and can serve as an independent prognostic factor in NSCLC patients undergoing neoadjuvant chemotherapy. Our data suggest that at least three slides should be read to accurately determine tumor response. The results of our study clearly demonstrate that MPR is a significant predictor of OS in neoadjuvant chemotherapy-treated NSCLC patients.
Introduction
Neoadjuvant chemotherapy followed by surgical resection has been increasingly used in patients with locally advanced non-small cell lung cancer (NSCLC).1–4 In a previous study, we evaluated the utility of histopathological response criteria for predicting outcome and observed that major pathological response (MPR) indicated as percentage of viable tumor cells was associated with long-term overall survival (OS) in NSCLC patients who underwent neoadjuvant chemotherapy.5–7 We and others have suggested that MPR in resected specimens could serve as a surrogate endpoint for survival and provide a more accurate and rapid means of comparing different neoadjuvant treatment regimens, shortening the period needed to evaluate novel chemotherapeutic and biological therapies in clinical trials.7–10 We also reported that combination of MPR with biomarker is a significant predictor of prognosis in NSCLC patients who received neoadjuvant chemotherapy.11
The purpose of this retrospective study was to confirm that MPR is predictive of long-term OS in patients with NSCLC treated with neoadjuvant chemotherapy and surgical resection. We also assessed interobserver agreement on MPR between two observers and determined the minimum number of slides needed to obtain an accurate determination of tumor response. We confirmed that MPR is associated with OS and can serve as an independent prognostic factor in NSCLC patients undergoing neoadjuvant chemotherapy. The assessment of MPR was reproducible between observers. The number of slides needed to accurately assess the MPR depended on the magnitude of the true mean. Our data suggest that at least three slides should be read to accurately determine tumor response. The results of our study clearly demonstrate that MPR is a significant predictor of OS in neoadjuvant chemotherapy-treated NSCLC patients.
Patients and Methods
Patients
This study included 151 NSCLC patients who had been treated with neoadjuvant chemotherapy followed by complete surgical resection from 2008 to 2012 at The University of Texas MD Anderson Cancer Center (Houston, Texas). Patients were selected for analysis if their resection specimens were available in the files of the Department of Pathology. Clinical and demographic data were extracted from the patients’ medical records. The study population consisted of 76 men (50.3%) and 75 women(49.7%), with a median age of 61 years (range: 30–80 years). The patients’ histological tumor types were adenocarcinoma (n = 96), squamous cell carcinoma (n = 51), large cell carcinoma (n = 2), and pleomorphic carcinoma (n = 2). The majority of the patients received a platinum-based chemotherapy regimen (n = 149 [98.7%]). The median number of treatment cycles was 3 (range: 1–6). The study was approved by MD Anderson’s Institutional Review Board.
Histopathological Evaluation
Paraffin-embedded hematoxylin and eosin (H&E)-stained slides of tumor sections were reviewed for this analysis. For training purposes and to maximize interobserver consensus, initial evaluation of the slides included the following steps:
Step 1: Observer 1 (experienced observer) and observer 2 (new observer) co-examine 5–10 slide samples.
Step 2: Observer 2 (new observer) examines 5–10 slide samples and then reviews together with observer 1 (experienced observer).
Step 3: Observer 1 (experienced observer) and observer 2 (new observer) examine 5–10 slide samples independently and then review together.
During this initial review, certain critical issues were discussed, for instance not to score necrosis that was intrinsic to the tumor (ie. comedo-type necrosis) and unrelated to treatment change; likewise, foamy macrophages were only scored if present in the stroma and not counting alveolar macrophages related to postobstructive changes. Following these initial steps, the slides were then reviewed independently by two pathologists blinded to the patient outcome. The number of slides examined in each case ranged from 2 to 12. The percentage of residual tumor was estimated by comparing the estimated cross-sectional area of the viable tumor foci to the estimated cross-sectional areas of fibrosis and necrosis (tumor bed) on each slide.5 Histologic parameters analyzed included percentage of fibrosis and necrosis; the presence of giant cell reaction, cholesterol cleft granulomas, foamy macrophages, and inflammation was assessed using a score from 0–3, depending on the degree of those changes. For each patient, the results for all slides were averaged together to determine a mean value of treatment response.
Statistical Analysis
OS was defined as the time from the date of the surgery until death from any cause. Survival probability as a function of time was computed using the Kaplan-Meier estimator. The log-rank test was used to compare patient survival times between groups. A univariable Cox proportional hazards regression model was used to examine the association between OS and histopathological features and various clinical factors. The variables that were found to be significant on univariable analysis (p value < 0.25) were evaluated by multivariable analysis using the Cox proportional hazards model after backward stepwise Wald elimination. A p value of less than 0.05 on multivariable analysis was considered significant. The statistical analyses were performed using SPSS software (version 15; SPSS, Inc., Chicago, IL). The scores from two observers were compared graphically with the agreement of the mean scores, as evaluated by the Pearson’s correlation coefficient. Simulation studies were conducted by generating the scores for each patient, assuming that the scores were Gaussian distributed; the mean and standard deviation were calculated from the observed data. Scores from 1 to 12 sections were generated for each patient. The whole process was repeated 10,000 times.
Results
Patient Demographics and Treatment Characteristics
Table 1 shows the demographics of the 151 NSCLC patients who had been treated with neoadjuvant chemotherapy. Similar to our previous results, there was evidence of clinical downstaging in the resected specimens (presurgical stage IA/B = 17%, postsurgical stage = IA/B 29%; p < 0.05). All patients received a platinum (149 patients [99%]) or taxane-based regimen (97 patients [64%]); some received both (Table 1). The median number of treatment cycles was 3 (range: 1–6).
Table 1.
Patient Demographics and Treatment Characteristics
| Characteristic | No. (%)of Patients (N=151) |
|---|---|
| Age Mean (Range) | 61 (30–80) |
| Gender | |
| Male | 76 (50%) |
| Female | 75 (50%) |
| Histology | |
| Adenocarcinoma | 96 (63%) |
| Squamous cell carcinoma | 51 (34%) |
| Others | 4 (3%) |
| Tumor Size (cm) | |
| 0.0–2.0 | 29 (19%) |
| 2.1–3.0 | 36 (24%) |
| 3.1–5.0 | 43 (28%) |
| >5.0 | 43 (29%) |
| Pre-Surgical Stage | |
| IA/IB | 25(17%) |
| IIA/IIB | 52 (34%) |
| IIIA/IIIB | 67 (44%) |
| IV | 7 (5%) |
| Post-Surgical Stage | |
| 0/IA/IB | 44 (29%) |
| IIA/IIB | 44 (29%) |
| IIIA/IIIB | 60 (40%) |
| IV | 3 (2%) |
| Neoadjuvant Chemotherapy | |
| Cisplatin or Carboplatin | 149 (99%) |
| Taxol or Taxotere | 97 (64%) |
| Treatment Cycle Mean (Range) | 3 (1–6) |
Histopathological Agreement between Pathologists
The percentage of viable tumor cells was examined independently by two pathologists (experienced and trained). The number of slides read ranged from 2 to 12, with a mean of 5.4 and a standard deviation of 2.5. The first quartile, median, and third quartile were 4, 5, and 7, respectively. The scores of observer 1 and observer 2 for each patient are plotted in Figure 1A and Figure 1B, respectively, sorted by the ascending order of the mean score. The scores for both observers are plotted in Figure 2A. The majority of scores (73.6%) were identical between the two readers. Among the 812 pairs, the scores differed by, at most, 5% in 95.9% of the readings, and only 4.1% of the scores differed by 10% or more. In a few readings, observer 2 provided much higher scores than observer 1. Figure 2B shows the scatter plot of the mean scores of observer 1 and observer 2. The statistical analysis revealed a significant correlation between the two observers. Their mean scores agreed with each other very well. The Pearson correlation coefficient was 0.994 (95% confidence interval: 0.991–0.996) (Fig 2B, R2 = 0.994).
Figure 1.
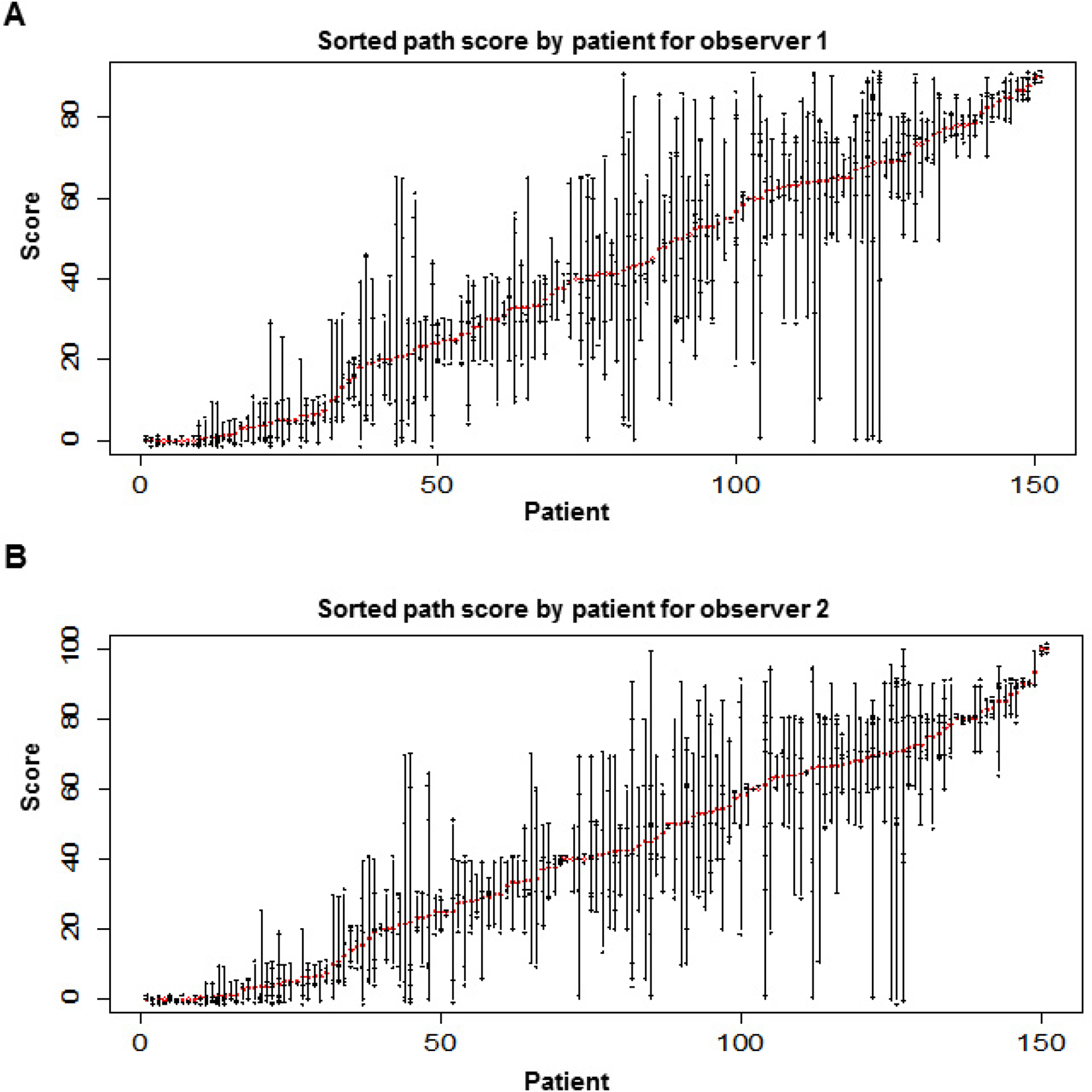
Score of the percentage of viable tumor cells, sorted by the ascending order of the mean score for each patient. The mean score is shown by the red circle. Each reading is marked by a ‘+’ sign. The scores were slightly jittered to break the ties. A and B show the results for observer 1 and observer 2, respectively.
Figure 2.
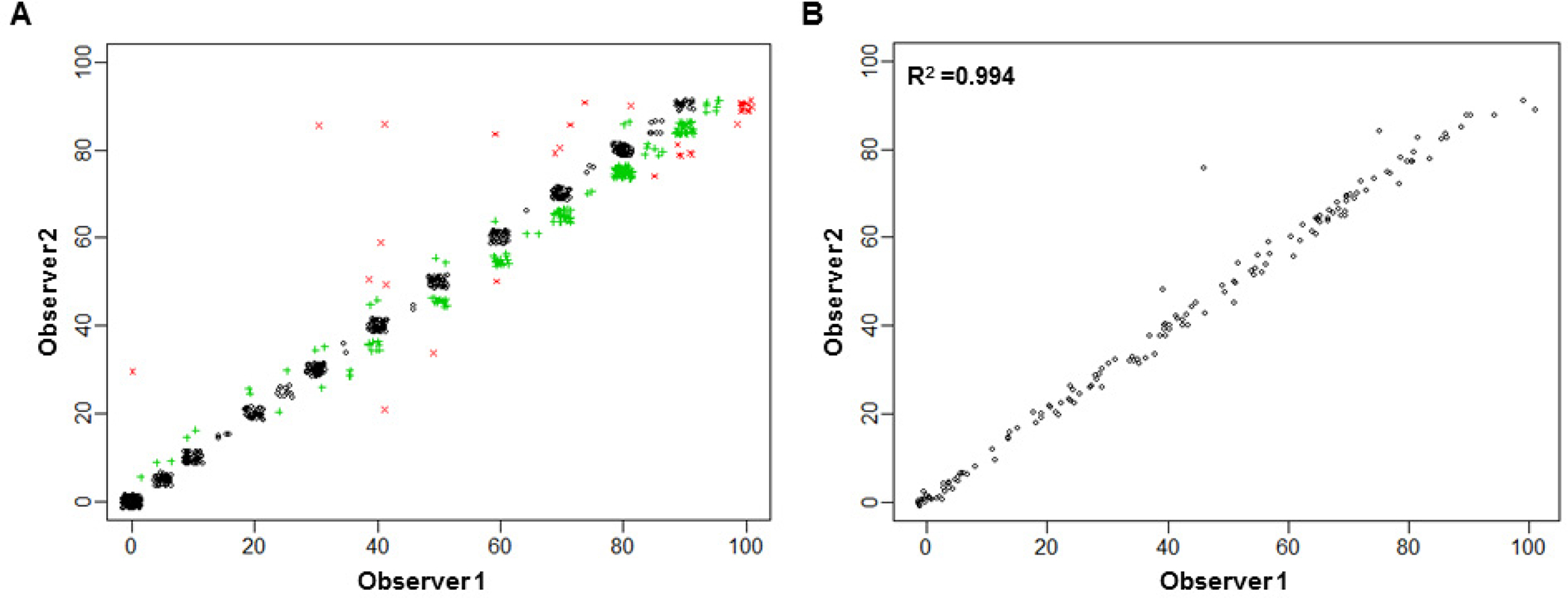
The percentage of viable tumor cells on each slide for individual patients, as examined by observers 1 and 2, for 151 patients. (A) Scatter plot of the score of the percentage of viable tumor cells for observer 1 versus observer 2 on all slides from all patients. The actual scores were jittered slightly to break the ties. Black circles show that the two scores are identical. Green “+” symbols show that the two scores differ by 5%. Red “x” symbols show that the two scores differ by 10% or more. (B) The statistical analysis indicated a significant correlation between observers 1 and 2.
Correlation between MPR and Clinicopathological Features and Disease Outcomes
We compared patients who had MPR+ (≤10%) with those who had MPR- (>10%) and did not detect any statistically significant correlations between MPR and age, sex, histological tumor type, clinical stage, or presence of lymph node metastasis (data not shown). We also did not detect any statistically significant correlations between MPR and chemotherapy treatment or treatment cycles (data not shown). The Kaplan-Meier survival curves, shown in Figure 3, reveal that MPR, examined either by observer 1 (Figure 3A, p = 0.001) or by observer 2 (Figure 3B, p = 0.002), was significantly predictive of long-term OS after neoadjuvant chemotherapy.
Figure 3.
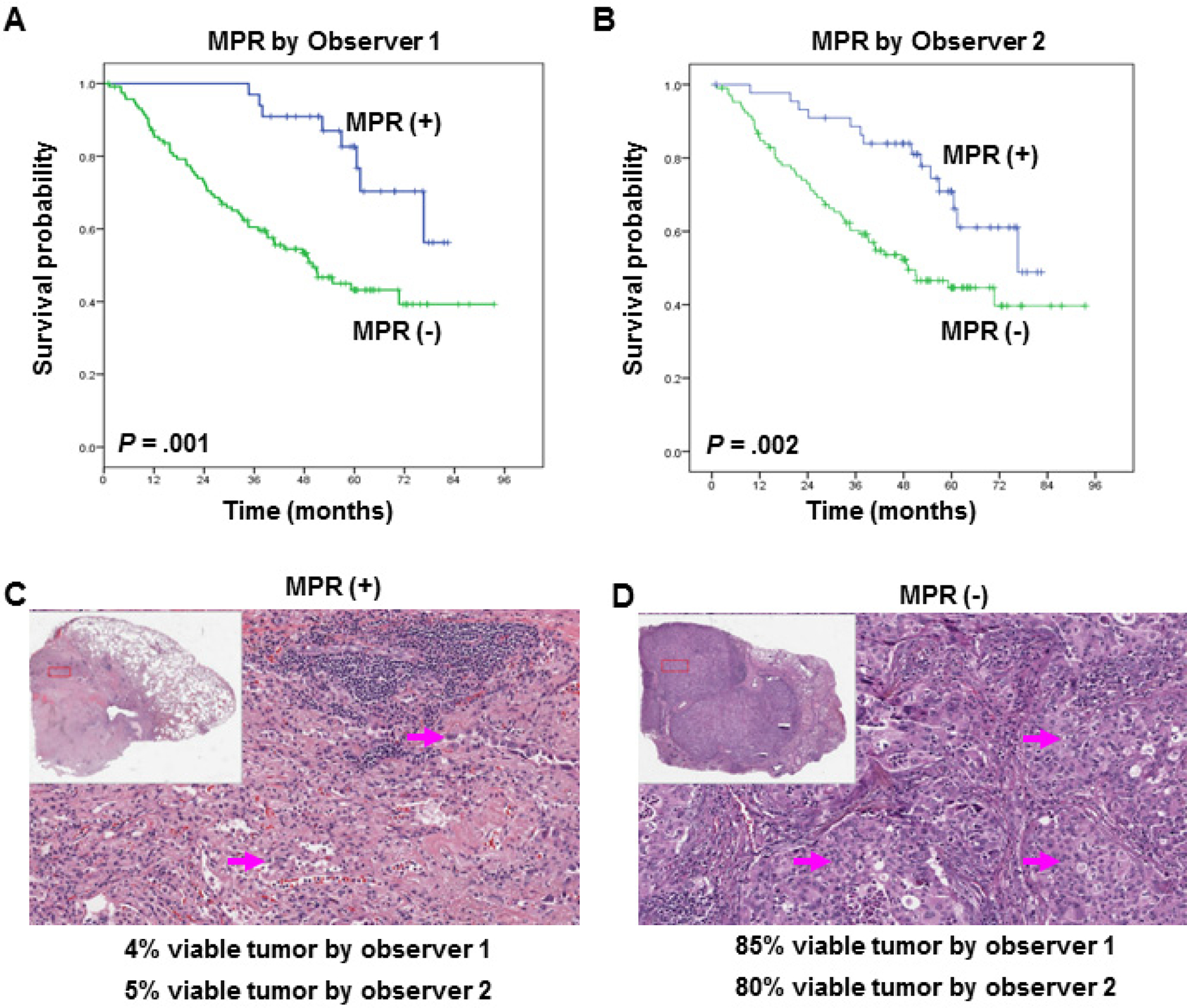
Kaplan-Meier estimates of overall survival on the basis of the percentage of viable tumor cells, as determined by observer 1 (A) and observer 2 (B). (A and B) Overall survival was significantly longer in patients with ≤10% viable tumor cells than in those with >10% viable tumor cells. Patients with ≤10% viable tumor cells are indicated by MPR (+), and patients with >10% viable tumor cells are indicated by MPR (−). Representative examples are shown of the histopathological features of tumors associated with extensive response to treatment (C) or no response to treatment (D) and of the percentage of viable tumor cells for individual patients, as determined by observers 1 and 2. Arrows indicate viable tumor cells.
We observed many histopathological patterns, including fibrosis, necrosis, cholesterol cleft granuloma, giant cell reaction fibrosis, macrophages, and inflammation in the tumor specimens. Figures 3C and 3D show typical examples of the histopathological features of tumors associated with extensive (3C) or no (3D) response to neoadjuvant chemotherapy and the percentage of viable tumor cells for individual patients, as examined by observers 1 and 2. We next used univariable Cox proportional hazard regression models to determine the effects of covariates on OS duration. The factors that significantly affected OS were pathological stage and MPR, as examined by observer 1 (Table 2). A multivariable Cox proportional hazards regression analysis showed that MPR, as examined by observer 1, was significantly associated with OS after accounting for the effects of pathological stage (HR = 2.68, p = 0.01). The results of a multivariable analysis indicated that MPR, as examined by observer 2, was also a significant predictor of OS duration (HR = 2.72, p = 0.01, Table 2).
Table 2.
Univariate and Multivariate Analyses for Overall Survival on NSCLC Patients treated with Neoadjuvant Chemotherapy
| Characteristics | No. of Patients | HR (95% Cl) | P Value |
|---|---|---|---|
| A. Univariate Cox Model | |||
| Age (Continuous) | 151 | 1.01 (0.98–1.04) | 0.53 |
| Gender | 0.51 | ||
| Female (Reference) | 76 | 1.00 | |
| Male | 75 | 1.17 (0.73–1.89) | |
| Histology | 0.35 | ||
| Adenocarcinoma (Reference) | 96 | 1.00 | |
| Squamous Cell Carcinoma | 51 | 1.23 (0.74–2.04) | 0.42 |
| Other | 4 | 2.02 (0.72–5.65) | 0.18 |
| Post-Surgical Stage | <0.0001 | ||
| 0/IA/IB (Reference) | 44 | 1.00 | |
| IIA/IIB | 44 | 1.86 (0.89–3.89) | 0.1 |
| IIIA/IIIB | 60 | 2.96 (1.52–5.77) | 0.001 |
| IV | 3 | 17.75 (4.76–66.18) | <0.0001 |
| MPR (Observer 1) | 0.001 | ||
| (+) ≤10% | 33 | 1.00 | |
| (−) >10% | 118 | 3.36 (1.60–7.05) | |
| MPR (Observer 2) | 0.001 | ||
| (+) ≤10% | 33 | 1.00 | |
| (−) >10% | 118 | 3.31 (1.59–7.12) | |
| B. Multivariate Cox Model | |||
| Post-Surgical Stage | 0.001 | ||
| 0/IA/IB (Reference) | 44 | 1.00 | |
| IIA/IIB | 44 | 1.51 (0.71–3.20) | 0.28 |
| IIIA/IIIB | 60 | 2.34 (1.18–4.65) | 0.02 |
| IV | 3 | 12.80 (3.39–48.38) | <0.0001 |
| MPR (Observer 1) | 0.01 | ||
| (+) ≤10% | 33 | 1.00 | |
| (−) >10% | 118 | 2.68 (1.25–5.73) | |
| MPR (Observer 2) | 0.01 | ||
| (+) ≤10% | 33 | 1.00 | |
| (−) >10% | 118 | 2.72 (1.22–5.70) |
Abbreviations: HR, Hazard Ratio; CI, Confidence Interval. (AJCC7)
Statistical Analysis of Pathological Reading of Multiple Slides
Because of potential tumor heterogeneity, multiple sections of tumor were taken at the time of grossing to determine the percentage of viable tumor cells. As preparing and reading a large number of slides per surgical specimen is time consuming, it is important to determine how many slides are needed to accurately determine tumor response. Because of the excellent agreement between the scores of observer 1 and observer 2, we only used the data from observer 1 to determine how many slides were needed. For each patient, we calculated the mean and standard deviation of the scores for all slides. Figure 4A shows the standard deviation versus the mean score for all 151 patients. When the mean score was ≤20, the standard deviation was smaller than when the mean score was >20. As the mean score approached 0% or 100%, the standard deviation became smaller. The results were consistent with the proportion for data from the binomial distribution, which suggested that, for an accurate assessment for the score, a smaller number of slides is required when the score is close to 0%. As shown in Figure 4A, the number of slides needed to accurately assess the percentage of viable tumor cells depended on the magnitude of the true mean. Therefore, we grouped the true mean as follows: group 1 = true mean, 0–5; group 2 = 6–15; group 3 = 16–25; and group 4 = 36–55. When the viable tumor cell proportion was 50%, the grouping beyond 50% was omitted because of its symmetric nature and the reduced importance of an accurate assessment.
Figure 4.
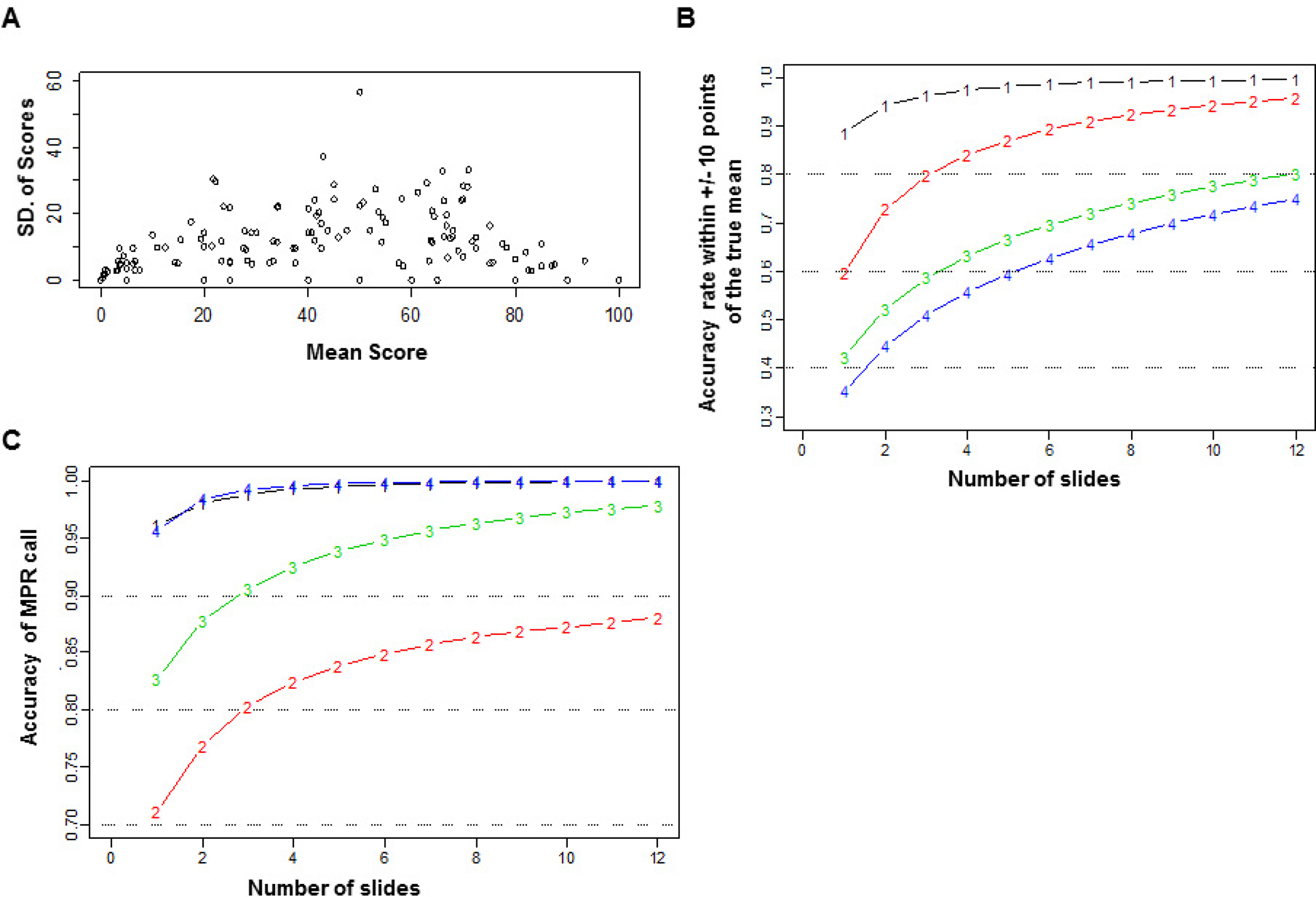
Statistical analysis of pathological readings of multiple slides. (A) Standard deviation versus mean score for all patients. (B) Accuracy of estimating the mean percentage of viable tumor cells within 10% of the true mean by the number of slides read, coded by the true mean group. Group 1 = true mean, 0–5; group 2 = 6–15; group 3 = 16–25; and group 4 = 36–55. (C) Accuracy of major pathological response (MPR) by the number of slides read, coded by the true mean group. Group 1 = true mean, 0–5; group 2 = 6–15; group 3 = 16–25; and group 4 = 36–55.
Figure 4B plots the accuracy of estimating the score within 10% of the true mean versus the number of slides. For group 1, only two slides were needed to achieve an accuracy of 90%. For group 2, three slides were needed for an accuracy of 80%. The number of slides required to reach 80% accuracy grew to 11 for group 3, while even with 12 slides, group 4 had an accuracy of less than 80%. The number of slides needed for an accurate MPR call depends on the magnitude of the true mean (Figure 4C). For group 1, only one slide was needed to achieve 90% accuracy and two to achieve 95% accuracy. For group 2, three slides were needed to achieve 80% accuracy. For group 3, only three were needed for 90% accuracy. For group 4, only one slide was needed for 95% accuracy because the patient would almost certainly not have achieved MPR. These results show that group 2 required the largest number of slides for an accurate call of MPR.
Discussion
In this study, we showed that the percentage of viable tumor cells, as determined by independent observers, is a significant predictor of long-term OS in neoadjuvant therapy-treated NSCLC patients. Neoadjuvant chemotherapy has been shown to improve the OS of NSCLC patients by lowering the distant metastasis rate.9, 12 The radiological measurements of tumor size before and after therapy in lung cancer patients is typically used to evaluate response to neoadjuvant chemotherapy.13 In a previous study, we and others were unable to show that CT RECIST assessment was a reliable predictor of OS in NSCLC patients undergoing surgical resection after neoadjuvant chemotherapy because of the difficulty in differentiating fibrosis from viable tumor radiographically.6, 14, 15
Consistent with the results of our previous study, there was an improvement in survival duration in patients with therapy-induced histological tumor regression (≤10% viable tumor cells). A statistical evaluation revealed that patients with an MPR+ had significantly longer OS durations than did those with MPR- (p < 0.001). The MPR was significantly associated with OS, even after accounting for the effects of pathological stage. Our data suggest that the initial evaluation should be based on three histological tumor slides. If the percentage of viable tumor in those slides is consistently >20%, no further slides need to be evaluated, as it is unlikely that the mean score will be <10%. On the other hand, if the scores are between 5% and 20%, more slide readings (seven or more) will be required to reach at least 90% accuracy.
There are several limitations to our study. The retrospective nature of our analysis means that we had no influence on the manner and extent of tumor sampling at the time of gross examination or availability of all slides for review. Traditionally, standard pathological approach has been to sample 1 section per cm of maximum tumor diameter and only if tumors were of small size (≤3 cm) was the entire tumor submitted for histological examination. In addition, it has been custom to preferentially select areas of viable tumor rather than necrotic or fibrotic tissue. Although this approach works very well for standard pathological processing, in the neoadjuvant setting, such method is suboptimal as ideally the entire remaining tumor mass should be submitted to most accurately analyze the treatment effect. On the other hand, this raises practical challenges, especially for large lesions that would require a significant number of sections to be submitted and examined, rendering the process rather impractical for daily use. To address these issues, we are currently planning to perform a prospective study with the goal to standardize the gross examination of neoadjuvant-treated NSCLC in a comprehensive yet practical manner. This means that the number of slides currently recommended for initial reading based on the present analysis may change once more information becomes available.
There are several potential advantages to developing neoadjuvant treatment strategies and evaluating MPR criteria for surgically resected NSCLC: 1) It will allow to design more efficient clinical trials, incorporating novel neoadjuvant therapies and evaluating MPR as a surrogate marker of improved long-term outcomes; 2) it will allow for an early readout of efficacy and could lead to streamlined drug development; and 3) it will allow us to investigate intensification of adjuvant treatment in patients who do not experience an adequate response to neoadjuvant therapy in an attempt to improve long-term outcomes.
Variations in histological features can occur in grading systems. Shannon and others have reported acceptable agreement on histological features among pathologists.16, 17 In an attempt to decrease interobserver variability in our study, histological evaluation of the resected specimens were performed by two pathologists independently. The two observers’ scores agreed with each other very well given a short training period. Because digital imaging technologies can be used as tools in pathological analysis, we plan to compare eye-based analysis with digital analysis in a future study.
In summary, our results confirm that MPR, as indicated by the percentage of viable tumor cells in the resected specimen, is correlated with long-term OS in NSCLC patients treated with neoadjuvant chemotherapy. The MPR may serve as a surrogate endpoint to evaluate novel chemotherapies and immunotherapy response in biomarker-driven translational clinical trials. The endpoint of pathological response may ultimately be a better and faster surrogate for treatment response than long-term OS.
Clinical Practice Points.
Neoadjuvant chemotherapy followed by surgical resection has been increasingly used in patients with locally advanced non-small cell lung cancer.
We and others have suggested that MPR in resected specimens could serve as a surrogate endpoint for survival and provide a more accurate and rapid means of comparing different neoadjuvant treatment regimens, shortening the period needed to evaluate novel chemotherapeutic and biological therapies in clinical trials
There are no large outcome studies that assessment of MPR was reproducible between observers.
Our data suggest that at least three slides should be read to accurately determine tumor response. The results of our study clearly demonstrate that MPR is a significant predictor of OS in neoadjuvant chemotherapy-treated NSCLC patients.
Acknowledgments
We thank Ann Sutton from the Department of Scientific Publications at MD Anderson Cancer Center for her assistance in preparing the manuscript.
Grant Support: This work was supported in part by the National Institutes of Health through MD Anderson’s Cancer Center Support Grant, CA 016672 (Lung Program), and by the Homer Flower Research Fund, the Charles Rogers Gene Therapy Fund, the Margaret Wiess Elkins Endowed Research Fund, the Flora and Stuart Mason Lung Cancer Research Fund, and the Phalan Thoracic Gene Therapy Fund.
Abbreviations:
- MPR
major pathologic response
- FFPE
formalin-fixed paraffin-embedded
- NSCLC
non-small cell lung cancer
- OS
overall survival
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: The authors declare no conflicts of interest.
References
- 1.Roth JA, Atkinson EN, Fossella F, et al. Long-term follow-up of patients enrolled in a randomized trial comparing perioperative chemotherapy and surgery with surgery alone in resectable stage IIIA non-small-cell lung cancer. Lung cancer (Amsterdam, Netherlands) 1998;21:1–6. [DOI] [PubMed] [Google Scholar]
- 2.Rosell R, Gomez-Codina J, Camps C, et al. Preresectional chemotherapy in stage IIIA non-small-cell lung cancer: a 7-year assessment of a randomized controlled trial. Lung cancer (Amsterdam, Netherlands) 1999;26:7–14. [DOI] [PubMed] [Google Scholar]
- 3.Pisters KM, Ginsberg RJ, Giroux DJ, et al. Induction chemotherapy before surgery for early-stage lung cancer: A novel approach. Bimodality Lung Oncology Team. The Journal of thoracic and cardiovascular surgery 2000;119:429–439. [DOI] [PubMed] [Google Scholar]
- 4.Depierre A, Milleron B, Moro-Sibilot D, et al. Preoperative chemotherapy followed by surgery compared with primary surgery in resectable stage I (except T1N0), II, and IIIa non-small-cell lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2002;20:247–253. [DOI] [PubMed] [Google Scholar]
- 5.Pataer A, Kalhor N, Correa AM, et al. Histopathologic response criteria predict survival of patients with resected lung cancer after neoadjuvant chemotherapy. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 2012;7:825–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.William WN Jr., Pataer A, Kalhor N, et al. Computed tomography RECIST assessment of histopathologic response and prediction of survival in patients with resectable non-small-cell lung cancer after neoadjuvant chemotherapy. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 2013;8:222–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hellmann MD, Chaft JE, William WN Jr., et al. Pathological response after neoadjuvant chemotherapy in resectable non-small-cell lung cancers: proposal for the use of major pathological response as a surrogate endpoint. Lancet Oncol 2014;15:e42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Funt SA, Chapman PB. The Role of Neoadjuvant Trials in Drug Development for Solid Tumors. Clin Cancer Res 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song WA, Zhou NK, Wang W, et al. Survival benefit of neoadjuvant chemotherapy in non-small cell lung cancer: an updated meta-analysis of 13 randomized control trials. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer 2010;5:510–516. [DOI] [PubMed] [Google Scholar]
- 10.Yamane Y, Ishii G, Goto K, et al. A novel histopathological evaluation method predicting the outcome of non-small cell lung cancer treated by neoadjuvant therapy: the prognostic importance of the area of residual tumor. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 2010;5:49–55. [DOI] [PubMed] [Google Scholar]
- 11.Pataer A, Shao R, Correa AM, et al. Major pathologic response and RAD51 predict survival in lung cancer patients receiving neoadjuvant chemotherapy. Cancer Med 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siegenthaler MP, Pisters KM, Merriman KW, et al. Preoperative chemotherapy for lung cancer does not increase surgical morbidity. The Annals of thoracic surgery 2001;71:1105–1111; discussion 1111–1102. [DOI] [PubMed] [Google Scholar]
- 13.Birchard KR, Hoang JK, Herndon JE Jr., et al. Early changes in tumor size in patients treated for advanced stage nonsmall cell lung cancer do not correlate with survival. Cancer 2009;115:581–586. [DOI] [PubMed] [Google Scholar]
- 14.Tanvetyanon T, Eikman EA, Sommers E, et al. Computed tomography response, but not positron emission tomography scan response, predicts survival after neoadjuvant chemotherapy for resectable non-small-cell lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2008;26:4610–4616. [DOI] [PubMed] [Google Scholar]
- 15.Choi H, Charnsangavej C, Faria SC, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2007;25:1753–1759. [DOI] [PubMed] [Google Scholar]
- 16.Swisher SK, Wu Y, Castaneda CA, et al. Interobserver Agreement Between Pathologists Assessing Tumor-Infiltrating Lymphocytes (TILs) in Breast Cancer Using Methodology Proposed by the International TILs Working Group. Annals of surgical oncology 2016;23:2242–2248. [DOI] [PubMed] [Google Scholar]
- 17.Wu TT, Chirieac LR, Abraham SC, et al. Excellent interobserver agreement on grading the extent of residual carcinoma after preoperative chemoradiation in esophageal and esophagogastric junction carcinoma: a reliable predictor for patient outcome. The American journal of surgical pathology 2007;31:58–64. [DOI] [PubMed] [Google Scholar]


