Abstract
Background
Vitamin D deficiency is a common, potentially reversible contributor to morbidity and mortality among critically ill patients. The potential benefits of vitamin D supplementation in acute critical illness require further study.
Methods
We conducted a randomized, double-blind, placebo-controlled, phase 3 trial of early vitamin D3 supplementation in critically ill, vitamin D-deficient patients who were at high risk for death. Randomization occurred within 12 hours after the decision to admit the patient to an intensive care unit. Eligible patients received a single enteral dose of 540,000 IU of vitamin D3 or matched placebo. The primary end point was 90-day all-cause, all-location mortality.
Results
A total of 1360 patients were found to be vitamin D-deficient during point-of-care screening and underwent randomization. Of these patients, 1078 had baseline vitamin D deficiency (25-hydroxyvitamin D level, <20 ng per milliliter [50 nmol per liter]) confirmed by subsequent testing and were included in the primary analysis population. The mean day 3 level of 25-hydroxyvitamin D was 46.9±23.2 ng per milliliter (117±58 nmol per liter) in the vitamin D group and 11.4±5.6 ng per milliliter (28±14 nmol per liter) in the placebo group (difference, 35.5 ng per milliliter; 95% confidence interval [CI], 31.5 to 39.6). The 90-day mortality was 23.5% in the vitamin D group (125 of 531 patients) and 20.6% in the placebo group (109 of 528 patients) (difference, 2.9 percentage points; 95% CI, −2.1 to 7.9; P=0.26). There were no clinically important differences between the groups with respect to secondary clinical, physiological, or safety end points. The severity of vitamin D deficiency at baseline did not affect the association between the treatment assignment and mortality.
Conclusions
Early administration of high-dose enteral vitamin D3 did not provide an advantage over placebo with respect to 90-day mortality or other, nonfatal outcomes among critically ill, vitamin D-deficient patients. (Funded by the National Heart, Lung, and Blood Institute; VIOLET ClinicalTrials.gov number, NCT03096314.)
Vitamin d may improve outcomes in critically ill patients. Preclinical data suggest that vitamin D is a potent immunomodulatory agent that is essential for lung development and function.1–7 Observational data and initial clinical trial data indicate that vitamin D deficiency is common among critically ill patients and constitutes a potentially modifiable risk factor associated with longer lengths of stay in the hospital and intensive care unit (ICU), lung and other organ injury, prolonged mechanical ventilation, and death.8–14 However, vitamin D level is considered a marker of coexisting conditions and frailty, and residual confounding may drive these associations.15
In a previous phase 2 trial (Correction of Vitamin D Deficiency in Critically Ill Patients [VITdAL-ICU], involving 475 patients), vitamin D supplementation administered to vitamin D-deficient, critically ill patients was associated with lower observed mortality than placebo at 28 days (21.9% vs. 28.6%, P = 0.14) and at 6 months (35.0% vs. 42.9%, P = 0.09), although the trial was underpowered for analysis of the mortality end point.16 Such findings, along with meta-analyses of previous trials in critical illness suggesting benefit of vitamin D treatment,17,18 support the need for a larger, phase 3 trial to evaluate the effect of short-term vitamin D supplementation on mortality among critically ill patients.
Accordingly, the National Heart, Lung, and Blood Institute (NHLBI) Prevention and Early Treatment of Acute Lung Injury (PETAL) Network conducted the Vitamin D to Improve Outcomes by Leveraging Early Treatment (VIOLET) trial. We hypothesized that early administration of high-dose vitamin D3 (cholecalciferol) would reduce 90-day all-cause, all-location mortality among critically ill, vitamin D-deficient patients who were at high risk for death.
Methods
Trial Design and Oversight
We designed the present multicenter, double-blind, placebo-controlled, phase 3 trial to have many similarities to the previous phase 2 trial,16 including the vitamin D3 regimen (a single enteral dose of 540,000 international units [IU]), the threshold for vitamin D deficiency (25-hydroxyvitamin D level, <20 ng per milliliter [50 nmol per liter]), and a focus on critically ill patients. Key differences in the present trial included early intervention (often before ICU admission), a focus on patients with specific higher-risk conditions, and a primary analysis based on measurement of 25-hydroxyvitamin D by the criterion standard of liquid chromatography-tandem mass spectrometry.19
The members of the writing committee vouch for the accuracy and completeness of the data and for the fidelity of the trial to the protocol and statistical analysis plan, which are available with the full text of this article at NEJM.org. Oversight was provided by a central institutional review board and data and safety monitoring board of the sponsoring network, which were appointed by the NHLBI. The trial was conducted under an investigational new drug application with the Food and Drug Administration (FDA). The study network coordinating center managed and analyzed the data. We obtained written informed consent from patients (when possible) or from their authorized representatives. Sekisui Diagnostics supplied the FastPack IP systems and Bio-Tech Pharmacal developed and produced the high-dose vitamin D3 and placebo used in the trial, but neither company had any role in the trial design or conduct, data analysis, or data interpretation.
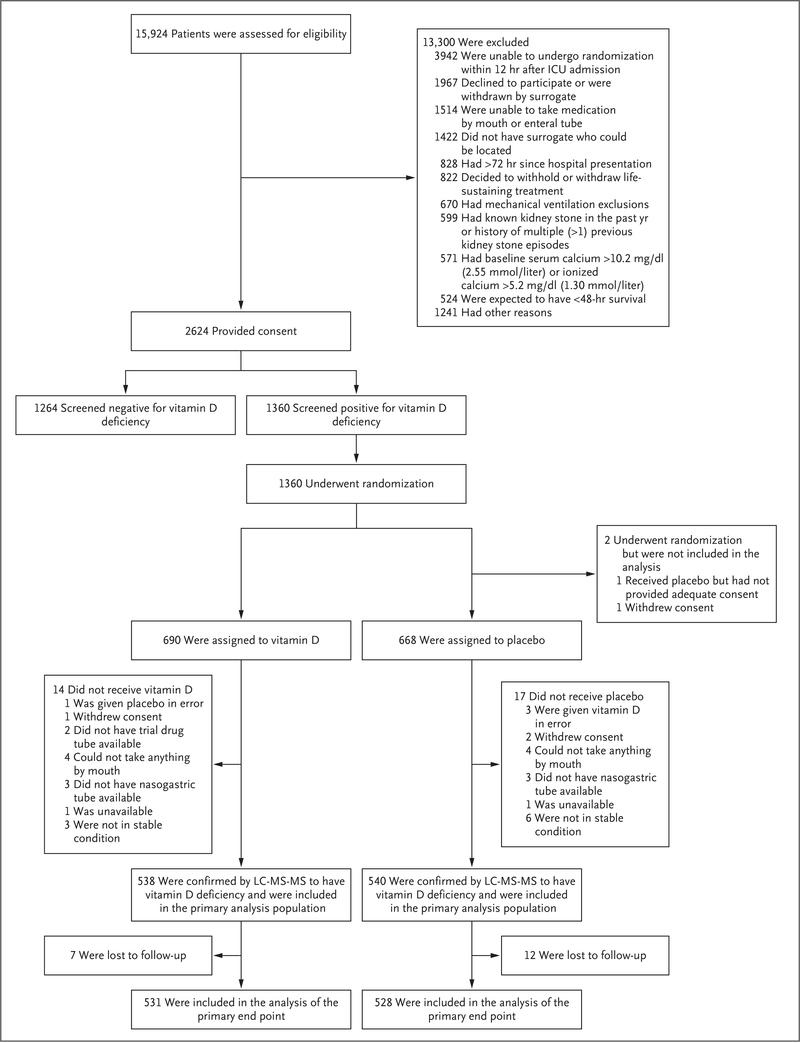
Patients
We enrolled each patient within 12 hours after the clinician’s decision to admit the patient to the ICU from the emergency department, hospital ward, operating room, or outside facility. Eligible patients were adults and had one or more acute risk factors for death or lung injury that contributed directly to the need for ICU admission (pneumonia, sepsis, shock, mechanical ventilation for acute respiratory failure, aspiration, smoke inhalation, pancreatitis, or lung contusion). The complete list of exclusion criteria is shown in Figure 1, and in the Supplementary Appendix, available at NEJM.org; the most common reasons for patients being excluded were an inability to take an enteral drug, a history of kidney stones, the presence of hypercalcemia at baseline, and informed consent not being obtained in a timely manner. After written informed consent was obtained, eligible patients underwent an FDA-approved test to screen for vitamin D deficiency — either a test conducted by the enrolling hospital clinical laboratory or a point-of-care test (FastPack IP, Sekisui Diagnostics) performed by research staff. Eligible patients had a plasma 25-hydroxyvitamin D level of less than 20 ng per milliliter as measured by either test. This “screened-deficient” population, which included all patients who underwent randomization, subsequently underwent confirmatory liquid chromatography-tandem mass spectrometry testing, which was completed at the University of Washington reference laboratory on batched plasma specimens that were collected at the same time as the initial screening test (before randomization). Patients with a 25-hydroxyvitamin D level of less than 20 ng per milliliter as measured by liquid chromatography-tandem mass spectrometry were considered to have confirmed vitamin D deficiency and made up the primary analysis population. We also obtained results for the screened-deficient population as secondary analyses. Figure S1 in the Supplementary Appendix shows the flow of patients through the trial.
Figure 1 (facing page). Screening, Enrollment, and Follow-up.
Patients may have had more than one reason for being excluded after the assessment of eligibility, and patients who underwent randomization may have had more than one reason for not receiving the assigned intervention. ICU denotes intensive care unit, and LC-MS-MS liquid chromatography-tandem mass spectrometry.
Randomization
We used a central electronic system and permuted blocks to randomly assign eligible patients in a 1:1 ratio, stratified according to site, to receive either a single enteral (administered orally or through a nasogastric or orogastric tube) dose of 540,000 IU of vitamin D3 or matched placebo, in liquid form, administered within 2 hours after randomization. We did not mandate other aspects of clinical care, because our intention was to evaluate the intervention in the context of usual practice. We recommended that treating clinicians avoid vitamin D testing or additional vitamin D supplementation in the 1 month after administration of vitamin D or placebo.
End Points
The primary end point was 90-day all-cause, all-location mortality in the primary analysis population (i.e., patients with vitamin D deficiency confirmed by liquid chromatography-tandem mass spectrometry). Secondary clinical end points were hospital length of stay to day 90, health care facility length of stay to day 90, proportions of patients alive and at home (previous level of care) at day 90, ventilator-free days to day 28, time to death to day 90, and quality of life to day 90. Secondary physiological end points were the severity of hypoxemia, acute respiratory distress syndrome, acute kidney injury, and cardiovascular failure to day 7, as well as 25-hydroxyvitamin D levels at day 3 (measured in a subgroup of the first 25% of patients per protocol). Safety end points were total and ionized calcium levels to day 14, incident kidney stones to day 90, and fall-related fractures to day 90.
Statistical Analysis
We based the sample size on a comparison of binomial proportions with an overall two-sided alpha level of 0.05. Under assumptions that 90-day mortality would be 20% in the placebo group and 15% in the vitamin D group, that three interim data analyses would be conducted, and that vitamin D deficiency would be confirmed by liquid chromatography-tandem mass spectrometry in 80% of the patients undergoing randomization, we calculated that the trial would have 87% power if 3000 patients underwent randomization. The design allowed stopping for efficacy on the basis of the Lan-DeMets alpha spending function.20 The futility stopping rules incorporated the observed mortality and the proportion of patients who underwent randomization who had 25-hydroxyvitamin D levels of less than 20 ng per milliliter as measured by liquid chromatography-tandem mass spectrometry to calculate the predictive probability of vitamin D supplementation being shown to be significantly superior to placebo with 3000 patients. We adopted a futility boundary of 10% posterior probability of superiority at interim analyses.
For the primary analysis, we compared 90-day mortality on the risk-difference scale using a generalized linear model with a binomial distribution and identity link function. We used quadratic smoothing splines with prespecified knots at plasma 25-hydroxyvitamin D levels (measured by liquid chromatography-tandem mass spectrometry) of 5, 10, 15, 20, 25, and 30 ng per milliliter and pointwise 95% bootstrap confidence intervals to estimate the relationship between the treatment effect and the baseline 25-hydroxyvitamin D level.21 We compared time to death to day 90 using Kaplan-Meier curves. We compared adverse events with the event as the unit of analysis using weighted Poisson regression with serious events given a weight twice that of the nonserious events.
We present other secondary end points with observed differences and 95% Wald confidence intervals. For the comparison of the highest creatinine levels and highest cardiovascular Sequential Organ Failure Assessment (SOFA) scores to day 7, controlling for baseline values, we used repeated-measures analysis of variance with a treatment-by-time interaction and shared intercept at baseline. We analyzed hospital and health care facility length of stay among patients who survived to day 90 and changes in quality of life as measured with the European Quality of Life-5 Dimensions 5-Level questionnaire (EQ-5D-5L)22 (score at day 90 minus score at baseline) using survivor average causal effect methods, including a model for predicting survival.23 In each treatment group, the estimated outcome in those who would survive in both treatment groups is a weighted average of the observed outcomes, with weights proportional to the estimated probability of survival in the other treatment group. The prespecified covariates were age, sex, race or ethnic group, Charlson comorbidity index, SOFA score, and baseline 25-hydroxyvitamin D level measured by liquid chromatography-tandem mass spectrometry. Beyond the survivor average causal effect models, we conducted all analyses using a complete case analysis approach, assuming that data were missing completely at random.
The main analyses used intention-to-treat principles. We considered a two-sided P value of less than 0.05 to indicate statistical significance for the primary analysis. Other reported P values (shown only for safety end points) and confidence intervals were not adjusted for multiple comparisons and should not be used to infer effects. We used SAS software, version 9.4 (SAS Institute), for the analyses.
Results
Patients
From April 2017 through July 2018 at 44 U.S. hospitals, we obtained consent from 2624 patients; 1360 patients who were screened as vitamin D-deficient underwent randomization, and 1078 of these patients had vitamin D deficiency confirmed by liquid chromatography-tandem mass spectrometry and were included in the primary analysis population (Fig. 1). After the first interim analysis, the data and safety monitoring board recommended that the trial be stopped for futility, primarily on the basis of a predictive probability of less than 2% that vitamin D treatment would be found to be superior to placebo with full trial enrollment.
Overall, 690 patients were assigned to the vitamin D group and 668 patients were assigned to the placebo group. In the primary analysis population, 538 patients were in the vitamin D group and 540 were in the placebo group. Baseline characteristics were similar in the two treatment groups in both the screened-deficient population and the primary analysis population (Table 1 and Table S1). The most common qualifying conditions were pneumonia, shock, and sepsis. Randomization was performed at a mean (±SD) of 6.7±3.5 hours after the clinician’s decision to admit the patient to the ICU (Table S2).
Table 1.
Baseline Characteristics of the Patients in the Primary Analysis Population.*
| Characteristic | Vitamin D (N = 538) | Placebo (N = 540) | ||
|---|---|---|---|---|
| Value | No. of Patients with Data | Value | No. of Patients with Data | |
| Demographic | ||||
| Age — yr | 56.5±15.9 | 538 | 54.6±16.7 | 540 |
| Female sex — no. (%) | 229 (42.6) | 538 | 238 (44.1) | 540 |
| Race or ethnic group — no. (%)† | ||||
| Non-Hispanic white | 280 (52.0) | 538 | 287 (53.1) | 540 |
| Black | 130 (24.2) | 538 | 122 (22.6) | 540 |
| Nonblack Hispanic | 33 (6.1) | 538 | 31 (5.7) | 540 |
| Other | 15 (2.8) | 538 | 12 (2.2) | 540 |
| Not available | 80 (14.9) | 538 | 88 (16.3) | 540 |
| Facility residence before hospitalization — no. (%) | 33 (6.1) | 538 | 35 (6.5) | 540 |
| EQ-5D-5L score‡ | 0.7±0.3 | 507 | 0.7±0.3 | 504 |
| Clinical | ||||
| Charlson comorbidity index§ | 4.0±2.9 | 522 | 3.5±2.9 | 521 |
| Body-mass index¶ | 29.8±10.1 | 524 | 31.0±11.4 | 529 |
| Acute risk factors for death — no. (%)∥ | ||||
| Pneumonia | 204 (37.9) | 538 | 181 (33.5) | 540 |
| Shock | 192 (35.7) | 538 | 197 (36.5) | 540 |
| Sepsis | 185 (34.4) | 538 | 174 (32.2) | 540 |
| Mechanical ventilation for acute respiratory failure | 119 (22.1) | 538 | 121 (22.4) | 540 |
| Aspiration | 27 (5.0) | 538 | 35 (6.5) | 540 |
| Lung contusion | 15 (2.8) | 538 | 18 (3.3) | 540 |
| Pancreatitis | 17 (3.2) | 538 | 19 (3.5) | 540 |
| Smoke inhalation | 1 (02) | 538 | 2 (0.4) | 540 |
| Medical ICU admission — no. (%) | 447 (83.1) | 538 | 462 (85.6) | 540 |
| Illness severity | ||||
| Total SOFA score** | 5.6±3.6 | 538 | 5.4±3.7 | 540 |
| LIPS†† | 5.3±2.9 | 538 | 5.3±3.1 | 540 |
| Mechanical ventilation — no. (%) | 173 (32.2) | 538 | 184 (34.1) | 540 |
| ARDS — no. (%) | 44 (8.2) | 538 | 44 (8.1) | 540 |
| Vasopressor use at baseline — no. (%) | 169 (31.4) | 538 | 177 (32.8) | 540 |
| Vitamin D–related | ||||
| Vitamin D supplement use in past week — no. (%) | 31 (5.8) | 538 | 24 (4.4) | 540 |
| Multivitamin use in past week — no. (%) | 38 (7.1) | 538 | 37 (6.9) | 540 |
| Estimated average daily vitamin D dose — IU | 3269±13,118 | 57 | 4252±15,094 | 54 |
| 25-hydroxyvitamin D level — ng/ml | 11.2±4.8 | 11.0±4.7 | ||
| Total calcium level — mg/dl | 8.3±0.9 | 528 | 8.3±0.9 | 526 |
| Ionized calcium level — mg/dl | 4.3±1.4 | 210 | 4.3±0.9 | 212 |
| Creatinine level — mg/dl | 2.2±2.3 | 535 | 2.0±2.0 | 539 |
| eGFR — ml/min/1.73 m2 | 60±39.3 | 535 | 60.9±36.9 | 539 |
Plus–minus values are means ±SD. The primary analysis population included all patients who underwent randomization and had vitamin D deficiency confirmed by liquid chromatography–tandem mass spectrometry. Percentages may not total 100 because of rounding. To convert the values for 25-hydroxyvitamin D to nanomoles per liter, multiply by 2.496. To convert the values for calcium to millimoles per liter, multiply by 0.250. To convert the values for creatinine to micromoles per liter, multiply by 88.4. ARDS denotes acute respiratory distress syndrome, and eGFR estimated glomerular filtration rate.
Race and ethnic group were reported by the patient or the patient’s surrogate.
Scores on the EuroQol–5 Dimensions 5-Level quality-of-life assessment (EQ-5D-5L) range from −0.11 to 1.00, with higher scores indicating better health.
Charlson comorbidity index scores range from 0 to 37, with higher scores indicating more coexisting conditions.
Body-mass index is the weight in kilograms divided by the square of the height in meters.
Patients may have had more than one risk factor.
The total Sequential Organ Failure Assessment (SOFA) score ranges from 0 to 24, with higher scores indicating more severe organ failure.
The Lung Injury Prediction Score (LIPS) ranges from 0 to 36, with higher scores indicating a higher risk of lung injury.
Plasma Vitamin D Levels
In the primary analysis population of 1078 patients, 532 (98.9%) of those who were randomly assigned to the vitamin D group and 532 (98.5%) of those who were randomly assigned to the placebo group received the assigned treatment, a mean of 1.2±1.1 hours after randomization (Table S2). The mean baseline 25-hydroxyvitamin D level as measured by liquid chromatography-tandem mass spectrometry was 11.2±4.8 ng per milliliter (28±12 nmol per liter) in the vitamin D group and 11.0±4.7 ng per milliliter (27±12 nmol per liter) in the placebo group (Table 1). In the first 25% of patients who had day 3 plasma specimens (per-protocol subgroup), the mean day 3 level of 25-hydroxyvitamin D as measured by liquid chromatography-tandem mass spectrometry was 46.9±23.2 ng per milliliter (117±58 nmol per liter) in the vitamin D group and 11.4±5.6 ng per milliliter (28±14 nmol per liter) in the placebo group (difference, 35.5 ng per milliliter; 95% confidence interval [CI], 31.5 to 39.6) (Table 2). In the vitamin D group, most patients (74.5%) had reached the target 25-hydroxyvitamin D level of 30 to less than 120 ng per milliliter (75 to <300 nmol per liter) at day 3, with the levels in few patients (12.4%) remaining below 20 ng per milliliter (Table 2). In the placebo group, 94.0% of patients had 25-hydroxyvitamin D levels that remained below 20 ng per milliliter at day 3. These results were similar in the screened-deficient population (Tables S1 and S3).
Table 2.
End Points in the Primary Analysis Population.*
| End Point | Vitamin D (N = 538) | Placebo (N = 540) | P Value or Difference (95% CI) | ||
|---|---|---|---|---|---|
| Value | No. of Patients with Data | Value | No. of Patients with Data | ||
| Primary end point | |||||
| Death from any cause in any location to day 90 — no. (%) | 125 (23.5) | 531 | 109 (20.6) | 528 | 0.26 |
| Secondary clinical end points | |||||
| Death from any cause in any location to day 28 — no. (%) | 92 (17.3) | 531 | 69 (13.1) | 528 | 4.3 (−0.1 to 8.6) |
| Death in the hospital to day 90 — no. (%) | 92 (17.1) | 538 | 72 (13.4) | 539 | 3.7 (−0.5 to 8.0) |
| Alive and at home under previous level of care at day 90 — no. (%) | 348 (65.9) | 528 | 345 (65.6) | 526 | 0.3 (−5.4 to 6.0) |
| Hospital length of stay to day 90 — days | |||||
| Mean ±SD | 9.1±9.2 | 406 | 10.4±11.0 | 418 | −1.4 (−2.7 to 0.0) |
| Mean ±SE† | 9.0±0.4 | 406 | 9.9±0.4 | 418 | −0.9 (−1.9 to 0.1) |
| Discharged to other health care facility — no. (%) | 71 (17.5) | 406 | 89 (21.2) | 419 | −3.8 (−9.1 to 1.6) |
| Health care facility length of stay — days | |||||
| Mean ±SD | 6.0±17.5 | 402 | 8.1±20.4 | 416 | −2.2 (−4.8 to 0.4) |
| Mean ±SE† | 5.5±0.7 | 402 | 7.5±0.7 | 416 | −1.9 (−3.8 to −0.1) |
| Mean ±SD ventilator-free days to day 28 | 21.3±11.3 | 523 | 22.1±10.5 | 534 | −0.8 (−2.1 to 0.5) |
| Change in EQ-5D-5L from baseline to day 90 | |||||
| Mean ±SD | 0.0±0.2 | 340 | 0.0±0.2 | 346 | 0.0 (0.0 to 0.1) |
| Mean ±SE† | 0.0±0.0 | 340 | 0.0±0.0 | 346 | 0.0 (0.0 to 0.1) |
| Secondary physiological end points | |||||
| New, postrandomization mechanical ventilation — no. (%) | 39 (10.7) | 365 | 29 (8.2) | 354 | 2.5 (−1.8 to 6.8) |
| Mean ±SD lowest Pao2:Fio2 to day 7 | 179.8±102.6 | 122 | 185.8±110.4 | 136 | −5.9 (−32.2 to 20.3) |
| New ARDS to day 7 — no. (%) | 20 (4.9) | 411 | 17 (4.1) | 412 | 0.7 (−2.1 to 3.6) |
| ARDS severity to day 7 — no. (%) | |||||
| Mild | 6 (30.0) | 20 | 4 (23.5) | 17 | 6.5 (−22.0 to 34.9) |
| Moderate | 9 (45.0) | 20 | 12 (70.6) | 17 | −25.6 (−56.3 to 5.1) |
| Severe | 5 (25.0) | 20 | 1 (5.9) | 17 | 19.1 (−2.9 to 41.1) |
| Worst severity of acute kidney injury to day 7 — no. (%) | |||||
| None | 285 (58.9) | 484 | 297 (60.0) | 495 | −1.1 (−7.3 to 5.0) |
| Mild | 70 (14.5) | 484 | 77 (15.6) | 495 | −1.1 (−5.6 to 3.4) |
| Moderate | 48 (9.9) | 484 | 52 (10.5) | 495 | −0.6 (−4.4 to 3.2) |
| Severe | 81 (16.7) | 484 | 69 (13.9) | 495 | 2.8 (−1.7 to 7.3) |
| New renal-replacement therapy to day 7 — no. (%) | 20 (4.1) | 489 | 18 (3.6) | 500 | 0.5 (−1.9 to 2.9) |
| Mean ±SE highest creatinine level to day 7 — mg/dl‡ | 2.2±0.1 | 518 | 2.1±0.1 | 528 | 0.0 (−0.2 to 0.1) |
| New vasopressor use to day 7 — no. (%) | 43 (12.0) | 357 | 42 (11.7) | 360 | 0.4 (−4.4 to 5.1) |
| Mean ±SE highest cardiovascular SOFA score to day 7‡ | 1.4±0.1 | 523 | 1.3±0.1 | 534 | −0.1 (−0.3 to 0.0) |
| Mean ±SD 25-hydroxyvitamin D level at day 3 — ng/ml | 46.9±23.2 | 145 | 11.4±5.6 | 133 | 35.5 (31.5 to 39.6) |
| 25-Hydroxyvitamin D level category at day 3 — no. (%) | |||||
| <20 ng/ml | 18 (12.4) | 145 | 125 (94.0) | 133 | −81.6 (−88.3 to −74.9) |
| 20 to <30 ng/ml | 18 (12.4) | 145 | 8 (6.0) | 133 | 6.4 (−0.3 to 13.1) |
| 30 to <120 ng/ml | 108 (74.5) | 145 | 0 | 133 | 74.5 (67.4 to 81.6) |
| ≥120 ng/ml | 1 (0.7) | 145 | 0 | 133 | 0.7 (−0.7 to 2.0) |
| Mean ±SD interleukin-6 level at day 3 — pg/ml | 216±1574 | 141 | 298±2219 | 125 | −82 (−543 to 378) |
| Secondary safety end points | |||||
| Serious adverse events — no. | 13 | 17 | 0.47 | ||
| Hypercalcemia to day 14 — no. (%) | 14 (2.7) | 513 | 11 (2.1) | 523 | 0.51 |
| Mean ±SD highest total calcium level to day 14 — mg/dl | 8.9±0.8 | 507 | 8.8±0.7 | 513 | 0.004 |
| Mean ±SD highest ionized calcium level to day 14 — mg/dl | 4.7±0.8 | 153 | 4.6±0.8 | 177 | 0.67 |
| Kidney stones to day 90 — no. (%) | 0 | 507 | 3 (0.6) | 507 | 0.25 |
| Falls to day 90 — no. (%) | 36 (7.1) | 507 | 27 (5.3) | 507 | 0.24 |
| Fall-related fractures to day 90 — no. (%) | 4 (0.8) | 507 | 2 (0.4) | 507 | 0.69 |
To convert the values for calcium to millimoles per liter, multiply by 0.250. Pao2:Fio2 denotes the ratio of the partial pressure of arterial oxygen to the fraction of inspired oxygen.
Values were calculated from a survivor average causal effect model.
Cardiovascular SOFA scores range from 0 to 4, with higher scores indicating more severe organ failure. Values were controlled for the baseline value with the use of repeated-measures analysis of variance with a treatment-by-time interaction and shared intercept at baseline.
Primary End Point
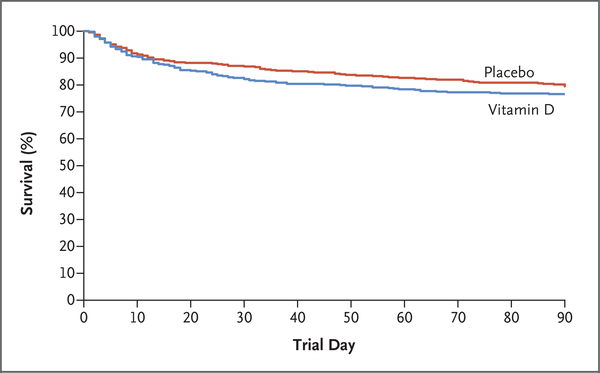
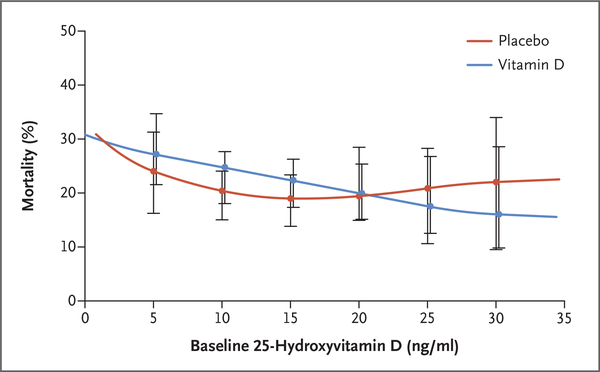
In the primary analysis population, 90-day all-cause, all-location mortality was 23.5% in the vitamin D group (125 of 531 patients) and 20.6% in the placebo group (109 of 528 patients) (difference, 2.9 percentage points; 95% CI, −2.1 to 7.9; P = 0.26) (Table 2 and Fig. 2). In the screened-deficient population, 90-day all-cause, all-location mortality was 23.3% in the vitamin D group (159 of 681 patients) and 20.9% in the placebo group (137 of 656 patients) (difference, 2.5 percentage points; 95% CI, −2.0 to 6.9; P = 0.28) (Table S3 and Fig. S2). On the basis of the range of baseline 25-hydroxyvitamin D levels and prespecified thresholds for this measurement, there was no apparent interaction between treatment group and the baseline 25-hydroxyvitamin D level (Fig. 3 and Figs. S2 and S3 and Table S4). The observed mortality was higher in the vitamin D group than in the placebo group for several subgroups: patients with sepsis or infection in the primary analysis population and prehospital facility residence, pneumonia, infection, and prerandomization acute respiratory distress syndrome in the screened-deficient population.
Figure 2. Survival to Day 90 in the Primary Analysis Population.
This figure is descriptive and not intended for inference of effects.
Figure 3. Mortality to Day 90 According to Baseline 25-Hydroxyvitamin D Level among All Patients Who Underwent Randomization.
I bars represent 95% confidence intervals. Plasma 25-hydroxyvitamin D concentrations were measured by liquid chromatography-tandem mass spectrometry. Estimates were obtained from the quadratic smoothing spline in each treatment group with prespecified knots at plasma 25-hydroxyvitamin D levels of 5, 10, 15, 20, 25, and 30 ng per milliliter and pointwise 95% bootstrap confidence intervals. To convert the values for 25-hydroxyvitamin D to nanomoles per liter, multiply by 2.496.
Secondary End Points
In the primary analysis population, mortality to day 28, hospital mortality to day 90, hospital and health care facility length of stay, ventilator-free days, and change in EQ-5D-5L score did not differ significantly between the groups (Table 2). The postrandomization incidence of acute respiratory distress syndrome did not differ significantly between the two treatment groups (4.9% in the vitamin D group and 4.1% in the placebo group; difference, 0.7 percentage points; 95% CI, −2.1 to 3.6). Other physiological end points, including respiratory, kidney, and cardiovascular failure, were also similar in the two groups. Results for secondary end points were similar in the screened-deficient population.
Safety and Adverse Events
Safety and adverse events are summarized in Table 2 and Tables S5 through S7. Although there were 296 total deaths reported in the trial, none were adjudicated as being causally related to vitamin D or placebo. Prespecified vitamin D-related adverse events (hypercalcemia, kidney stones, and fall-related fractures) were similar in the two groups. There was a small increase in the highest total calcium level to day 14 in the vitamin D group. Similarly, there were small increases in total and ionized calcium levels according to trial day in the vitamin D group in the primary analysis population and the screened-deficient population. Reported serious and nonserious adverse events were uncommon and similar in the vitamin D and placebo groups across the populations.
Discussion
A single 540,000 IU enteral dose of vitamin D3 administered early during critical illness rapidly corrected vitamin D deficiency but did not provide an advantage over placebo with respect to mortality or other clinically important end points. The very low likelihood of finding a benefit justified stopping the trial for futility before the pretrial sampling target of up to 3000 patients had been reached. We enrolled the intended population in a blinded fashion, with 90-day mortality similar to the predefined estimated rate, and a robust vitamin D response was achieved, with few adverse events. No predefined subgroups appeared to benefit from the vitamin D supplementation, including those with more severe vitamin D deficiency and those with specific acute risk factors for death. Furthermore, the higher observed mortality in the vitamin D group among patients with infectious causes of illness and patients with prerandomization acute respiratory distress syndrome was unexpected and contrary to the reported immunomodulatory effects of vitamin D. This observation may reflect differences between the use of vitamin D for prevention in previous studies24 and the use of vitamin D as treatment during acute illness in the present trial, but it also may be the result of chance.
There are several important differences between the current phase 3 trial and the previous, phase 2 trial (VITdAL-ICU).16,25 First, we enrolled patients early in their critical illness, often before arrival in the ICU, to correct vitamin D deficiency before established critical illness. The phase 2 trial enrolled patients a mean of 3 days after admission to the ICU. Second, the current trial primarily enrolled typical medical patients in the ICU (e.g., patients with pneumonia, sepsis, shock, or respiratory failure), whereas more than three quarters of the patients in the phase 2 trial were surgical or neurologic patients in the ICU. Third, we did not provide additional vitamin D supplementation after the initial loading dose, on the basis of the expected 2-to-3-week half-life of 25-hydroxyvitamin D,11,15,16 which we believed was adequate. Fourth, to maximize the inclusion of patients who were most likely to benefit from vitamin D supplementation, our primary analysis was based on liquid chromatography-tandem mass spectrometry testing, the criterion standard for 25-hydroxyvitamin D measurement. However, the results were not materially different in the screened-deficient population. Fifth, the population in the present trial had racial and ethnic diversity representative of the U.S. population; the phase 2 trial was conducted in Austria, and more than 99% of the patient population was white. Given known differences in vitamin D metabolism and response genes according to race and ethnic group,26–28 such differences may affect the results.
The results of the present trial do not support early testing for or treatment of vitamin D deficiency in critically ill patients. Ongoing studies will evaluate the effect of vitamin D supplementation in patients with severe vitamin D deficiency (ClinicalTrials.gov number, NCT03188796), other subgroups of patients that may be more likely to benefit (National Institutes of Health project number R01HL144566), and long-term outcomes (NCT03733418).
The strengths of our trial included a large, diverse, and representative population of patients with critical illness who were efficiently enrolled early during their critical illness. Our trial also achieved strong separation between the groups, with rapid correction of vitamin D deficiency. The trial also had certain limitations. One was the exclusion of patients later in the course of critical illness, which may have biased the trial population toward patients with less severe illness because of an inability to obtain timely informed consent from patients who had more severe illness. We did not follow the outcomes among patients who did not undergo randomization because they were found not to be vitamin D-deficient during screening. Finally, we did not provide additional vitamin D supplementation after the loading dose, since our intent was early correction of vitamin D deficiency.
In this phase 3 trial, early administration of high-dose enteral vitamin D3 did not provide an advantage over placebo with respect to 90-day mortality or other measures of nonfatal outcomes among critically ill patients with vitamin D deficiency.
Supplementary Material
Acknowledgments
Supported by grants from the National Heart, Lung, and Blood Institute (U01HL123009, U01HL122998, U01HL123018, U01HL123023, U01HL123008, U01HL123031, U01HL123004, U01HL123027, U01HL123010, U01HL123033, U01HL122989, U01HL123022, and U01HL123020). Sekisui Diagnostics supplied the FastPack IP systems, and Bio-Tech Pharmacal developed and produced the high-dose vitamin D3 and placebo used in the trial.
We thank the participating patients and their families, the clinical and research staff at the participating sites, the staff at the Prevention and Early Treatment of Acute Lung Injury (PETAL) coordinating center, and members of the PETAL data and safety monitoring board.
Appendix
The affiliations of the members of the writing committee are as follows: the Department of Emergency Medicine, University of Colorado School of Medicine, Aurora (A.A.G., L.F.); the Department of Medicine, Johns Hopkins University School of Medicine, Baltimore (R.G.B.); the Department of Emergency Medicine, Ohio State University, Columbus (J.M.C.); the Departments of Anesthesia, Critical Care, and Pain Medicine (V.M.B.-G., D.T.) and Emergency Medicine (N.I.S.), Beth Israel Deaconess Medical Center, and the Biostatistics Center (D.H.) and the Department of Medicine (N.R., B.T.T.), Massachusetts General Hospital — all in Boston; the Department of Medicine, Intermountain Medical Center and the University of Utah, Salt Lake City (C.K.G.); the Department of Medicine, University of Washington, Seattle (C.L.H.); the Departments of Medicine (R.C.H.) and Surgery (P.K.P.), University of Michigan, Ann Arbor; the Department of Emergency Medicine and Surgery, Henry Ford Hospital, Detroit (E.P.R.); the Department of Medicine, Oregon Health and Science University, Portland (A.K.); the Department of Medicine, Stanford University, Palo Alto, CA (J.E.L.); the Department of Emergency Medicine, Vanderbilt University Medical Center, Nashville (W.H.S.); and the Department of Emergency Medicine, University of Pittsburgh School of Medicine, Pittsburgh (D.M.Y.)
Footnotes
References
- 1.Zhang Y, Leung DY, Richers BN, et al. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J Immunol 2012;188:2127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Di Rosa M, Malaguarnera M, Nicoletti F, Malaguarnera L. Vitamin D3: a helpful immuno-modulator. Immunology 2011;134:123–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takano Y, Mitsuhashi H, Ueno K. 1α,25-Dihydroxyvitamin D3 inhibits neutrophil recruitment in hamster model of acute lung injury. Steroids 2011;76:1305–9. [DOI] [PubMed] [Google Scholar]
- 4.Brockman-Schneider RA, Pickles RJ, Gern JE. Effects of vitamin D on airway epithelial cell morphology and rhinovirus replication. PLoS One 2014;9(1):e86755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dancer RC, Parekh D, Lax S, et al. Vitamin D deficiency contributes directly to the acute respiratory distress syndrome (ARDS). Thorax 2015;70:617–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Phokela SS, Peleg S, Moya FR, Alcorn JL. Regulation of human pulmonary surfactant protein gene expression by 1alpha,25-dihydroxyvitamin D3. Am J Physiol Lung Cell Mol Physiol 2005;289:L617–L626. [DOI] [PubMed] [Google Scholar]
- 7.Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 2006;311:1770–3. [DOI] [PubMed] [Google Scholar]
- 8.Thomas MK, Lloyd-Jones DM, Thadhani RI, et al. Hypovitaminosis D in medical inpatients. N Engl J Med 1998;338:777–83. [DOI] [PubMed] [Google Scholar]
- 9.Ginde AA, Camargo CA Jr, Shapiro NI. Vitamin D insufficiency and sepsis severity in emergency department patients with suspected infection. Acad Emerg Med 2011;18:551–4. [DOI] [PubMed] [Google Scholar]
- 10.Nair P, Venkatesh B, Center JR. Vitamin D deficiency and supplementation in critical illness — the known knowns and known unknowns. Crit Care 2018;22:276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amrein K, Sourij H, Wagner G, et al. Short-term effects of high-dose oral vitamin D3 in critically ill vitamin D deficient patients: a randomized, double-blind, placebo-controlled pilot study. Crit Care 2011;15:R104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leaf DE, Raed A, Donnino MW, Ginde AA, Waikar SS. Randomized controlled trial of calcitriol in severe sepsis. Am J Respir Crit Care Med 2014;190:533–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quraishi SA, De Pascale G, Needleman JS, et al. Effect of cholecalciferol supplementation on vitamin D status and cathelicidin levels in sepsis: a randomized, placebo-controlled trial. Crit Care Med 2015;43:1928–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han JE, Jones JL, Tangpricha V, et al. High dose vitamin D administration in ventilated intensive care unit patients: a pilot double blind randomized controlled trial. J Clin Transl Endocrinol 2016;4:59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab 2011;96:53–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amrein K, Schnedl C, Holl A, et al. Effect of high-dose vitamin D3 on hospital length of stay in critically ill patients with vitamin D deficiency: the VITdAL-ICU randomized clinical trial. JAMA 2014;312:1520–30. [DOI] [PubMed] [Google Scholar]
- 17.Weng H, Li JG, Mao Z, Zeng XT. Randomised trials of vitamin D3 for critically ill patients in adults: systematic review and meta-analysis with trial sequential analysis. Intensive Care Med 2017;43:277–8. [DOI] [PubMed] [Google Scholar]
- 18.Putzu A, Belletti A, Cassina T, et al. Vitamin D and outcomes in adult critically ill patients: a systematic review and meta-analysis of randomized trials. J Crit Care 2017;38:109–14. [DOI] [PubMed] [Google Scholar]
- 19.Garg U 25-Hydroxyvitamin D testing: immunoassays versus tandem mass spectrometry. Clin Lab Med 2018;38:439–53. [DOI] [PubMed] [Google Scholar]
- 20.Lan KKG, DeMets DL. Discrete sequential boundaries for clinical trials. Biometrika 1983;70:659–63. [Google Scholar]
- 21.Eilers PHC, Marx BD. Flexible smoothing with B-splines and penalties. Stat Sci 1996;11:89–121. [Google Scholar]
- 22.EuroQol Group. EuroQol — a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199–208. [DOI] [PubMed] [Google Scholar]
- 23.Hayden D, Pauler DK, Schoenfeld D. An estimator for treatment comparisons among survivors in randomized trials. Biometrics 2005;61:305–10. [DOI] [PubMed] [Google Scholar]
- 24.Martineau AR, Jolliffe DA, Hooper RL, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ 2017;356:i6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martucci G, McNally D, Parekh D, et al. Trying to identify who may benefit most from future vitamin D intervention trials: a post hoc analysis from the VITDAL-ICU study excluding the early deaths. Crit Care 2019;23:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Powe CE, Evans MK, Wenger J, et al. Vitamin D-binding protein and vitamin D status of black Americans and white Americans. N Engl J Med 2013;369:1991–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Batai K, Murphy AB, Shah E, et al. Common vitamin D pathway gene variants reveal contrasting effects on serum vitamin D levels in African Americans and European Americans. Hum Genet 2014;133:1395–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hong J, Hatchell KE, Bradfield JP, et al. Transethnic evaluation identifies low-frequency loci associated with 25-hydroxyvitamin D concentrations. J Clin Endocrinol Metab 2018;103:1380–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.