Abstract
Background
Preparation of endometrial thickness in frozen-thawed embryo transfer (FET) is extremely important, particularly in repeated implantation failure (RIF) patients.
Objective
This study aimed to investigate the clinical outcomes of FET cycles among RIF women, based on the effects of administering gonadotropin-releasing hormone (GnRH) agonist prior to estrogen-progesterone preparation of the endometrium.
Materials and Methods
In this randomized clinical trial, 67 infertile women who were candidates for FET were divided into two groups: A) case group (n = 34), treated with GnRH agonist prior to endometrial preparation and B) control group (n = 33), which received the routine protocol. (6 mg daily estradiol started from second day) The clinical outcomes) including chemical and clinical pregnancy, in addition to implantation rates, were compared between the two groups.
Results
The results showed no significant differences in women's age (p = 0.558), duration (p = 0.540), type (p = 0.562), and cause of infertility (p = 0.699). Regarding pregnancy and implantation rates, there was a trend toward an increase in the case group; however, differences were not statistically significant.
Conclusion
Although our results showed no significant differences between groups. Because there are trends to better results in case group larger sample size may show significant difference.
Keywords: Implantation failure, Gonadotropin-releasing hormone, Embryo transfer, Pregnancy, Implantation.
1. Introduction
Ovarian stimulation commonly increases the number of available embryos for embryo transfer (ET). Despite advances in assisted reproductive technology (ART) over the past four decades, some cycles still fail in terms of achieving live birth following in vitro fertilization (IVF)/ET (1). Embryo cryopreservation can be applied as an important technique in ART programs to avoid multiple ovarian stimulation protocols in infertile couples. Embryo cryopreservation programs enable the creation of surplus embryos following IVF/intracytoplasmic sperm injection (ICSI) to be stored and utilized at a later date in a frozen-thawed ET (FET) cycle (2). Exact synchronization between endometrial maturation and embryo development is an essential factor for successful implantation in FET cycles. Successful FET was reported during a natural cycle following spontaneous ovulation, stimulated cycles (3), and hormone supplement cycles (4). Available hormonal supplement protocols introduced for endometrial preparation differ in terms of drug type, administration route, dosage, and whether or not gonadotropin-releasing hormone (GnRH) analogs are used (5). Recently, in the most common protocols, spontaneous ovulation prior to estradiol and progesterone administration was avoided by pituitary down-regulation with a GnRH agonist (6).
One of the main challenges of ART programs is repeated implantation failure (RIF). Several treatment strategies have been suggested for this problem (7). FET cycles can be timed with ovulation in natural cycles or after endometrial preparation following exogenous hormone therapy (3, 6). Another protocol for controlled endometrial preparation is using the GnRH agonist prior to steroid administration, resulting in ovarian function suppression, subsequently creating synchronization between endometrial and embryo development (8). Several studies showed significant improvements in pregnancy rates for both fresh (9, 10) and frozen cycles (11) following GnRH agonist administration in infertile women with endometriosis or adenomyosis. Specifically, in FET cycles, it was reported that pretreatment with a GnRH agonist in patients with adenomyosis improved endometrial receptivity, as well as pregnancy outcomes (11).
The present study was conducted to investigate the effects of GnRH agonist administration on the implantation rate in the FET cycles of women with RIF, prior to estrogen-progesterone preparation of the endometrium.
2. Materials and Methods
Participants
In the present randomized clinical trial, 67 infertile women with history of idiopathic RIF (at least two implantation failures), undergoing FET cycles in Yazd Reproductive Sciences Institute, Yazd, Iran between August and November 2017 were randomly enrolled in two groups. Randomization was done as computer generated.
The case group (n = 34) received 0.1 mg/day of the GnRH agonist (Variopeptyl, VarianDarou, Iran), subcutaneous, from day 21 of the cycle preceding the actual FET cycle. On the second day of the cycle, the dose of GnRH agonist was reduced to 0.05 mg and 6 mg/day oral estradiol valerate (2 mg, Aburaihan Co., Tehran, Iran) was also started. When the endometrial thickness reached to 7.5 mm, vaginal supplementation of CyclogestⓇ pessaries (Cox Pharmaceuticals, Barnstaple, UK) at 400 mg twice daily was started and the GnRH agonist was also stopped.
The control group (n = 33), received 6 mg/day oral estradiol valerate (2 mg, Aburaihan Co., Tehran, Iran) from the second day of the cycle without the GnRH agonist. In the two groups, frozen-thawed embryos were transferred on the fourth day of progesterone treatment. All women with endometrial polyp, uterine myoma, and uterine anomaly were excluded from the study (Figure 1).
Embryo vitrification and thawing
Vitrification and warming procedures were effected using RapidVit Cleave and RapidWarm Cleave kits, respectively (Vitrolife Inc., Gothenburg, Sweden), as per manufacturer instructions. The quality of embryos was evaluated 24 h later, and those with less than 50% fragmentation were selected for ET.
Clinical outcomes
Chemical pregnancy was defined by serum β hCG 50 IU/L, 14 days after ET. Clinical pregnancy was approved by the detection of a fetal heartbeat 2 wk after positive β hCG. Implantation rate was defined as the number of gestational sacs per embryos transferred. Moreover, endometrial thickness was observed in sagittal view via sonography on the day that progesterone was started.
Figure 1.
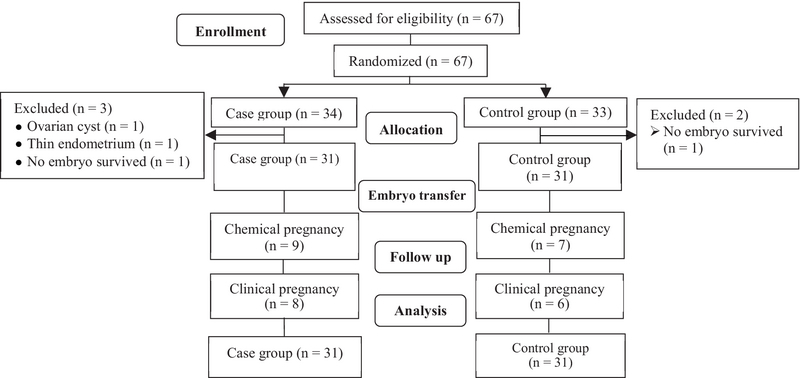
Study design flowchart.
Ethical consideration
The study protocol was approved by the Ethics Committee of Yazd Reproductive Sciences Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran (Code: IR.SSU.RSI.Rec.1396.1). Written informed consent was provided by all participants.
Statistical analysis
Statistical analysis was carried out using the statistical package for the social sciences (SPSS v. 14, Chicago, IL). Both Mann-Whitney U and chi-square tests were performed where appropriate. The level of significance was set at p-value 0.05.
3. Results
In the case group, three cycles were canceled due to ovarian cyst (37 mm), endometrial failure to reach a thickness of at least 7.5 mm, and no embryo survival after thawing procedure. Two cycles were also canceled in the control group because no embryo survived following the thawing procedure.
Finally, the number of cycles remaining was 31 in each group. No participants were lost to follow-up in either group. No significant differences in the demographic characteristics were observed between the two groups (Table I). The two groups were similar in endometrial thickness at the start of progesterone therapy (8.70 1.08 vs. 8.94 1.33, p = 0.521) and in the number and quality of transferred embryo per women (1.90 30 vs. 1.83 .52, p = 0.554) (Table I).
As presented in Table II, the ART outcomes in the two groups were not significantly different. Data showed higher chemical and clinical pregnancy rates for the case group than the control group (29% vs. 22.6% and 25.8% vs. 19.4%, respectively); however, differences were not significant. Moreover, implantation rate showed no statistically significant difference between groups; however, there was a trend toward greater implantation rate in the case group (13.55% vs. 10.52%, p = 0.521).
Table 1.
Comparison of demographic characteristics in two study groups
|
| ||||||
| Variables | Case group (n = 31) | Control group (n = 31) | p-value | |||
| Age (y)* | 32.61 4.40 | 31.96 4.22 | 0.558 | |||
| Duration of infertility (y*) | 6.93 3.94 | 7.48 2.99 | 0.540 | |||
| Type of infertility ** | ||||||
| Primary | 22 (71.0) | 24 (77.4) | ||||
| Secondary | 9 (29.0) | 7 (22.6) | <brow>-2</erow> 0.562 | |||
| Cause of infertility * | ||||||
| Male factor | 9 (29.0) | 13 (41.9) | ||||
| Poly cystic ovarian syndrome | 5 (16.1) | 4 (12.9) | ||||
| Tubal factor | 3 (9.7) | 1 (3.2) | ||||
| MIX | 9 (29.0) | 8 (25.8) | ||||
| Unexplained | 5 (16.1) | 4 (12.9) | ||||
| Ovarian factor | 0 (0.0) | 1 (3.2) | <brow>-6</erow> 0.699 | |||
| Endometrial thickness (mm)* | 8.70 1.08 | 8.94 1.33 | 0.521 | |||
| Cycle day* | 15.38 2.29 | 16.38 1.92 | 0.068 | |||
| No. of transferred embryos* | 1.90 30 | 1.83 0.52 | 0.554 | |||
| Embryo quality ** | ||||||
| Grade A | 22 (71) | 20 (64.5) | ||||
| Grade B | 7 (22.6) | 5 (16.1) | ||||
| Grade C | 2 (6.5) | 6 (19.4) | <brow>-3</erow> 0.297 | |||
| * Data presented as Mean SD. ** Data presented as n (%) a: Mann-Whitney U test, b: Chi-square | ||||||
Table 2.
ART outcomes for the case group vs. control group
|
| |||
| Variables | Case group (n = 31) | Control group (n = 31) | p-value |
| Chemical pregnancy | 9 (29.0) | 7 (22.6) | 0.562 |
| Clinical pregnancy | 8 (25.8) | 6 (19.4) | 0.544 |
| Implantation rate | 8/52 (13.55) | 6/57 (10.52) | 0.521 |
| Data presented as n (%). Analyzed by Chi-square test | |||
4. Discussion
The present study found that pituitary down-regulation prior to frozen ET did not lead to significantly higher chemical and clinical pregnancy rates in women with a history of RIF.
RIF is an important challenge in ART programs for both patients and physicians. An important factor in implantation is synchronization between embryo development and endometrial maturation. One study have evaluated other factors that may affect or improve ART outcome of frozen-thawed embryos (1).
The programmed cycle using a GnRH agonist prior to estrogen and progesterone administration aims to effect pituitary down-regulation, thereby avoiding spontaneous ovulation and cycle cancelation, the primary drawbacks of a natural cycle protocol (12). Our study revealed that the GnRH agonist in women with RIF may improve implantation and pregnancy rates; however, indicated differences showed no level of significance. Hebisha and colleagues showed that daily subcutaneous administration of 0.1 mg GnRH agonist (Triptorelin) prior to estrogen and progesterone (starting from the mid-luteal phase of the previous cycle) increased implantation and pregnancy rates (13). A recent study related to sample size also delivered meaningful results. The implementation of a smaller case group in our study was the reason we did not report significant statistical results. Furthermore, using the GnRH agonist prior to frozen ET in polycystic ovary syndrome, women indicated an improvement in ongoing pregnancy rate (14).
Similarly, Xing and associates found that hormonally controlled endometrial preparation with prior GnRH agonist suppression can be used for patients who had experienced repeated IVF treatment failures, despite having morphologically optimal embryos. This study administered two intramuscular injections of Diphereline (2.5 mg), with a duration time of 28 days between the first and second injection. The comparison showed that clinical outcomes were significantly better with GnRH agonist protocols when compared with endometrial preparation with estrogen and progesterone only protocols in the same patients (15). Generally, the underlying mechanism of a GnRH agonist is not yet clearly understood. Probably It seems that induces pituitary suppression and decreased circulating estrogen, a GnRH agonist may reduce concentrations of peritoneal fluid metalloproteinase tissue inhibitors, down-regulate peritoneal fluid inflammatory proteins, and promote apoptosis and expression of pro-apoptotic proteins (16, 17). Niu and colleagues showed using long-term GnRH agonist prior to IVF/ICSI in infertile women with endometriosis or adenomyosis significantly enhanced the chances of pregnancy in both fresh and frozen cycles (11). In fact, it was found that women with repeated implantation failure have concomitant uterine adenomyosis and a prominent aggregation of macrophages within the superficial endometrial glands, that interrupted with embryo implantation. Tremellen and colleagues showed that pituitary suppression with a GnRH agonist increased implantation rate (18).
In agreement with our result, Dal and colleagues reported no difference in implantation and pregnancy rates with or without the use of a GnRH agonist for pituitary suppression in FET cycles. They administered 3.75 mg Decapeptyl in the mid-luteal phase of the cycle. Their results indicated that endometrial preparation with GnRH agonist pretreatment did not increase the success rate of FET cycles (19). Previously, we found no significant different implantation or pregnancy rates when using exogenous steroids with or without GnRH agonist during FET cycles (20).
5. Conclusion
In conclusion, our study found that the use of a GnRH agonist in women with RIF may improve endometrial receptivity and increase pregnancy rate. However, differences were not statistically significant. Therefore, additional large prospective studies are necessary on this topic.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgments
The authors thank the staff of the infertility Clinic as well as the operating room personnel at Yazd Reproductive Sciences Institute, Yazd, Iran for their valuable contributions to all procedures. Financial support was done by Shahid Sadoughi University of Medical Sciences, Yazd Iran.
References
- 1.Go Kathryn J. Gardner DK, Weissman A, Howles CM, Shoham Z, editors. Textbook of Assisted Reproductive Techniques: Laboratory and Clinical Perspectives. Fertility and Sterility. 2002;78(6):1357–1358. doi: 10.1016/s0015-0282(02)04247-4. [DOI] [Google Scholar]
- 2.Yarali Hakan, Polat Mehtap, Mumusoglu Sezcan, Yarali Irem, Bozdag Gurkan. Preparation of endometrium for frozen embryo replacement cycles: a systematic review and meta-analysis. Journal of Assisted Reproduction and Genetics. 2016;33(10):1287–1304. doi: 10.1007/s10815-016-0787-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shapiro Bruce S., Daneshmand Said T., Garner Forest C., Aguirre Martha, Hudson Cynthia, Thomas Shyni. Evidence of impaired endometrial receptivity after ovarian stimulation for in vitro fertilization: a prospective randomized trial comparing fresh and frozen–thawed embryo transfer in normal responders. Fertility and Sterility. 2011;96(2):344–348. doi: 10.1016/j.fertnstert.2011.05.050. [DOI] [PubMed] [Google Scholar]
- 4.Ming Li, Liu Ping, Qiao Jie, Lian Ying, Zheng Xiaoying, Ren Xiulian, Huang Jin, Wu Yuqi. Synchronization between embryo development and endometrium is a contributing factor for rescue ICSI outcome. Reproductive BioMedicine Online. 2012;24(5):527–531. doi: 10.1016/j.rbmo.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Groenewoud Eva R., Cantineau Astrid E.P., Kollen Boudewijn J., Macklon Nick S., Cohlen Ben J. What is the optimal means of preparing the endometrium in frozen–thawed embryo transfer cycles? A systematic review and meta-analysis. Human Reproduction Update. 2013;19(5):458–470. doi: 10.1093/humupd/dmt030. [DOI] [PubMed] [Google Scholar]
- 6.Ozgur Kemal, Berkkanoglu Murat, Bulut Hasan, Humaidan Peter, Coetzee Kevin. Agonist depot versus OCP programming of frozen embryo transfer: a retrospective analysis of freeze-all cycles. Journal of Assisted Reproduction and Genetics. 2015;33(2):207–214. doi: 10.1007/s10815-015-0639-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Makrigiannakis Antonis, Benkhalifa Moncef, Vrekoussis Thomas, Mahjub Sami, Kalantaridou Sophia N., Gurgan Timur. Repeated implantation failure: a new potential treatment option. European Journal of Clinical Investigation. 2015;45(4):380–384. doi: 10.1111/eci.12417. [DOI] [PubMed] [Google Scholar]
- 8.Roque Matheus, Lattes Karinna, Serra Sandra, Solà Ivan, Geber Selmo, Carreras Ramón, Checa Miguel Angel. Fresh embryo transfer versus frozen embryo transfer in in vitro fertilization cycles: a systematic review and meta-analysis. Fertility and Sterility. 2013;99(1):156–162. doi: 10.1016/j.fertnstert.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 9.S Guo, D Zhang, Z Niu, Y Sun, Fengy Pregnancy outcomes and neonatal outcomes after pituitary down-regulation in patients with adenomyosis receiving IVF/ICSI and FET. Results of a retrospective cohort study. Int J Clin Exp Med 2016; 9: 14313-14320.
- 10.Surrey Eric S., Silverberg Kaylen M., Surrey Mark W., Schoolcraft William B. Effect of prolonged gonadotropin-releasing hormone agonist therapy on the outcome of in vitro fertilization-embryo transfer in patients with endometriosis. Fertility and Sterility. 2002;78(4):699–704. doi: 10.1016/s0015-0282(02)03373-3. [DOI] [PubMed] [Google Scholar]
- 11.Niu Zhihong, Chen Qian, Sun Yijuan, Feng Yun. Long-term pituitary downregulation before frozen embryo transfer could improve pregnancy outcomes in women with adenomyosis. Gynecological Endocrinology. 2013;29(12):1026–1030. doi: 10.3109/09513590.2013.824960. [DOI] [PubMed] [Google Scholar]
- 12.X Yang, X Dong, K Huang, L Wang, T Xiong, L Ji, et al. The effect of accompanying dominant follicle development/ovulation on the outcomes of frozen-thawed blastocyst transfer in HRT cycle. Int J Clin Exp Pathol 2013; 6: 718-723. [PMC free article] [PubMed]
- 13.Hebisha S. A., Adel H. M. GnRh Agonist Treatment Improves Implantation and Pregnancy Rates of Frozen–Thawed Embryos Transfer. The Journal of Obstetrics and Gynecology of India. 2016;67(2):133–136. doi: 10.1007/s13224-016-0936-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsai Hsiao-Wen, Wang Peng-Hui, Lin Li-Te, Chen San-Nung, Tsui Kuan-Hao. Using gonadotropin-releasing hormone agonist before frozen embryo transfer may improve ongoing pregnancy rates in hyperandrogenic polycystic ovary syndrome women. Gynecological Endocrinology. 2017;33(9):686–689. doi: 10.1080/09513590.2017.1307961. [DOI] [PubMed] [Google Scholar]
- 15.Yang Xing, Huang Rui, Wang Yan-Fang, Liang Xiao-Yan. Pituitary suppression before frozen embryo transfer is beneficial for patients suffering from idiopathic repeated implantation failure. Journal of Huazhong University of Science and Technology [Medical Sciences] 2016;36(1):127–131. doi: 10.1007/s11596-016-1554-2. [DOI] [PubMed] [Google Scholar]
- 16.Ferrero S, Gillott D, Remorgida V, Anserini P, Price K, Ragni N, Grudzinskas J. Haptoglobin β chain isoforms in the plasma and peritoneal fluid of women with endometriosis. Fertility and Sterility. 2005;83(5):1536–1543. doi: 10.1016/j.fertnstert.2004.11.064. [DOI] [PubMed] [Google Scholar]
- 17.S Ferrero, Dj Gillott, V Remorgida, P Anserini, N Ragni, Jg Grudzinskas. GnRH analogue remarkably down-regulates inflammatory proteins in peritoneal fluid proteome of women with endometriosis. J Reprod Med 2009; 54: 223-231. [PubMed]
- 18.Tremellen Kelton, Russell Peter. Adenomyosis is a potential cause of recurrent implantation failure during IVF treatment. Australian and New Zealand Journal of Obstetrics and Gynaecology. 2011;51(3):280–283. doi: 10.1111/j.1479-828x.2010.01276.x. [DOI] [PubMed] [Google Scholar]
- 19.Prato Luca Dal, Borini Andrea, Cattoli Monica, Bonu Maria Antonietta, Sciajno Raffaella, Flamigni Carlo. Endometrial preparation for frozen-thawed embryo transfer with or without pretreatment with gonadotropin-releasing hormone agonist. Fertility and Sterility. 2002;77(5):956–960. doi: 10.1016/s0015-0282(02)02960-6. [DOI] [PubMed] [Google Scholar]
- 20.. Robab Davar, . Maryam Eftekhar, . Naeimeh Tayebi. Transfer of Cryopreserved-Thawed Embryos in a Cycle Using Exogenous Steroids with or Without Prior Gonadotropihin-Releasing Hormone Agonist. Journal of Medical Sciences(Faisalabad) 2007;7(5):880–883. doi: 10.3923/jms.2007.880.883. [DOI] [Google Scholar]


