Graphical abstract
Keywords: SARS-CoV-2, COVID-19, Cardiovascular, ACE2, Cytokine storm
Abbreviations: ACE, Angiotensin-converting enzyme; Ang, Angiotensin; ARB, Angiotensin receptor blocker; ARDS, Acute respiratory distress syndrome; CAD, Coronary artery disease; COVID-19, Coronavirus disease 2019; CVD, Cardiovascular diseases; DIC, Disseminated intravascular coagulation; ECMO, Extracorporeal membranous oxygenation; HFpEF, Heart failure with preserved ejection fraction; ICU, Intensive care unit; IFN, Interferon; IL, Interleukin; IP-10, Interferon -γ inducible protein 10; MCP-1, monocyte chemoattractant protein 1; MERS, Middle East respiratory syndrome; MOF, Multiple organ failure; NT-proBNP, N-terminal pro-brain natriuretic peptide; RAAS, Renin-angiotensin-aldosteron system; RDRP, RNA-dependent RNA polymerase proteins; ROS, reactive oxygen species; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; TNF, Tumor necrosis factor
Abstract
The coronavirus disease 2019 (COVID-19), elicited by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, is a pandemic public health emergency of global concern. Other than the profound severe pulmonary damage, SARS-CoV-2 infection also leads to a series of cardiovascular abnormalities, including myocardial injury, myocarditis and pericarditis, arrhythmia and cardiac arrest, cardiomyopathy, heart failure, cardiogenic shock, and coagulation abnormalities. Meanwhile, COVID-19 patients with preexisting cardiovascular diseases are often at a much higher risk of increased morbidity and mortality. Up–to-date, a number of mechanisms have been postulated for COVID-19-associated cardiovascular damage including SARS-CoV-2 receptor angiotensin-converting enzyme 2 (ACE2) activation, cytokine storm, hypoxemia, stress and cardiotoxicity of antiviral drugs. In this context, special attention should be given towards COVID-19 patients with concurrent cardiovascular diseases, and special cardiovascular attention is warranted for treatment of COVID-19.
1. Introduction
The novel coronavirus infectious disease (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), first broke out in Wuhan, China in early December 2019, and subsequently quickly spread worldwide (over 7,700,000 confirmed cases as of 6/14/2020) [1]. Following purification and sequencing analysis in samples of bronchoalveolar lavage fluid, SARS-CoV-2 is suggested to be closely related to two bat-derived SARS-like coronaviruses (with 88% genomic homology), and SARS-CoV (approximately 79% identity homology) and more remotely from the Middle East respiratory syndrome (MERS)-CoV (approximately 50% identity) [2]. During the SARS outbreak in 2003, SARS-CoV infected over 8000 people, with 916 death cases in 29 countries [3]. These data suggested that SARS-CoV-2 possesses a much stronger contingency compared with SARS-CoV, with an estimated basic reproductive number R0 value (indicating as viral infectivity) of 2.28 [4]. On 30 January 2020, the WHO declared that COVID-19 outbreak had become a pandemic Public Health Emergency of International Concern. Rapidly rising number of COVID-19 cases with a high mortality rate makes it rather challenging for timely and tightly control of the disease. Up-to-date, no antiviral drug or vaccine has been approved for SARS-CoV-2 infection which can directly target SARS-CoV-2.
Based on clinical manifestation, nearly all SARS-CoV-2-infected patients develop some degree of pneumonia, and patients with severe conditions develop acute respiratory distress syndrome (ARDS). Respiratory failure caused by severe lung injury is perhaps the main cause of death in SARS-CoV-2-infected patients. The SARS-CoV-2 viral load from patient respiratory tracts is believed to be positively linked to lung disease severity [5]. According to the analysis of clinical features of 138 patients infected with SARS-CoV-2, common symptoms associated with COVID-19 include fever (98.6%), dry cough (59.4%), and fatigue (69.6%) [6]. Except for respiratory symptoms, many patients have cardiac symptoms including palpitation and chest tightness, and severe acute cardiovascular injury [7]. In addition, COVID-19 patients with pre-existing cardiovascular issues (coronary heart disease, hypertension) displayed more severe clinical outcomes and higher mortalities [7]. These clinical findings indicated pronounced cardiovascular sequelae for SARS-CoV-2 infection. Here we will summarize the relationship between SARS-CoV-2 and cardiovascular diseases, and discuss possible mechanisms of action behind SARS-CoV-2 infection-induced damage to cardiovascular system.
2. SARS-CoV-2 and cardiovascular abnormalities
Previous studies have depicted a close relationship between cardiovascular diseases and SARS or MERS. Patients with SARS-CoV often suffer from a wide variety of cardiovascular complications including hypotension (50.4%), tachycardia (71.9%), bradycardia (14.9%), reversible cardiomegaly (10.7%), and transient atrial fibrillation [8]. Meta-analysis including 637 cases suggested high prevalence of hypertension (approximately 50%) and heart diseases (30%) in patients with MERS [9]. Given that COVID-19 shares many aspects of pathogenesis and clinical symptoms reminiscent of SARS and MERS, cardiovascular complications might also occur in patients with COVID-19. Unlike SARS-CoV which tends to infect the young population, the susceptible groups for COVID-19 are believed to be middle-aged and elderly with preexisting comorbidities. The median age is 56 year-old in patients infected with SARS-CoV-2 [6]. Not surprisingly, this is an age when many chronic comorbidities start to develop including myocarditis, heart failure, cardiomyopathy, arrhythmia, hypertension, and diabetes mellitus. The overall association between COVID-19 and cardiovascular abnormities is summarized in Table 1 . Particular forms of cardiovascular complications or aggravation of preexisting cardiovascular conditions in COVID-19 patients are discussed in detail here.
Table 1.
Cardiovascular (CV) comorbidities and complications in patients with COVID-19.
| Cases | Hospital | Age | Cardiovascular comorbidity | Cardiovascular complications | Ref |
|---|---|---|---|---|---|
| 41 | Jinyintan Hospital | 49 (41–58) | CVD (15%), hypertension (15%) | Acute cardiac injury* (12%) | [7] |
| 138 | Zhongnan Hospital | 56 (42–68) | Hypertension (31.2%), CVD (14.5%), cerebrovascular (5.1%) | Acute cardiac injury (7.2%), shock (8.7%) and arrhythmia (16.7%) | [6] |
| 1099 | 552 Hospitals in China | 47 (35–58) | Hypertension (15%), CAD (2.5%), cerebrovascular (1.4%) | Creatine kinase ≥ 200 U/L (13.7%), and septic shock (1.1%) | [11] |
| 21 | Evergreen Hospital | 70 (43–92) | Congestive heart failure (42.9%), troponin level > 0.3 ng/mL (14%) | Cardiomyopathy** (33.3%) | [29] |
| 137 | 9 Tertiary Hospitals in Hubei | 57 (20–83) | Hypertension (9.5%) and CVD (7.3%) | Symptom of heart palpitation (7.3%) and comorbid organ dysfunction (18.9%) | [22] |
| 149 | 3 Tertiary Hospitals Wenzhou | 45 (32–58) | Cardio-cerebrovascular disease (18.79%) | Symptoms of Chest pain (3.36%) and chest tightness (10.74%), increased creatine kinase (8.05%) | [88] |
| 140 | No.7 Hospital of Wuhan | 57 (25–87) | Hypertension (30%), CAD (5%), hyperlipidemia (5%), arrhythmia (3.6%), stroke (2.1%), aorta sclerosis (1.4%) | Symptom of dyspnea/chest tightness (36.7%), increased creatine kinase (6.7%) | [89] |
| 80 | 3 Hospitals in Jiangsu | 46 (31–62) | CVD and cerebrovascular disease (31.25%) | Symptom of chest pain (3.75%) and increased creatine kinase-MB (20%) | [90] |
| 187 | The 7th Hospital of Wuhan | 59 (44–73) | Hypertension (32.6%), coronary heart disease (11.2%), and cardiopathy (4.3%) | Myocardial injury (27.8%), ventricular tachycardia/ fibrillation (5.9%), acute coagulopathy (34.1%) | [24] |
*Acute cardiac injury is defined as the increased of biomarkers of myocardial injury or new abnormalities in electrocardiogram and echocardiogram. **Cardiomyopathy is defined as decreased of left ventricular ejection fraction to clinical symptoms of cardiogenic shock, an increase of myocardial biomarkers, or a decrease of central venous oxygen saturation (<70%) with no past history of contraction dysfunction.
2.1. Myocardial injury
Myocardial injury, characterized by elevated levels of cardiac biomarkers, results from myocardial ischemia and non-ischemic causes including myocarditis [10]. Several studies have noted acute myocardial injury in patients with COVID-19. Among one of the initial 41 cases of COVID-19 in Wuhan, 6 patients (15%) had cardiovascular diseases and hypertension, and 5 (12%) developed acute myocardial injury, which were mainly manifested as follows (1) cardiac biomarkers (hypersensitive cardiac troponin I) > 99th percentile upper reference limit; or (2) new abnormalities in electrocardiogram or echocardiogram [7]. In addition, 4 out of 5 patients with cardiac injury received intensive care unit (ICU) care, indicating the importance of myocardial injury in poor prognosis of COVID-19. In addition to acute myocardial injury, COVID-19 patients admitted to ICU displayed a significantly higher systolic blood pressure compared with those non-ICU patients [7]. In another multi-centered study involving 1099 COVID-19 cases with preexisting anomalies including diabetes (7.4%), hypertension (15%), coronary heart disease (2.5%), and cerebrovascular disease (1.4%), increased level of creatine kinase (≥200 U/L) in patients in severe condition accounted for a much higher percentage than non-severe patients (19.0% versus 12.5%) [11]. In 138 hospitalized COVID-19 patients, acute cardiac injury was observed in 10 patients (7.2%), among which majority of patients required ICU care (80% ICU versus 20% non-ICU, P < 0.001) [6]. In a meta-analysis involving 1527 patients from 6 independent studies, incidences of cardiocerebrovascular disease, hypertension and diabetes were 16.4%, 17.1%, and 9.7%, respectively, in COVID-19 patients [12]. Moreover, prevalence of cardiometabolic diseases was much higher in ICU patients compared with non-ICU patients. This study also revealed presence of acute cardiac injury in >8% patients infected with SARS-CoV-2, of which incidence in ICU cases was approximately 13 folds higher than non-ICU cases [12]. These findings indicated that patients with preexisting cardiovascular diseases are more sensitive to SARS-CoV-2 infection, and patients with COVID-19 combined with cardiovascular diseases might be associated with a higher ICU rate and mortality.
One burning issue remains uncertain is whether SARS-CoV-2 would lead to long-term damage in cardiovascular system. However, a 12-year follow-up of 25 recovered SARS patients suggested that patients had several cardiovascular and metabolic disorders, including cardiovascular abnormalities (44%), hyperlipidemia (68%), and abnormal glucose metabolism (60%) [13]. The precise mechanism of SARS-induced disturbed metabolism of glucose and lipid still remains elusive. During a 10-year follow-up of 591 patients with pneumonia, 206 (34.9%) had cardiovascular events including life-threatening coronary heart disease, myocardial infarction and stroke [14]. Hospitalization for pneumonia is deemed closely related to risks of short-term and long-term cardiovascular diseases. Furthermore, the administration of corticosteroids in severe pneumonia patients to avoid immunopathological lung injury increases overall adverse cardiovascular disease sequelae [13], [15]. Given that SARS-CoV shares similar structure and genomic identity with SARS-CoV-2, patients with COVID-19 should be expected to develop chronic cardiovascular damage, thus special attention is needed for the clinical preservation of cardiovascular function.
2.2. Myocarditis and pericarditis
Earlier studies have demonstrated the occurrence of myocarditis in patients with MERS using cardiac magnetic resonance [16]. Limited COVID-19 autopsy cases have revealed substantial interstitial infiltration of proinflammatory mononuclear cells in heart tissues,validating presence of myocardial inflammation and injury with SARS-CoV-2 infection [17]. Recently, Tavazzi and colleagues reported the first case with SARS-CoV-2 viral particles in the heart, and cardiomyocyte necrosis using endomyocardial biopsy. These data suggested that heart can be directly infected with SARS-CoV-2 [18]. Several cases of myocarditis were reported after SARS-CoV-2 infection [19], [20]. In a 53-year-old COVID-19 patient admitted to ICU for systolic dysfunction, myocarditis was confirmed as evidenced by (1) increased levels of N-terminal pro-brain natriuretic peptide (NT-proBNP) and cardiac biomarkers (creatine kinase-MB, high-sensitivity troponin T), and (2) diffused biventricular hypokinesis and interstitial edema, and circumferential pericardial effusion using cardiac magnetic resonance imaging [19]. In an analysis of 68 fatal cases with COVID-19, 5 patients (7%) were found with fatal fulminant myocarditis in combination with circulatory failure, and 22 fatalities (33%) were attributed to both myocarditis and respiratory failure [21]. Unfortunately, specifics of incidence rate of myocarditis in COVID-19 patients have not been reported in any large-scale studies. The occurrence of myocarditis in COVID-19 patients may be attributable to direct localization of SARS-CoV-2 in myocardium and systemic inflammatory response.
2.3. Arrhythmia and cardiac arrest
According to a cohort of 137 COVID-19 cases in Hubei province, 10 patients (7.3%) presented heart palpitations as early symptom [22]. Among 138 hospitalized patients with COVID-19, 16.7% developed cardiac arrhythmia, with much more prevalent cases in ICU (44.4% in ICU versus 6.9% in non-ICU, P < 0.001) [6]. Du and colleagues reported that arrhythmia occurred in 51 of 85 fatal cases of COVID-19 from Wuhan, and 2 patients died of malignant arrhythmias [23]. However, none of these studies were able to discern the specific nature of arrhythmias in COVID-19. In another study involving 187 patients confirmed with COVID-19 infection, malignant life-threatening ventricular arrhythmias such as ventricular tachycardia and ventricular fibrillation, were noted in 11 patients (5.9%) [24]. Moreover, patients with elevated troponin T experienced higher risk of ventricular arrhythmias (17.3% in high troponin T group versus 1.5% in normal troponin T group, P < 0.001). In addition to acquired arrhythmia, patients with inherited arrhythmia syndromes, including long and short QT syndrome, Brugada syndrome, and catecholaminergic polymorphic ventricular tachycardia, are believed to be more susceptible to pro-arrhythmic effects of SARS-CoV-2 such as stress, fever, use of antiviral drugs and electrolyte disturbance [25].
Cardiac arrest triggered sudden death appears to be a common cause of death of patients with COVID-19. In 85 fatal cases of COVID-19, cardiac arrest is the direct cause of death of 7 patients [23]. In a recent study including 99 cases of COVID-19, the first fatality case was a 61-year-old man who developed heart failure, respiratory failure and sudden cardiac arrest [26]. Some critically ill COVID-19 patients developed fatal cardiac arrest on transplantation or immediately upon admission to ICU [27]. Survival of severe COVID-19 patients who underwent an in-hospital cardiac arrest is generally considered rather poor [28]. Nonetheless, no direct evidence for cardiac arrest is present as a complication of COVID-19.
2.4. Cardiomyopathy and heart failure
Several studies have noted the occurrence of cardiomyopathy in patients with COVID-19. Among 21 critically ill patients with COVID-19, cardiomyopathy developed in 7 (33.3%) patients [29]. Meanwhile, in a single-centered observational study, 8 of 187 patients with confirmed COVID-19 had preexisting cardiomyopathy although little follow evaluation was performed on the COVID-19 outcome in these patients [24]. It is noteworthy that a number of medications employed in COVID-19 may also lead to cardiomyopathy, including chloroquine, interferon, and bevacizumab [30]. Heart failure is a common complication of COVID-19, due to deterioration of preexisting cardiac dysfunction and newly developed cardiomyopathy and myocarditis. In a multi-centered cohort study involving 191 COVID-19 patients, heart failure was noted in 23% of patients, and more prevalent in non-survivor patients compared with survivors (52% versus 12%, P < 0.0001) [31]. Heart failure is characterized by decreased left ventricular ejection fraction and drastically elevated NT-proBNP. Guo and colleagues reported patients with elevated troponin T have a higher level of cardiac biomarkers and NT-proBNP [24]. Moreover, a tight correlation was identified between NT-proBNP and troponin T levels, indicating that patients with myocardial injury are at higher risks of cardiac dysfunction or heart failure [24]. Although COVID-19 patients often display comorbidities affecting cardiac diastolic function including diabetes, obesity and hypertension, few studies have revealed a relationship between heart failure with preserved ejection fraction (HFpEF) and COVID-19. Sinkey and colleagues reported that HFpEF was developed in a postpartum patient with COVID-19 and preeclampsia [32]. Notably, loss of angiotensin-converting enzyme 2 (ACE2), the receptor for SARS-CoV-2, increases the proinflammatory macrophage phenotype in the heart from patients with HFpEF [33]. Further study is warranted to explore the precise interplay between SARS-CoV-2 and HFpEF. Heart failure in COVID-19 patients is attributable to myocardial injury, systemic inflammatory response, pulmonary hypertension and ARDS, renal dysfunction, retention of water and sodium, and imbalance of myocardial oxygen demand and supply.
2.5. Cardiogenic shock
Although little direct evidence is readily available for the incidence rate of cardiogenic shock in patients infected with SARS-CoV-2, cardiogenic shock was demonstrated a severe complication of COVID-19. In a 69-year-old patient with confirmed COVID-19, elevated inflammatory markers and increased hypersensitive troponin I were noted, prior to the development of severe cardiogenic shock [18]. Cardiogenic shock may be mixed with other types of shock following SARS-CoV-2 infection, such as septic shock. In a study involving 138 cases with COVID-19, shock was confirmed in 8.7% of patients, and was more common in patients admitted to ICU compared with those non-ICU patients (30.6% versus 1.0%, P < 0.001) [6]. However, subtypes of shock were not reported in this study. Notably, circulatory and respiratory support with extracorporeal membranous oxygenation (ECMO) should be considered in COVID-19 patients with cardiogenic shock.
2.6. Coagulation abnormalities
Abnormal coagulation parameters (D-dimer, fibrin degradation products, prothrombin time, and activated partial thromboplastin time) were noted in patients with COVID-19. In particular, elevated levels of D-dimer and fibrin degradation products were suggested to be closely linked with poor prognosis [31], [34]. In a multi-centered retrospective cohort study, an elevated level of D-dimer (>1 g/L) was tightly tied with in-hospital mortality of COVID-19, even in multivariate analysis [31]. Thromboembolic anomalies and coagulopathy, including venous thromboembolism, pulmonary embolism and disseminated intravascular coagulation (DIC), are believed to be highly prevalent in COVID-19 patients. For example, a mass of pulmonary embolism was noted in COVID-19 patients, and the prevalence of pulmonary embolism was twice higher in ICU COVID-19 as all ICU or influenza ICU patients [35], [36]. Another independent report noted 71.4% incidence of disseminated intravascular coagulation (DIC) in non-survivors accompanied with coagulation abnormalities in terminal COVID-19 cases [31]. High prevalence of coagulation abnormalities in COVID-19 may be attributable to vascular inflammation and endothelial defect, as SARS-CoV-2 can directly attack endothelial cells expressing high levels of ACE2. In addition, SARS-CoV-2 virus has been noted within endothelial cells and infiltration of proinflammatory cells, contributing to the onset and development of endothelial dysfunction and defective coagulation [37]. At this point, optimal thromboembolic prophylactic therapy has not been well established for COVID-19 patients. However, interactions between antiviral drugs for SARS-CoV-2 and antiplatelet agents and anticoagulants should be considered [38].
3. Possible mechanisms of action under COVID-19-associated cardiovascular anomalies
3.1. ACE2
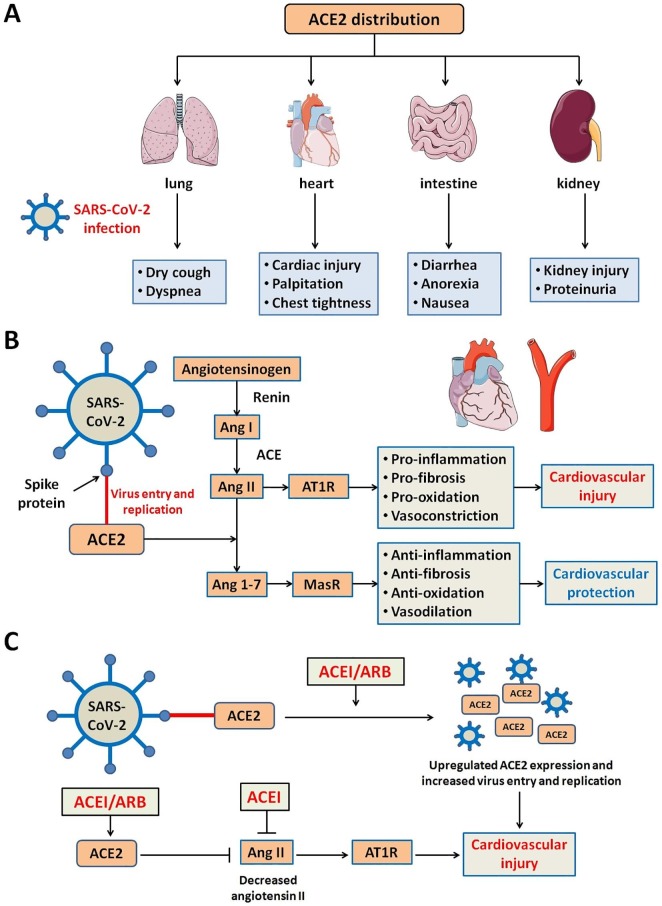
Ample evidence has suggested that ACE2 functions as a target receptor for SARS-CoV-2. ACE2 is known to be a membrane-bound aminopeptidase mainly in hearts, lungs, intestines and kidneys [39]. Organ distribution of ACE2 seems to be closely related to the clinical sequelae of COVID-19 (Fig. 1 ). It is noteworthy that SARS-CoV-2 possesses a 10-fold greater affinity for ACE2 than that of SARS-CoV, making it a much more potent virus. ACE2 is distinct from angiotensin-converting enzyme (ACE) in that it lacks cleavage for dipeptidases, but only single peptidases, and is not subject to inhibition by ACE inhibitors. ACE2 level was upregulated in diabetes mellitus and cardiovascular diseases, including heart failure and ischemic cardiomyopathy [40], [41], [42]. Given that SARS-CoV-2 is a substrate for ACE2, patients with preexisting cardiovascular diseases with elevated ACE2 levels are thus more susceptible to SARS-CoV-2 and presented a poor prognosis. ACE2 is reported to counter angiotensin II (Ang II) from RAAS in cardiovascular diseases. Binding of SARS-CoV-2 to ACE2 prevents the enzyme from converting Ang II to Ang 1–7, potentiating Ang II-induced biological effect to worsen pulmonary and cardiovascular outcomes. Naïve ACE2 is known to offer an array of cardiovascular benefits including anti-inflammation, anti-fibrosis, anti-oxidation, and vasodilation [43]. This is supported by the findings that ACE2 knockout provoked Ang II accumulation and compromised cardiac contractile function [44]. Murine models and human autopsy samples revealed that pulmonary infection of SARS-CoV leads to downregulated cardiac and pulmonary ACE2 signaling, favoring proinflammatory response and acute respiratory failure [45]. In this context, overt cardiovascular injuries in COVID-19 patients may also result from loss of ACE2-mediated cardiovascular protection (Fig. 1). Meanwhile, approaches targeting ACE2 downstream signaling may help alleviate pulmonary and cardiovascular injury. Ang 1–7 has been shown to protect against cardiac and pulmonary injury through suppressing alveolar cell apoptosis, alleviating alveolar cell activation, and exerting anti-fibrotic, anti-inflammatory, and vasodilatory effects. This is consistent with its utility in a clinical trial on ARDS patients [42], [46], [47], [48]. Thus, these favorable effects of Ang 1–7 should demonstrate the therapeutic potential to counter organ pathologies in patients infected with SARS-CoV-2. Increment of Ang 1–7 levels may be of significant clinical value in the prevention against cardiovascular and lung injury in the face of SARS-CoV-2 infection [48].
Fig. 1.
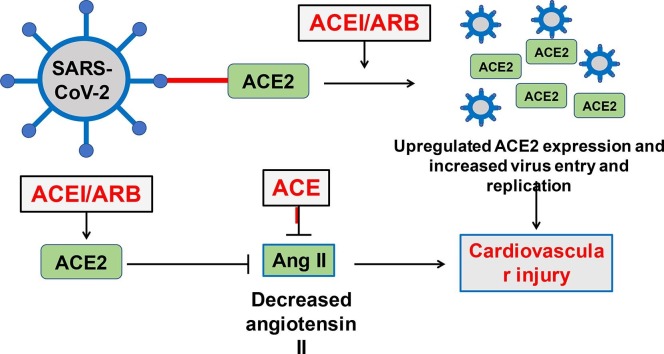
Relationship between ACE2 and SARS-CoV-2-related cardiovascular injury. (A) Organ distribution of ACE2 may be associated with clinical symptoms of COVID-19 patients. (B) Potential mechanism of cardiovascular injury induced by ACE2-mediated SARS-CoV-2 infection. SARS-CoV-2 uses ACE2 receptor for viral entry and replication. ACE2, but not ACE, is downregulated through binding of the spike protein of SARS-CoV-2 and ACE2. This leads to an increased level of Ang II and subsequent cardiovascular injury. (C) Impact of RAAS Blockers (ACEI and ARB) on cardiovascular system of COVID-19 patients. On the one hand, RAAS blockers upregulate the expression of ACE2, thereby leading to increased viral entry and replication and cardiovascular injury. On the other hand, RAAS blockers contribute to Ang II inhibition directly or indirectly (caused by upregulated ACE2), which may attenuate cardiovascular injury. AT1R, Ang II type 1 receptor; MasR, mitochondrial assembly receptor.
The following scheme is believed the modality for viral entry and replication: The spike glycoprotein of SARS-CoV and SARS-CoV-2 recognizes ACE2 on cell surface and binds with ACE2, to allow viral entry into cells to release viral particles. Viral RNA is translated using the host ribosomes. Viral proteins are then packaged in the Golgi apparatus and rough endoplasmic reticulum, before release of virus. Here recognition and binding of spike protein and ACE2 are considered the most critical process for viral entry and replication, and may be facilitated and interrupted by ACE2 and ACE2 neutralizing antibody, respectively [49]. A number of maneuvers are speculated to counter ACE2-mediated multi-organ dysfunction including cardiovascular complications in the face of SARS-CoV-2 infection [39], including (1) spike glycoprotein-based vaccine; (2) ACE2 receptor blockade; (3) delivering excessive soluble ACE2 to neutralize SARS-CoV-2 virus; and (4) suppression of transmembrane protease serine 2, among which spike protein priming seems crucial for interaction with ACE2 [50].
Administration of renin-angiotensin-aldosterone system (RAAS) inhibitors including ACE inhibitors and angiotensin receptor blocker (ARB) are known to upregulate ACE2, which is expected to promote SARS-CoV-2 entry and aggravation of lung and cardiovascular injury in COVID-19 patients. Nonetheless, other studies suggested that RAAS inhibitors may rather enhance the pulmonary protective role of ACE2 and alleviate inflammatory response and cytokine release in SARS-CoV-2 or other viral infection [51], [52], [53]. As for cardiovascular system, RAAS inhibitors may benefit cardiovascular function through direct inhibition of Ang II production or indirect inhibition of Ang II through upregulation of ACE2 (Fig. 1). In a multi-centered study involving 1128 COVID-19 patients combined with hypertension, administration of ACE inhibitors or ARBs was associated with a lower mortality rate (3.7% in ACEI/ARB groups versus 9.8% in non-ACEI/ARB group, P = 0.01) [54]. In another study involving 362 patients with hypertension hospitalized for COVID-19, there was no difference in severe infections and mortality rate during hospitalization between patients treated with and without ACE inhibitors or ARBs [55]. According to the statement of the European Society of Hypertension, treatment with ACE inhibitors and ARB should be encouraged in patients with stable COVID-19 or at risk for SARS-CoV-2 infection [56]. Despite that, it remains controversial whether RAAS inhibitors should be administrated to COVID-19 patients with existing cardiovascular diseases.
3.2. Cytokine storm
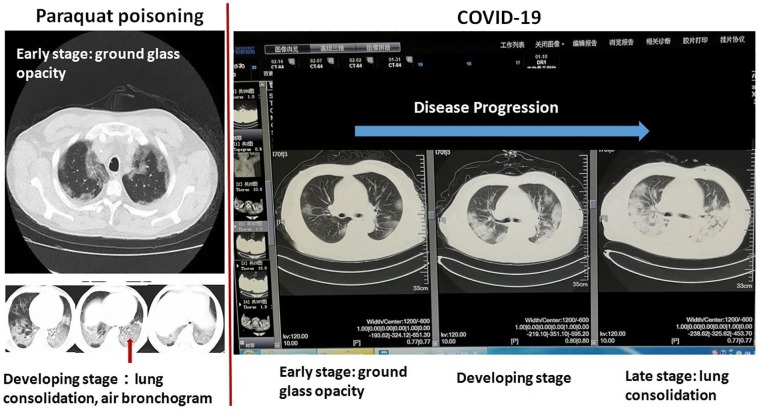
Clinical observation noted that COVID-19 patients exhibit signs of overt cytokine storm (profound immune and inflammatory responses), reminiscent of those seen in pesticide paraquat toxicity ( Fig. 2 ). COVID-19 patients with cytokine storm are likely to develop multiple organ failure (MOF) and sudden death, which greatly worsen the overall survival in COVID-19 patients [57]. Cytokine storm syndrome (as often seen in paraquat toxicity) denotes a severe life-threatening condition manifested by a sharp rise in proinflammatory cytokines, overwhelming inflammation, hyperferritinemia, hemodynamic instability, and MOF, and is potentially fatal if untreated [57]. The hallmark of cytokine storm is an uncontrolled and dysfunctional immune response involving continued activation of lymphocytes, macrophages, and natural killer cells [58]. These cells release abundant pro-inflammatory cytokines including interferon (IFN)-γ, tumor necrosis factor (TNF)-α, interleukin (IL)-1, IL-6, IL-18, IL-7 and IL-10, granulocyte-colony stimulating factor, IFN-γ inducible protein 10 (IP-10), monocyte chemoattractant protein 1 (MCP-1), macrophage inflammatory protein 1-α, which cheat more immune cells to form a positive feedback cycle causing a cytokine storm. Cytokine storm has attracted more attention as it directly correlates with COVID-19 mortality. In the severe stage of the disease, patients with COVID-19 mostly develop ARDS, MOF, with heavy involvement of cytokine storms [57].
Fig. 2.
Resemblance in lung injury between paraquat poisoning and COVID-19 infection. In both cases, there is gradual ground glass opacity slowly progressed into the advanced stages of lung tissue consolidation (solidifying process). Imaged taken from a COVID-19 patient in Wuhan, courtesy of Dr. Hu Peng, ICU physician in Wuhan. The COVID-19 patient received written consent and was recovered from COVID-19 later. Classical paraquat image was from China-Radiology https://mp.weixin.qq.com/s/MMSq1ufNkWIUyAKEPcvxQQ.
In terms of proinflammatory mediators, SARS-CoV-2 shows remarkable similarities to SARS-CoV. An outbreak of SARS-CoV in 2003 revealed markedly high INF-γ, IL-1β, IL-6, IL-10, and IL-12. Polymorphonuclear neutrophil (PMN) chemokines IL-8, MCP-1, and IP-10 were also elevated [59]. Patients infected with SARS-CoV-2 showed similar elevations of IFN-γ, IL-1β, IP-10 and MCP-1 [7]. INF-γ from natural killer cells assists mature dendritic cells, to release more interferon, and macrophage activation. IL-1β is an “early response cytokine” generated by inflammasomes to provoke B and T cell proliferation, epithelial cell activation and vascular leakage [58], [59]. IL-6 contributes to pulmonary inflammation and fever, in addition to its known effect in cardiac remodeling and injury [60]. Under cytokine storm, reactive oxygen species (ROS) accumulate to trigger apoptosis of cells within the infected area, as well as degradation of extra-cellular matrices. ROS production in the face of cytokine storm moves the immune response from a protective to a pre-injury state [61]. IL-10, produced by Th2 cells, serves as a negative feedback cue to counteract secretion of pro-inflammatory cytokines. It was noted that SARS-CoV-2 infection did upregulate Th2 cells as well, which improves overall cytokine balance and mitigates hyperinflammatory state induced by cytokine storm [7]. When the balance of pro-inflammatory and anti-inflammatory mediators is thrown off, immune response can become harmful [58]. Patients with cytokine storm present high fever, enlarged spleen (an accessory lymphoid organ), excessive bleeding and anemia due to vascular malfunction. Due to tissue destruction and inadequate blood flow, patients start to develop organ failure. The aforementioned cytokines and inflammatory mediators are also capable of activating capillary endothelial cells, thus rising capillary permeability for cellular migration. Although such mechanism is meant to deliver immune cells quickly to the site of infection, it also provokes fluid buildup within lungs resulting in poor oxygen transport and hypoxemia, as seen in SARS-CoV-2 infection [16]. Recently, a newly discovered multisystem inflammatory syndrome was observed in a 14-year-old teenager with COVID-19, showing resemblance to Kawasaki disease, an inflammatory disease in infants and toddlers [62]. The syndrome mainly impairs the cardiovascular system and manifests as severe heart failure and cardiogenic shock, accompanied with extracardiovascular symptoms including fever, lymphadenectasis, rash on hands and feet, and stomachache [62]. The pathophysiology of the syndrome cannot be comprehensively explained currently, but steroids administrated to the patient seem to be effective. Further study should be conducted to figure out this inflammatory syndrome.
It is noteworthy that increased levels of Ang II caused by SARS-CoV-2 infection may play a role in the immune response and inflammatory damage in COVID-19 patients. While classical RAAS is responsible for the maintenance of blood pressure and hemostasis, immune cells possess several Ang II-related intracellular actions in parallel with RAAS [63]. (1) Ang II activates the proinflammatory mediator NF-κB, which promotes monocytes to produce chemoattractant proteins such as MCP-1, IL-6 and TNF-α, for immune cell recruitment, and initiation of cytokine storm in COVID-19 patients [7], [63], [64]. (2) Ang II may stimulate production of adhesion molecules such as VCAM-1 and ICAM-1, to recruit immune cells including dendritic cells and T lymphocytes [65]. Upon binding with Ang II, dendritic cells (with both Ang II receptors) exhibit high levels of maturation and migration [63]. (3) Ang II causes profound ROS production, serving as proinflammatory mediators to provoke damage of surrounding tissues, endothelial activation, vascular leakage and immune cell recruitment [63]. Vascular damage from oxidative stress is well perceived in atherosclerosis, hypertension and other cardiovascular pathologies [64]. Worsening oxidative damage to the vasculature is a major contributor for unfavorable cardiovascular outcomes in COVID-19 patients. Ang II-evoked oxidative damage, proinflammatory stimulation, and immune cell recruitment collectively underscore the global pathological outcomes for SARS-CoV-2, encompassing stroke, cardiac, pulmonary, vascular and kidney injuries [7], [63], [64], [66]. Such scenario may likely explain towards why patients with preexisting pathologies involving RAAS system such as hypertension, chronic heart failure and diabetes mellitus fair worse outcomes from COVID-19 insults [7], [29], [66]. It is possible that these patients may be hit much harder by the COVID-19 virus due to an upregulation of ACE2 receptors (which would ease viral entry) and preexisting systemic inflammation and cardiovascular dysregulation by excitement of the parallel RAAS signaling within immune cells.
3.3. Hypoxemia
Due to inflammation and lung injury, SARS and MERS patients can develop hypoxemia, or low circulating oxygen levels [6], [16], [30]. As delineated earlier, an acute attack on respiratory system provokes damage within vasculature and tissues. Tissue breakdown and vascular leakage dampen the ability of heart and lungs to perfuse properly, leading to hypoxemia, dyspnea or shortness of breath. All of these events contribute to myocardial defect, including arrhythmia and shock [6]. MERS patients presented pneumonia accompanied by shortness of breath and left sided chest pain. Further diagnostics revealed myocardial edema and acute myocardial injury due to viral infection rather than ischemic injury [16]. According to a study involving 41 patients confirmed with COVID-19, 32% of patients developed various degree of hypoxemia and required oxygen therapy [7]. Due to severe pulmonary damage, hypoxemia is believed to cause the reduced energy supply of cardiomyocyte, leading to intracellular acidosis and ROS to destroy the cell membrane [12]. In addition, influx of calcium ions can be induced by hypoxemia and cause apoptosis and injury of cardiomyocytes [12]. Although not all hypoxemic patients will require intense therapy such as ventilation, a burning concern for many health organizations is how to properly manage a large number of severely hypoxemic SARS-CoV-2 patients with only limited ventilators [67]. Of course, an alternate option for the treatment of hypoxemia is ECMO. At the University of Minnesota Medical Center, a SARS-CoV-2 patient arrived with profound signs of dyspnea and severe hypoxemia, and was successfully treated with 12 days of ECMO followed by decannulation [68]. Likewise, ECMO was successfully applied in many SARS-CoV-2 cases in China although more in depth scrutiny is warranted to better fine the use of ECMO in the treatment of ARDS from SARS-CoV-2.
3.4. Drug-induced cardiovascular toxicity
Cardiovascular toxicities of several anti-SARS-CoV-2 drugs are listed in Table 2 . At this point, antiviral drug-induced cardiovascular toxicity in the COVID-19 treatment should not be ignored. Antiviral drugs including IFN-α, ribavirin, chloroquine phosphate, lopinavir/ritonavir, arbidol and remdesivir have all been included in the treatment of COVID-19 [69]. Several antiviral drugs exert cardiotoxicity or elicit interactions with other cardiovascular medications. For instance, lopinavir/ritonavir may lead to a prolongation of PR and QT intervals and influence serum levels of antiplatelet drugs through CYP3A4 inhibition [30], [70]. Remdesivir, previously administrated to patients with Ebola viral infection, is used clinically in COVID-19 patients. During Ebola outbreak, one patient (among a total of 175 patients) administrated with loading dose of remdesivir developed severe hypotension and sudden cardiac arrest [71]. In systemic lupus erythematosus and rheumatoid arthritis therapy, cardiotoxity including cardiac arrhythmias, dilated or restrictive cardiomyopathy, decreased myocardial function, vasodilation, and hypotension is often noted with frequent administration of chloroquine [72], [73]. In addition, chloroquine affects beta-receptor blockers through inhibition of CYP2D6 [30]. Therefore, blood pressure and heart rate must be closely monitored when co-administration of β-blockers and chloroquine in COVID-19 patients.
Table 2.
Mechanisms, cardiovascular adverse effects, advantages and disadvantages of several medications used in SARS-CoV-2 infection.
| Medication | MOA | Effect on SARS-CoV-2 | CV toxicity | Advantages | Disadvantages | Ref |
|---|---|---|---|---|---|---|
| Remdesivir | RDRP inhibitor | Reduces symptoms in SARS-CoV-2 patients, inhibits SARS-CoV-2 infection in vitro | Unknown | Established safety profile, resistant to nsp14-ExoN | Causes viral resistance, and must be injected | [17], [77] |
| Chloroquine/Hydroxychloroquine | Raises endosomal pH and anti-inflammation | Blocks SARS-CoV-2 from early endosomes to endolysosomes, blocks glycosylation of ACE2 | Myocardial toxicity, QT prolongation, altered cardiac conductivity | High oral bioavailability, concentrates in lungs | concentrates in the liver, spleen and kidney and efficacy has been debated | [17], [79], [80] |
| Nitazoxanide | Blocks pyruvate ferredoxin oxidoreductase in anaerobes | Inhibits growth of SARS-CoV-2 and cytokine production from PMNs and IL-6 production | Unknown | parent drug and metabolite are active, can be given orally | Expensive, and safety profile is less understood | [17] |
| Lopinavir/Ritonavir | Protease inhibitor/CYP450 inhibitor | Shortens median hospital stay in SARS-CoV-2 infected patients | QT interval prolongation, and high degree atrioventricular block | Well studied, oral route | Does not reduce SARS-CoV-2 mortality in recent studies | [78], [84] |
| Ribavirin | RDRP inhibitor | Inhibits in vitro growth of SARS-CoV-2 at high concentrations | Unknown | Low cost and well-studied | worsens in some patients outcomes and efficacy is debated | [17], [76] |
| Convalescent plasma | Performs neutralizing immunoglobulin targeting SARS-CoV-2 | Lowers viral load and lead to quick improvement of symptoms in critically ill COVID-19 patients | Unknown | Immunomodulatory effects: potentially mitigate cytokine storm | Ineffective in MERS-CoV prophylaxis and must be injected | [87] |
| Corticosteroids | Reduces inflammatory mediators | combat the damage from cytokine storm, reducing lung injury | Immunosuppression, cardiovascular and metabolic disorders | Useful in later stages of infection | Must be injected, raises mortality and adverse effect risk if used inappropriately | [5], [78] |
| Ammonium chloride | Raises endosomal pH | Blocks glycosylation of ACE2 receptors and inhibited viral growth in vitro | Ammonia toxicity can lead to bradyarrhythmia | Cheap, few apparent drug interactions | Not well studied and uncommonly used in humans | [17] |
| ACEI/ARB | Reduces Ang II effect and prevents vasoconstriction | Upregulates ACE2 and prevents overproduction of Ang II, reduces cardiac and lung injury | Hypotension | Well studied, low side effect profile, cheap | not directly target virus and ACE2 upregulation provides virus with more sites to attack | [53] |
| Tocilizumab | Inhibits IL-6 receptor | combat the damage from cytokine storm | Hypertension, and increased serumcholesterol | Inhibit IL-6 and mitigate cytokine storm | Expensive and does not target SARS-CoV-2 | [30] |
3.5. Other possible mechanisms
Other than aforementioned mainstream mechanisms for COVID-19-induced defects in cardiovascular system, a number of additional scenarios should not be underestimated. For example, psychological stress is deemed a possible contributing factor that SARS-CoV-2 may lead to cardiovascular damage. SARS-CoV-2 infection, especially those with severe infection, is obviously an acute stress for patients. With SARS-CoV-2 infection, stress contributes to the activation of autonomic nervous system, increases in blood pressure and heart rate, disorders in thrombus, and coronary vasoconstriction [74], [75]. Moreover, stress may promote platelet aggregation, compromise vascular endothelial function and promote the risk of ischemia and thrombosis [74]. To this end, COVID-19 patients undergoing psychological stress process are at a higher risk of cardiovascular diseases including hypertension, cardiac arrhythmias, and myocardial ischemia/infarction.
4. Therapeutic options and considerations
Table 2 demonstrates several medications used in SARS-CoV-2 infection. SARS-CoV-2 has proven to be in the same lineage of coronavirus as SARS-CoV and MERS-CoV, with 80% genetic compatibility to SARS-CoV [76], [77]. The binding domain of the SARS-CoV-2 protein spike with ACE2 is distinct from that of the SARS-CoV protein spike, even though the spikes themselves have 76% compatibility. Medications targeting such protein spike may not share the same efficacy against both viruses [78]. On the other hand, RNA-dependent RNA polymerase proteins (RDRP) of SARS-CoV-2 have 96% compatibility with those of SARS-CoV [78]. Medications which successfully targeted this polymerase in SARS-CoV are likely effective for SARS-CoV-2. Remdesivir was one of these medications and showed some promises (although inconsistently) to inhibit SARS-CoV-2 infection in vitro, and is suspected to manage COVID-19 symptoms [17], [77]. Ribavirin, another RDRP inhibitor, also displays efficacy against SARS-CoV-2, although it is limited by an intrinsic viral protein; nsp14-ExoN, which can cleave the drug out of the RNA chain prior to reaching the RDRP [78].
An alternative to these antiviral drugs is chloroquine or hydroxychloroquine, common anti-malarial agent that blocks viral entry through endosomal modifications, modulation of inflammatory mediators, and alterations to ACE2 [79]. Nonetheless, conflicting data have seen with regards to the efficacy of these medications in the treatment of SARS-CoV-2, in addition to valid concerns on chloroquine toxicity [80], [81]. One other therapy that may have a similar mechanism of action is ammonium chloride, an acidotic agent which inhibits SARS-CoV viral growth in vitro. This medication also exhibited alterations to the ACE2 receptor, potentially reducing viral ability to bind [79]. Ammonium chloride has limited uses in clinical treatment and is poorly examined, making it a major concern for treatment in vulnerable patients [81].
Other options which are being explored this time include lopinavir/ritonavir, which act synergistically via HIV protease inhibition and metabolic inhibition to lengthen drug half-life [81]. While the mechanism in SARS-CoV-2 is not clear, this medication may reduce viral titers and lower risk of death based on previous MERS-CoV and SARS-CoV studies [82], [83]. Use in SARS-CoV-2 patients has had unconvincing results, showing no mortality benefit and minimal symptom improvement [84]. Nitazoxanide, an enzyme inhibitor utilized in anaerobic infections, inhibited viral growth of SARS-CoV-2 in vitro and may be a viable candidate [17], [81]. More research is being conducted on this therapy [18].
Adjunctive treatments that may benefit SARS-CoV-2 patients include IL-1β, which has shown in vitro efficacy in MERS-CoV along with lopinavir/ritonavir [82]. Risks may outweigh benefits as both thrombocytopenia and worsened patient outcomes have been shown as a result of this medication [82]. In severely ill patients, corticosteroids have shown mixed benefits and risks [5], [85]. If used appropriately, corticosteroids could help reduce damage from the cytokine storm in severely ill patients [85]. Caution should be used with these medications as adverse effects can occur often [85]. A more promising therapy involves upregulation of the ACE2 receptor through the use of Ang II receptor blockers such as losartan [86]. This medication may potentiate the pulmonary protective effects of ACE2 by preventing excess production of Ang II. When produced at high levels, these molecules can lead to severe vasoconstriction and activation of endothelial cells, potentiating the lung damage seen in SARS-CoV-2 [53], [86]. A last resort, which has shown promise in SARS-CoV-2 patients is convalescent plasma taken from recovered patients. This plasma includes neutralizing antibodies which can target and help take down the active virus in a new patient. Though data is limited, potential mortality and symptom benefits have been shown in recent SARS-CoV-2 patients [25], [87].
5. Conclusions
The COVID-19 pandemic has impacted millions of patients and posed a tremendous threat to human health. Cardiovascular comorbidities, including pre-existing cardiovascular diseases and new-onset cardiovascular abnormalities, are prevalent in patients with SARS-CoV-2 infection, and these patients are at a higher risk of severe disease and mortality. COVID-19 is closely associated to a series of cardiovascular sequelae, including acute and chronic myocardial injury, myopericarditis, arrhythmia, cardiac arrest, cardiomyopathy, heart failure, and cardiogenic shock. These represent possible mechanisms underscoring the SARS-CoV-2-induced cardiovascular diseases. Further understanding of interactions among ACE2 protein, RAAS inhibitors and SARS-CoV-2 should be of great significance for patients with cardiovascular diseases and COVID-19. Besides, cytokine storm syndrome and immune dysfunction are also important causes of multiple organ failure (including cardiovascular dysfunction) and critical condition of patients with COVID-19. A number of promising antiviral drugs and vaccines are under investigation, but none has been proved to be clinical efficient to date. Clinical physicians should pay attention to the cardiovascular toxicity of medications used in COVID-19 patients. In addition, the therapeutic challenges posed by coexist of COVID-19 and cardiovascular diseases need to be adequately studied.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors appreciate the ICU unit from the Wuhan Third Hospital for their support when HP worked as an ICU physician during Feb – April 2020. The human subject protocol was approved by the Tenth Hospital from Tongji University (Shanghai).
References
- 1.Wu J.T., Leung K., Leung G.M. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: a modelling study. Lancet. 2020;395(10225):689–697. doi: 10.1016/S0140-6736(20)30260-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N., Bi Y., Ma X., Zhan F., Wang L., Hu T., Zhou H., Hu Z., Zhou W., Zhao L., Chen J., Meng Y., Wang J., Lin Y., Yuan J., Xie Z., Ma J., Liu W.J., Wang D., Xu W., Holmes E.C., Gao G.F., Wu G., Chen W., Shi W., Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yin Y., Wunderink R.G. MERS, SARS and other coronaviruses as causes of pneumonia. Respirology. 2018;23(2):130–137. doi: 10.1111/resp.13196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang S., Diao M., Yu W., Pei L., Lin Z., Chen D. Estimation of the reproductive number of novel coronavirus (COVID-19) and the probable outbreak size on the Diamond Princess cruise ship: a data-driven analysis. Int. J. Infect. Dis. 2020;93:201–204. doi: 10.1016/j.ijid.2020.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Russell C.D., Millar J.E., Baillie J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395(10223):473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Dawei, Hu Bo, Hu Chang, Zhu Fangfang, Liu Xing, Zhang Jing, Wang Binbin, Xiang Hui, Cheng Zhenshun, Xiong Yong, Zhao Yan, Li Yirong, Wang Xinghuan, Peng Zhiyong. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061. doi: 10.1001/jama.2020.1585. https://jamanetwork.com/journals/jama/fullarticle/2761044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu C.M., Wong R.S., Wu E.B., Kong S.L., Wong J., Yip G.W., Soo Y.O., Chiu M.L., Chan Y.S., Hui D., Lee N., Wu A., Leung C.B., Sung J.J. Cardiovascular complications of severe acute respiratory syndrome. Postgrad. Med. J. 2006;82(964):140–144. doi: 10.1136/pgmj.2005.037515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Badawi A., Ryoo S.G. Prevalence of comorbidities in the Middle East respiratory syndrome coronavirus (MERS-CoV): a systematic review and meta-analysis. Int. J. Infect. Dis. 2016;49:129–133. doi: 10.1016/j.ijid.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sarkisian L., Saaby L., Poulsen T.S., Gerke O., Jangaard N., Hosbond S., Diederichsen A.C., Thygesen K., Mickley H. Clinical characteristics and outcomes of patients with myocardial infarction, myocardial injury, and nonelevated troponins. Am. J. Med. 2016;129(4) doi: 10.1016/j.amjmed.2015.11.006. 446 e5-446 e21. [DOI] [PubMed] [Google Scholar]
- 11.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., Liu L., Shan H., Lei C.L., Hui D.S.C., Du B., Li L.J., Zeng G., Yuen K.Y., Chen R.C., Tang C.L., Wang T., Chen P.Y., Xiang J., Li S.Y., Wang J.L., Liang Z.J., Peng Y.X., Wei L., Liu Y., Hu Y.H., Peng P., Wang J.M., Liu J.Y., Chen Z., Li G., Zheng Z.J., Qiu S.Q., Luo J., Ye C.J., Zhu S.Y., Zhong N.S. C. China Medical Treatment Expert Group for, Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li B., Yang J., Zhao F., Zhi L., Wang X., Liu L., Bi Z., Zhao Y. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin. Res. Cardiol. 2020 doi: 10.1007/s00392-020-01626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu Q., Zhou L., Sun X., Yan Z., Hu C., Wu J., Xu L., Li X., Liu H., Yin P., Li K., Zhao J., Li Y., Wang X., Li Y., Zhang Q., Xu G., Chen H. Altered Lipid metabolism in recovered SARS patients twelve years after infection. Sci. Rep. 2017;7(1):9110. doi: 10.1038/s41598-017-09536-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corrales-Medina V.F., Alvarez K.N., Weissfeld L.A., Angus D.C., Chirinos J.A., Chang C.C.H., Newman A., Loehr L., Folsom A.R., Elkind M.S., Lyles M.F., Kronmal R.A., Yende S. Association Between hospitalization for pneumonia and subsequent risk of cardiovascular disease (vol 313, pg 264, 2015) Jama-J. Ame. Med. Assoc. 2015;313(3) doi: 10.1001/jama.2014.18229. A264-A264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yam L.Y., Lau A.C., Lai F.Y., Shung E., Chan J., Wong V., S.C.G. Hong Kong Hospital Authority Corticosteroid treatment of severe acute respiratory syndrome in Hong Kong. J Infect. 2007;54(1):28–39. doi: 10.1016/j.jinf.2006.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alhogbani T. Acute myocarditis associated with novel Middle East respiratory syndrome coronavirus. Ann. Saudi Med. 2016;36(1):78–80. doi: 10.5144/0256-4947.2016.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tavazzi G., Pellegrini C., Maurelli M., Belliato M., Sciutti F., Bottazzi A., Sepe P.A., Resasco T., Camporotondo R., Bruno R., Baldanti F., Paolucci S., Pelenghi S., Iotti G.A., Mojoli F., Arbustini E. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur. J. Heart Fail. 2020 doi: 10.1002/ejhf.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inciardi R.M., Lupi L., Zaccone G., Italia L., Raffo M., Tomasoni D., Cani D.S., Cerini M., Farina D., Gavazzi E., Maroldi R., Adamo M., Ammirati E., Sinagra G., Lombardi C.M., Metra M. Cardiac Involvement in a patient with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu H., Ma F., Wei X., Fang Y. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. Eur. Heart J. 2020 doi: 10.1093/eurheartj/ehaa190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruan Q.R., Yang K., Wang W.X., Jiang L.Y., Song J.X. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020 doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu K., Fang Y.Y., Deng Y., Liu W., Wang M.F., Ma J.P., Xiao W., Wang Y.N., Zhong M.H., Li C.H., Li G.C., Liu H.G. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin. Med. J. (Engl.) 2020 doi: 10.1097/CM9.0000000000000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Du Y., Tu L., Zhu P., Mu M., Wang R., Yang P., Wang X., Hu C., Ping R., Hu P., Li T., Cao F., Chang C., Hu Q., Jin Y., Xu G. Clinical features of 85 fatal cases of COVID-19 from Wuhan: a retrospective observational study. Am. J. Respir. Crit. Care Med. 2020 doi: 10.1164/rccm.202003-0543OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo T., Fan Y., Chen M., Wu X., Zhang L., He T., Wang H., Wan J., Wang X., Lu Z. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duan K., Liu B., Li C., Zhang H., Yu T., Qu J., Zhou M., Chen L., Meng S., Hu Y., Peng C., Yuan M., Huang J., Wang Z., Yu J., Gao X., Wang D., Yu X., Li L., Zhang J., Wu X., Li B., Xu Y., Chen W., Peng Y., Hu Y., Lin L., Liu X., Huang S., Zhou Z., Zhang L., Wang Y., Zhang Z., Deng K., Xia Z., Gong Q., Zhang W., Zheng X., Liu Y., Yang H., Zhou D., Yu D., Hou J., Shi Z., Chen S., Chen Z., Zhang X., Yang X. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc. Natl. Acad. Sci. U.S.A. 2020 doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., Xia J., Yu T., Zhang X., Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., Wu Y., Zhang L., Yu Z., Fang M., Yu T., Wang Y., Pan S., Zou X., Yuan S., Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respiratory Med. 2020 doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shao F., Xu S., Ma X., Xu Z., Lyu J., Ng M., Cui H., Yu C., Zhang Q., Sun P., Tang Z. In-hospital cardiac arrest outcomes among patients with COVID-19 pneumonia in Wuhan, China. Resuscitation. 2020 doi: 10.1016/j.resuscitation.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arentz M., Yim E., Klaff L., Lokhandwala S., Riedo F.X., Chong M., Lee M. Characteristics and Outcomes of 21 Critically Ill Patients With COVID-19 in Washington State. JAMA. 2020 doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Driggin E., Madhavan M.V., Bikdeli B., Chuich T., Laracy J., Bondi-Zoccai G., Brown T.S., Nigoghossian C., Zidar D.A., Haythe J., Brodie D., Beckman J.A., Kirtane A.J., Stone G.W., Krumholz H.M., Parikh S.A. Cardiovascular considerations for patients, health care workers, and health systems during the coronavirus disease 2019 (COVID-19) pandemic. J. Am. Coll. Cardiol. 2020 doi: 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sinkey R.G., Rajapreyar I., Robbins L.S., Dionne-Odom J., Pogwizd S.M., Casey B.M., Tita A.T.N. Heart failure with preserved ejection fraction in a postpartum patient with superimposed preeclampsia and COVID-19. AJP Rep. 2020;10(2):e165–e168. doi: 10.1055/s-0040-1712926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patel V.B., Shah S., Verma S., Oudit G.Y. Epicardial adipose tissue as a metabolic transducer: role in heart failure and coronary artery disease. Heart Fail. Rev. 2017;22(6):889–902. doi: 10.1007/s10741-017-9644-1. [DOI] [PubMed] [Google Scholar]
- 34.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thrombosis Haemostasis: JTH. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Poissy J., Goutay J., Caplan M., Parmentier E., Duburcq T., Lassalle F., Jeanpierre E., Rauch A., Labreuche J., Susen S., I.C.U.H.C.-g. Lille Pulmonary embolism in COVID-19 patients: awareness of an increased prevalence. Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.047430. [DOI] [PubMed] [Google Scholar]
- 36.Danzi G.B., Loffi M., Galeazzi G., Gherbesi E. Acute pulmonary embolism and COVID-19 pneumonia: a random association? Eur. Heart J. 2020;41(19):1858. doi: 10.1093/eurheartj/ehaa254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., Mehra M.R., Schuepbach R.A., Ruschitzka F., Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bikdeli B., Madhavan M.V., Jimenez D., Chuich T., Dreyfus I., Driggin E., Nigoghossian C., Ageno W., Madjid M., Guo Y., Tang L.V., Hu Y., Giri J., Cushman M., Quere I., Dimakakos E.P., Gibson C.M., Lippi G., Favaloro E.J., Fareed J., Caprini J.A., Tafur A.J., Burton J.R., Francese D.P., Wang E.Y., Falanga A., McLintock C., Hunt B.J., Spyropoulos A.C., Barnes G.D., Eikelboom J.W., Weinberg I., Schulman S., Carrier M., Piazza G., Beckman J.A., Steg P.G., Stone G.W., Rosenkranz S., Goldhaber S.Z., Parikh S.A., Monreal M., Krumholz H.M., Konstantinides S.V., Weitz J.I., Lip G.Y.H., E.b.t.I.N.E. Global Covid-19 Thrombosis Collaborative Group, S.b.t.E.S.C.W.G.o.P.C. the Iua, F Right ventricular, COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J. Am. College Cardiol. 2020;75(23):2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang H., Penninger J.M., Li Y., Zhong N., Slutsky A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46(4):586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Turner A.J., Hiscox J.A., Hooper N.M. ACE2: from vasopeptidase to SARS virus receptor. Trends Pharmacol. Sci. 2004;25(6):291–294. doi: 10.1016/j.tips.2004.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zisman L.S., Keller R.S., Weaver B., Lin Q., Speth R., Bristow M.R., Canver C.C. Increased angiotensin-(1–7)-forming activity in failing human heart ventricles: evidence for upregulation of the angiotensin-converting enzyme Homologue ACE2. Circulation. 2003;108(14):1707–1712. doi: 10.1161/01.CIR.0000094734.67990.99. [DOI] [PubMed] [Google Scholar]
- 42.Pei Z., Meng R., Li G., Yan G., Xu C., Zhuang Z., Ren J., Wu Z. Angiotensin-(1–7) ameliorates myocardial remodeling and interstitial fibrosis in spontaneous hypertension: role of MMPs/TIMPs. Toxicol. Lett. 2010;199(2):173–181. doi: 10.1016/j.toxlet.2010.08.021. [DOI] [PubMed] [Google Scholar]
- 43.Ferrario C.M., Chappell M.C., Tallant E.A., Brosnihan K.B., Diz D.I. Counterregulatory actions of angiotensin-(1–7) Hypertension. 1997;30(3 Pt 2):535–541. doi: 10.1161/01.hyp.30.3.535. [DOI] [PubMed] [Google Scholar]
- 44.Crackower M.A., Sarao R., Oudit G.Y., Yagil C., Kozieradzki I., Scanga S.E., Oliveira-dos-Santos A.J., da Costa J., Zhang L., Pei Y., Scholey J., Ferrario C.M., Manoukian A.S., Chappell M.C., Backx P.H., Yagil Y., Penninger J.M. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature. 2002;417(6891):822–828. doi: 10.1038/nature00786. [DOI] [PubMed] [Google Scholar]
- 45.Oudit G.Y., Kassiri Z., Jiang C., Liu P.P., Poutanen S.M., Penninger J.M., Butany J. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur. J. Clin. Invest. 2009;39(7):618–625. doi: 10.1111/j.1365-2362.2009.02153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khan A., Benthin C., Zeno B., Albertson T.E., Boyd J., Christie J.D., Hall R., Poirier G., Ronco J.J., Tidswell M., Hardes K., Powley W.M., Wright T.J., Siederer S.K., Fairman D.A., Lipson D.A., Bayliffe A.I., Lazaar A.L. A pilot clinical trial of recombinant human angiotensin-converting enzyme 2 in acute respiratory distress syndrome. Crit. Care. 2017;21(1):234. doi: 10.1186/s13054-017-1823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shete A. Urgent need for evaluating agonists of angiotensin-(1–7)/Mas receptor axis for treating patients with COVID-19. Int. J. Infectious Dis. 2020;96:348–351. doi: 10.1016/j.ijid.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peiro C., Moncada S. Substituting angiotensin-(1–7) to prevent lung damage in SARS-CoV-2 infection? Circulation. 2020;141(21):1665–1666. doi: 10.1161/CIRCULATIONAHA.120.047297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., Somasundaran M., Sullivan J.L., Luzuriaga K., Greenough T.C., Choe H., Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Muller M.A., Drosten C., Pohlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020 doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Henry C., Zaizafoun M., Stock E., Ghamande S., Arroliga A.C., White H.D. Impact of angiotensin-converting enzyme inhibitors and statins on viral pneumonia. Proc (Bayl. Univ. Med. Cent.) 2018;31(4):419–423. doi: 10.1080/08998280.2018.1499293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Imai Y., Kuba K., Rao S., Huan Y., Guo F., Guan B., Yang P., Sarao R., Wada T., Leong-Poi H., Crackower M.A., Fukamizu A., Hui C.C., Hein L., Uhlig S., Slutsky A.S., Jiang C., Penninger J.M. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436(7047):112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gurwitz D. Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics. Drug Dev. Res. 2020 doi: 10.1002/ddr.21656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang P., Zhu L., Cai J., Lei F., Qin J.J., Xie J., Liu Y.M., Zhao Y.C., Huang X., Lin L., Xia M., Chen M.M., Cheng X., Zhang X., Guo D., Peng Y., Ji Y.X., Chen J., She Z.G., Wang Y., Xu Q., Tan R., Wang H., Lin J., Luo P., Fu S., Cai H., Ye P., Xiao B., Mao W., Liu L., Yan Y., Liu M., Chen M., Zhang X.J., Wang X., Touyz R.M., Xia J., Zhang B.H., Huang X., Yuan Y., Rohit L., Liu P.P., Li H. Association of inpatient use of angiotensin converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ. Res. 2020 doi: 10.1161/CIRCRESAHA.120.317242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li J., Wang X., Chen J., Zhang H., Deng A. Association of renin-angiotensin system inhibitors with severity or risk of death in patients with hypertension hospitalized for coronavirus disease 2019 (COVID-19) infection in Wuhan, China. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.ESH, ESH UPDATE ON COVID-19: Statement of the European Society of Hypertension (ESH) on hypertension, Renin Angiotensin System blockers and COVID-19, 2020. https://www.eshonline.org/spotlights/esh-stabtement-on-covid-19/.
- 57.Pedersen S.F., Ho Y.C. SARS-CoV-2: a storm is raging. J. Clin. Invest. 2020 doi: 10.1172/JCI137647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tisoncik J.R., Korth M.J., Simmons C.P., Farrar J., Martin T.R., Katze M.G. Into the eye of the cytokine storm. Microbiol. Mol. Biol. Rev.: MMBR. 2012;76(1):16–32. doi: 10.1128/MMBR.05015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wong C.K., Lam C.W., Wu A.K., Ip W.K., Lee N.L., Chan I.H., Lit L.C., Hui D.S., Chan M.H., Chung S.S., Sung J.J. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin. Exp. Immunol. 2004;136(1):95–103. doi: 10.1111/j.1365-2249.2004.02415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rose N.R. Critical cytokine pathways to cardiac inflammation. J. Interferon Cytokine Res. 2011;31(10):705–710. doi: 10.1089/jir.2011.0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.B. Kaplan, C. Sulentic, H. Haggerty, M. Holsapple, N. Kaminski, Toxic Responses of the Immune System. In Barnes and Davis (Eds). Toxicology (Ch 12). New York: McGraw Hill Education.
- 62.P. Belluck, 'Straight-Up Fire' in His Veins: Teen Battles New COVID Syndrome, 2020. https://www.yahoo.com/news/straight-fire-veins-teen-battles-122647600.html.
- 63.Benigni A., Cassis P., Remuzzi G. Angiotensin II revisited: new roles in inflammation, immunology and aging. EMBO Mol. Med. 2010;2(7):247–257. doi: 10.1002/emmm.201000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pacurari M., Kafoury R., Tchounwou P.B., Ndebele K. The Renin-Angiotensin-aldosterone system in vascular inflammation and remodeling. Int. J. Inflammation. 2014;2014 doi: 10.1155/2014/689360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ruster C., Wolf G. Renin-angiotensin-aldosterone system and progression of renal disease. J. Am. Soc. Nephrol.: JASN. 2006;17(11):2985–2991. doi: 10.1681/ASN.2006040356. [DOI] [PubMed] [Google Scholar]
- 66.S. Richardson, J.S. Hirsch, M. Narasimhan, J.M. Crawford, T. McGinn, K.W. Davidson, C.-R.C. and the Northwell, D.P. Barnaby, L.B. Becker, J.D. Chelico, S.L. Cohen, J. Cookingham, K. Coppa, M.A. Diefenbach, A.J. Dominello, J. Duer-Hefele, L. Falzon, J. Gitlin, N. Hajizadeh, T.G. Harvin, D.A. Hirschwerk, E.J. Kim, Z.M. Kozel, L.M. Marrast, J.N. Mogavero, G.A. Osorio, M. Qiu, T.P. Zanos, Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area, Jama (2020). [DOI] [PMC free article] [PubMed]
- 67.Murthy S., Gomersall C.D., Fowler R.A. Care for critically Ill patients with COVID-19. JAMA. 2020 doi: 10.1001/jama.2020.3633. [DOI] [PubMed] [Google Scholar]
- 68.M. Brunsvold, ECMO and COVID., University of Minnesota (2020).
- 69.Dong L., Hu S., Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19) Drug Discov. Ther. 2020;14(1):58–60. doi: 10.5582/ddt.2020.01012. [DOI] [PubMed] [Google Scholar]
- 70.Mueck W., Kubitza D., Becka M. Co-administration of rivaroxaban with drugs that share its elimination pathways: pharmacokinetic effects in healthy subjects. Br. J. Clin. Pharmacol. 2013;76(3):455–466. doi: 10.1111/bcp.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mulangu S., Dodd L.E., Davey R.T., Mbaya O.T., Proschan M., Mukadi D., Manzo M.L., Nzolo D., Oloma A.T., Ibanda A., Ali R., Coulibaly S., Levine A.C., Grais R., Diaz J., Lane H.C., Muyembe-Tamfum J.J., Sivahera B., Camara M., Kojan R., Walker R., Dighero-Kemp B., Cao H.Y., Mukumbayi P., Mbala-Kingebeni P., Ahuka S., Albert S., Bonnett T., Crozier I., Duvenhage M., Proffitt C., Teitelbaum M., Moench T., Aboulhab J., Barrett K., Cahill K., Cone K., Eckes R., Hensley L., Herpin B., Higgs E., Ledgerwood J., Pierson J., Smolskis M., Sow Y., Tierney J., Sivapalasingam S., Holman W., Gettinger N., Vallee D., Nordwall J., Grp P.W., Team P.C.S., Randomized A. Controlled trial of Ebola virus disease therapeutics. N. Engl. J. Med. 2019;381(24):2293–2303. doi: 10.1056/NEJMoa1910993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ben-Zvi I., Kivity S., Langevitz P., Shoenfeld Y. Hydroxychloroquine: from malaria to autoimmunity. Clin. Rev. Allergy Immunol. 2012;42(2):145–153. doi: 10.1007/s12016-010-8243-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Costedoat-Chalumeau N., Hulot J.S., Amoura Z., Delcourt A., Maisonobe T., Dorent R., Bonnet N., Sable R., Lechat P., Wechsler B., Piette J.C. Cardiomyopathy related to antimalarial therapy with illustrative case report. Cardiology. 2007;107(2):73–80. doi: 10.1159/000094079. [DOI] [PubMed] [Google Scholar]
- 74.Yaribeygi H., Panahi Y., Sahraei H., Johnston T.P., Sahebkar A. The impact of stress on body function: a review. EXCLI J. 2017;16:1057–1072. doi: 10.17179/excli2017-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rozanski A., Blumenthal J.A., Kaplan J. Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation. 1999;99(16):2192–2217. doi: 10.1161/01.cir.99.16.2192. [DOI] [PubMed] [Google Scholar]
- 76.Khalili J.S., Zhu H., Mak N.S.A., Yan Y., Zhu Y. Novel coronavirus treatment with ribavirin: groundwork for an evaluation concerning COVID-19. J. Med. Virol. 2020 doi: 10.1002/jmv.25798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cao Y.C., Deng Q.X., Dai S.X. Remdesivir for severe acute respiratory syndrome coronavirus 2 causing COVID-19: an evaluation of the evidence. Travel Med. Infect. Dis. 2020;101647 doi: 10.1016/j.tmaid.2020.101647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Morse J.S., Lalonde T., Xu S., Liu W.R. Learning from the past: possible urgent prevention and treatment options for severe acute respiratory infections caused by 2019-nCoV. ChemBioChem. 2020;21(5):730–738. doi: 10.1002/cbic.202000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vincent M.J., Bergeron E., Benjannet S., Erickson B.R., Rollin P.E., Ksiazek T.G., Seidah N.G., Nichol S.T. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol. J. 2005;2:69. doi: 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu J., Cao R., Xu M., Wang X., Zhang H., Hu H., Li Y., Hu Z., Zhong W., Wang M. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6:16. doi: 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lexi-Drugs., Lexi-Comp OnlineTM. Hudson (OH): Wolters Kluwer Clinical Drug Information, Inc.; 2020. Available from: http://online.lexi.com.
- 82.Sheahan T.P., Sims A.C., Leist S.R., Schafer A., Won J., Brown A.J., Montgomery S.A., Hogg A., Babusis D., Clarke M.O., Spahn J.E., Bauer L., Sellers S., Porter D., Feng J.Y., Cihlar T., Jordan R., Denison M.R., Baric R.S. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat. Commun. 2020;11(1):222. doi: 10.1038/s41467-019-13940-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li H., Wang Y.M., Xu J.Y., Cao B. Potential antiviral therapeutics for 2019 Novel Coronavirus. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43(3):170–172. doi: 10.3760/cma.j.issn.1001-0939.2020.03.004. [DOI] [PubMed] [Google Scholar]
- 84.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G., Ruan L., Song B., Cai Y., Wei M., Li X., Xia J., Chen N., Xiang J., Yu T., Bai T., Xie X., Zhang L., Li C., Yuan Y., Chen H., Li H., Huang H., Tu S., Gong F., Liu Y., Wei Y., Dong C., Zhou F., Gu X., Xu J., Liu Z., Zhang Y., Li H., Shang L., Wang K., Li K., Zhou X., Dong X., Qu Z., Lu S., Hu X., Ruan S., Luo S., Wu J., Peng L., Cheng F., Pan L., Zou J., Jia C., Wang J., Liu X., Wang S., Wu X., Ge Q., He J., Zhan H., Qiu F., Guo L., Huang C., Jaki T., Hayden F.G., Horby P.W., Zhang D., Wang C. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shang L., Zhao J., Hu Y., Du R., Cao B. On the use of corticosteroids for 2019-nCoV pneumonia. Lancet. 2020;395(10225):683–684. doi: 10.1016/S0140-6736(20)30361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.K. Glynn, University of Minnesota Launches COVID-19 Clinical Trials of Blood Pressure Drug Losartan. University of Minnesota Medical School. 2020 https://med.umn.edu/news-events/university-minnesota-launches-covid-19-clinical-trials-blood-pressure-drug-losarta, (2020).
- 87.Zhang B., Liu S., Tan T., Huang W., Dong Y., Chen L., Chen Q., Zhang L., Zhong Q., Zhang X., Zou Y., Zhang S. Treatment with convalescent plasma for critically Ill patients with SARS-CoV-2 infection. Chest. 2020 doi: 10.1016/j.chest.2020.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yang W., Cao Q., Qin L., Wang X., Cheng Z., Pan A., Dai J., Sun Q., Zhao F., Qu J., Yan F. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID-19): a multi-center study in Wenzhou city, Zhejiang China. J. Infect. 2020 doi: 10.1016/j.jinf.2020.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang J.J., Dong X., Cao Y.Y., Yuan Y.D., Yang Y.B., Yan Y.Q., Akdis C.A., Gao Y.D. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020 doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 90.Wu J., Liu J., Zhao X., Liu C., Wang W., Wang D., Xu W., Zhang C., Yu J., Jiang B., Cao H., Li L. Clinical characteristics of imported cases of COVID-19 in Jiangsu Province: a multicenter descriptive study. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa199. [DOI] [PMC free article] [PubMed] [Google Scholar]