The pancreatic islet is a highly vascularized endocrine mini-organ that depends on blood supply to function efficiently. As blood flows through islet capillaries reaching different endocrine cell types, it significantly impacts nutrient sensing, paracrine communication, and the final hormonal output. Thus, any change in blood flow, either induced physiologically (e.g., nervous input) or as a result of pathological changes (e.g., fibrosis), could affect islet function. It is not a stretch to state that the way the islet vasculature is arranged anatomically and regulated functionally must have consequences for glucose homeostasis.
Despite its potential impact for islet function, interest in the islet vasculature has been sporadic and is certainly not equal to that professed to the cells it serves. Still, there has been a substantive research effort in this arena. From beautiful scanning electron images of corrosion casts (1) to creative physiological experiments using perfused pancreases (2) and microbeads (3), investigators have employed various approaches to study the microcirculation of the islet. The results of these studies provided structural and functional insight but also raised questions. To settle a debate that had started in the mid-1960s, a group of prominent islet biologists decided to meet in 1996 to review existing notions about islet blood flow and “agreed to disagree” that there were three models (4). In model 1, non–β-cells are perfused before β-cells, allowing other endocrine cells to influence β-cells located downstream. In model 2, β-cells are perfused before the other endocrine cells and thus dominate islet function. In model 3, there is no apparent order of perfusion, but blood flows from the afferent to the efferent pole of the islet. The three flow patterns were confirmed more recently in in vivo studies in mice (5). Incidentally, the hierarchical organization based on blood flow may be possible in the mouse islet with its mantle versus core organization, but this scenario is highly unlikely in the desegregated human islet.
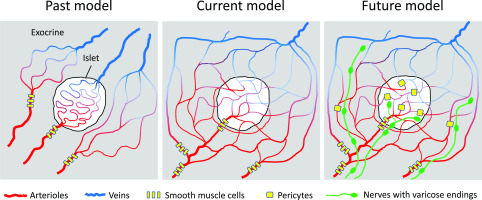
Their differences notwithstanding, all three models assumed that the islet microcirculation is self-contained, with each islet having its own vascular network comprising feeding arterioles, a glomerulus-like capillary net, and dedicated venous drainage (Fig. 1). This arrangement allows islet blood flow to be regulated independently from that of the exocrine pancreas. This notion is now being challenged by hot-off-the-press findings appearing in this issue of Diabetes. In their study, Dybala et al. (6) used intravital imaging of the exteriorized mouse pancreas to track individual red blood cells moving through islet capillaries in real time. In these technically challenging experiments, the authors followed red blood cells as they exited and entered the islet and found that blood flow is bidirectional and continuously integrated with that of the exocrine pancreas at multiple locations. The idea that islets, in particular smaller islets, could be incorporated into the exocrine capillary system is not completely new (7), but in their study, Dybala et al. moved anatomical guesswork into real-time in vivo physiology to show directly that the islet microcirculation is open and not isolated from that of the surrounding exocrine tissue.
Figure 1.
Cartoons depicting three different models of blood flow in the pancreas. Until recently, the microcirculation of the islet was considered to be independent of that of the surrounding exocrine tissues (left). In an article published in this issue of Diabetes, Dybala et al. (6) now show that the circulation of the islet is integrated with that of the exocrine tissue, with blood flowing bidirectionally between both compartments (center). In a more sophisticated model, regulatory elements such as nervous input and vascular gates can be incorporated into this scheme to allow for local control of blood flow (right).
The authors not only produced astonishingly detailed images of the pancreas vasculature but were further able to measure the basal velocity of individual red blood cells. The average speeds were similar inside and outside the islet, contradicting the prevalent view that islet blood flow is 5–10 times higher than in exocrine tissues. Because in vivo recordings of capillary blood flow are not yet possible in the human pancreas, the authors could only obtain structural data for human islets. The results are in line with previous observations made on corrosion casts that islets in the human pancreas are connected with the exocrine tissue through insulo-acinar portal vessels (1). Blood flow in the human islet can be expected to be integrated with its surroundings, as its vasculature already shows less of the tortuosity typical of the mouse islet vasculature (8,9). It is difficult to tell apart endocrine from exocrine regions based on vascular architecture in human pancreas sections. In addition, human islets do not have distinctive boundaries such as a capsule, which eliminates another barrier for full integration into the pancreatic vascular network.
The model proposed by Dybala et al. (6) still requires experimental confirmation by peers in the field before it becomes the new canon (Fig. 1). Nevertheless, from a physiological point of view, it makes sense that blood supply to endocrine and exocrine compartments is integrated. Secretion of digestive enzymes and insulin is simultaneously activated when nutrients need to be absorbed (i.e., the fed state), which requires coordinated increases in blood perfusion to both regions. The intimate relationship between islets and acinar tissues is evident in findings showing that diabetes is often associated with abnormal pancreatic exocrine function (10) and that pancreases of individuals at risk for or with type 1 diabetes are smaller (11). If these compartments are integrated through their vasculature, then acinar tissues should be exposed to high concentrations of islet secretory products, such as insulin, and vice versa. Indeed, insulin has been shown to regulate exocrine function by affecting protein biosynthesis and zymogen discharge, in particular of amylase (12). In general, however, there is relatively little interest in understanding how the exocrine and endocrine tissues of the pancreas influence each other. In view of the results shown here, the biology of the endocrine pancreas (studied by endocrinologists) should no longer be studied separately from that of the exocrine pancreas (the focus of gastroenterologists).
If endocrine and exocrine regions are really this integrated, is there still a chance for blood flow to be regulated selectively? Blood flow in the islet was proposed to be controlled by external gates at the level of the arteriole as well as by internal gates at the level of capillaries (4). These internal gates were described in early in vivo microscopy studies as “bulging endothelial cells” within islet capillaries that could influence the velocity and volume of blood flowing through capillaries (13). These internal gates are now known to be pericytes capable of changing islet capillary diameter and blood flow in response to increased β-cell activity or sympathetic nervous input (14). Thus, by responding to local and neural signals, the islet vasculature can alter blood flow selectively (Fig. 1). It is likely that similar mechanisms exist in acinar regions. Thus, we can imagine a scenario in which the perfusion of endocrine and exocrine compartments is regulated conjointly at the level of the pancreatic lobe while allowing for local control without the need for separate circulations. This should make everyone happy.
Article Information
Funding. This work was supported by National Institutes of Health grants K01DK111757 (J.A.), National Institute of Diabetes and Digestive and Kidney Diseases–supported Human Islet Research Network (UC4DK104162, New Investigator Pilot Award to J.A.), R01DK084321 (A.C.), R01DK111538 (A.C.), R01DK113093 (A.C.), U01DK120456 (A.C.), R33ES025673 (A.C.), and R21ES025673 (A.C.), and The Leona M. and Harry B. Helmsley Charitable Trust grants G-2018PG-T1D034 and G-1912-03552 (A.C.).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Footnotes
See accompanying article, p. 1439.
References
- 1.Murakami T, Hitomi S, Ohtsuka A, Taguchi T, Fujita T. Pancreatic insulo-acinar portal systems in humans, rats, and some other mammals: scanning electron microscopy of vascular casts. Microsc Res Tech 1997;37:478–488 [DOI] [PubMed] [Google Scholar]
- 2.Stagner JI, Samols E. Retrograde perfusion as a model for testing the relative effects of glucose versus insulin on the A cell. J Clin Invest 1986;77:1034–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carlsson PO, Andersson A, Jansson L. Pancreatic islet blood flow in normal and obese-hyperglycemic (ob/ob) mice. Am J Physiol 1996;271:E990–E995 [DOI] [PubMed] [Google Scholar]
- 4.Brunicardi FC, Stagner J, Bonner-Weir S, et al.; Long Beach Veterans Administration Regional Medical Education Center Symposium . Microcirculation of the islets of Langerhans. Diabetes 1996;45:385–392 [DOI] [PubMed] [Google Scholar]
- 5.Nyman LR, Wells KS, Head WS, et al. Real-time, multidimensional in vivo imaging used to investigate blood flow in mouse pancreatic islets. J Clin Invest 2008;118:3790–3797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dybala MP, Kuznetsov A, Motobu M, et al. Integrated pancreatic blood flow: bidirectional microcirculation between endocrine and exocrine pancreas. Diabetes 2020;69:1439–1450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonner-Weir S, Orci L. New perspectives on the microvasculature of the islets of Langerhans in the rat. Diabetes 1982;31:883–889 [DOI] [PubMed] [Google Scholar]
- 8.Cohrs CM, Chen C, Jahn SR, et al. Vessel network architecture of adult human islets promotes distinct cell-cell interactions in situ and is altered after transplantation. Endocrinology 2017;158:1373–1385 [DOI] [PubMed] [Google Scholar]
- 9.Brissova M, Shostak A, Fligner CL, et al. Human islets have fewer blood vessels than mouse islets and the density of islet vascular structures is increased in type 2 diabetes. J Histochem Cytochem 2015;63:637–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frier BM, Saunders JH, Wormsley KG, Bouchier IA. Exocrine pancreatic function in juvenile-onset diabetes mellitus. Gut 1976;17:685–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campbell-Thompson M, Wasserfall C, Montgomery EL, Atkinson MA, Kaddis JS. Pancreas organ weight in individuals with disease-associated autoantibodies at risk for type 1 diabetes. JAMA 2012;308:2337–2339 [DOI] [PubMed] [Google Scholar]
- 12.Adler G, Kern HF. Regulation of exocrine pancreatic secretory process by insulin in vivo. Horm Metab Res 1975;7:290–296 [DOI] [PubMed] [Google Scholar]
- 13.McCuskey RS, Chapman TM. Microscopy of the living pancreas in situ. Am J Anat 1969;126:395–407 [DOI] [PubMed] [Google Scholar]
- 14.Almaça J, Weitz J, Rodriguez-Diaz R, Pereira E, Caicedo A. The pericyte of the pancreatic islet regulates capillary diameter and local blood flow. Cell Metab 2018;27:630–644.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]