Abstract
Infants born to mothers with obesity have a greater risk for childhood obesity and metabolic diseases; however, the underlying biological mechanisms remain poorly understood. We used a Japanese macaque model to investigate whether maternal obesity combined with a Western-style diet (WSD) impairs offspring muscle insulin action. Adult females were fed a control or WSD prior to and during pregnancy through lactation, and offspring subsequently weaned to a control or WSD. Muscle glucose uptake and signaling were measured ex vivo in fetal (n = 5–8/group) and juvenile (n = 8/group) offspring. In vivo signaling was evaluated after an insulin bolus just prior to weaning (n = 4–5/group). Maternal WSD reduced insulin-stimulated glucose uptake and impaired insulin signaling at the level of Akt phosphorylation in fetal muscle. In juvenile offspring, insulin-stimulated glucose uptake was similarly reduced by both maternal and postweaning WSD and corresponded to modest reductions in insulin-stimulated Akt phosphorylation relative to controls. We conclude that maternal WSD leads to a persistent decrease in offspring muscle insulin-stimulated glucose uptake even in the absence of increased offspring adiposity or markers of systemic insulin resistance. Switching offspring to a healthy diet did not reverse the effects of maternal WSD on muscle insulin action, suggesting earlier interventions may be warranted.
Introduction
Obesity and associated metabolic disorders, including type 2 diabetes and cardiovascular disease, are major health issues worldwide, reaching epidemic proportions in Western populations. In the U.S., obesity continues to rise steadily, with recent reports that 39.8% of adults are obese (1). Reflecting this trend is the alarming rise in childhood obesity. While lifestyle factors clearly contribute to obesity, a growing body of data shows that maternal obesity or a Western-style diet (WSD) rich in saturated fat during pregnancy has adverse long-term metabolic effects on the offspring (2). Population-based studies demonstrate that obesity during pregnancy is associated with an almost fourfold greater risk of childhood obesity (3) and a twofold higher risk of developing metabolic syndrome in adolescents born to mothers with obesity (4–6). Genome-wide association studies find that only a fraction of intergenerational predisposition for obesity can be explained by genetic polymorphisms (7), suggesting that environmental exposures during development can program offspring metabolism later in life.
The developmental origin of health and disease (DOHaD) hypothesis postulates that exposure to environmental challenges during critical windows of development results in fetal adaptations that become maladaptive when exposed to subsequent metabolic and environmental stressors (8). Consistent with DOHaD, mesenchymal stem cells from infants exposed to maternal obesity have a greater propensity toward adipocyte versus myocyte differentiation, reduced lipid oxidative capacity when challenged with lipid, and hypermethylation of genes regulating fatty acid oxidation, relative to cells of infants from lean mothers, demonstrating functional consequences downstream of environmental stressors during development (9,10).
Skeletal muscle insulin resistance is a primary defect in the etiology of type 2 diabetes (11,12). Thus, it is important to understand the impact of maternal diet on skeletal muscle insulin response, especially in young offspring. Multiple studies have found that developmental exposure to maternal obesity or a maternal diet high in saturated fats during gestation predisposes offspring to obesity and insulin resistance (13); however, the mechanism by which early-life exposure to maternal obesity and WSD may alter skeletal muscle insulin-stimulated glucose transport and signal transduction is relatively untested.
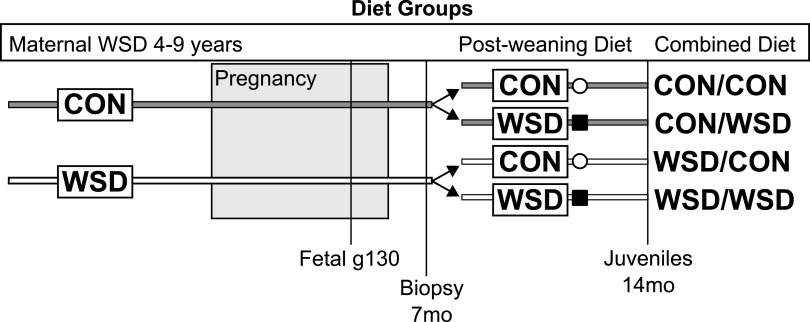
We used an established nonhuman primate model to investigate the impact of maternal obesity and a WSD on programming of offspring skeletal muscle insulin action (14–17). We hypothesized that exposure to maternal obesity concomitant with WSD feeding during fetal development and lactation would impair insulin-stimulated glucose uptake in offspring skeletal muscle independent of offspring adiposity and the effect would be exacerbated by a postweaning WSD. To test our hypothesis, we measured insulin-stimulated glucose uptake and canonical insulin signaling in isolated muscle strips from fetal and juvenile offspring, as well as insulin signaling activation in muscle biopsies from juvenile offspring of lean control (CON)-fed dams or obese WSD-fed dams, with crossover postweaning diets among the juvenile offspring (Fig. 1).
Figure 1.
Schematic of experimental design. Female Japanese macaques were placed on CON or WSD for 2–7 years prior to pregnancy and maintained on the same diet through pregnancy and weaning of offspring. Postweaning, offspring were either maintained on the same diet or switched to the alternate diet. Fetal samples were assayed at g130, juveniles were assayed at 14 months of age (14mo), and skeletal muscle biopsies were assayed from animals at 7 months of age (7mo).
Research Design and Methods
Experimental Animal Model
All animal procedures were approved by and conducted in accordance with the Institutional Animal Care and Use Committee of the Oregon National Primate Research Center and Oregon Health and Science University. The Oregon National Primate Research Center abides by the Animal Welfare Act and Regulations enforced by the U.S. Department of Agriculture and the Public Health Service Policy on Humane Care and Use of Laboratory Animals in accordance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health.
Adult Japanese macaques were group housed in indoor-outdoor enclosures and fed ad libitum a CON diet containing 15% calories from fat primarily from soybeans and corn (Monkey Diet, no. 5052; Purina Mills) or WSD with 36.6% calories from fat primarily from animal fat, egg, and corn oil (TAD Primate Diet, no. 5LOP; Purina Mills) for 4–9 years. The carbohydrate sources differed between the two diets, with sugars (primarily sucrose and fructose) comprising 19% of the WSD but only 3% of the CON. All animals fed the WSD were also given calorically dense treats once per day. Females were allowed to breed seasonally, and gestational age was determined by ultrasound (18,19). All offspring are from singleton births. Maternal body fat percent was determined by DEXA in nonpregnant females 3 months prior to the start of the breeding season (17). Intravenous glucose tolerance tests (IVGTT) were performed on adult females in the nonpregnant state (3 months prior to the start of the breeding season) and during the 3rd trimester of pregnancy as previously described (14,20). A detailed characterization of maternal metabolic profiles and maternal-fetal plasma measures, as well as changes in the placenta and fetal pancreas and liver, has previously been published (20,21). For fetal sample collection, dams were fasted overnight and anesthetized and fetuses were delivered by Cesarean section at gestational day 130 (g130) of 173.
A subset of offspring were delivered naturally and remained with the maternal group and on the maternal diet until weaning at 6–8 months of age. At weaning, offspring from both maternal diet groups (n = 16; 8 per maternal group) were combined into new social groups and housed in enriched, indoor-outdoor units with 6–10 similarly aged juveniles and 1–2 unrelated adult females per group. Juvenile offspring were fed CON or WSD postweaning with nutritional supplements as described above until necropsied at 14 months of age. One week prior to necropsy, fasting juvenile offspring underwent sedated IVGTT using 0.6 g/kg glucose. Baseline and timed samples of glucose and insulin were taken. Glucose area under the curve (AUC) and insulin AUC (IAUC) were calculated in GraphPad Prism, version 8.2, using fasting basal blood glucose as baseline.
Offspring Insulin-Stimulated Biopsy Collection
Prior to weaning, at ∼7 months of age, biopsy samples were collected from the soleus of fasted animals under anesthesia. Briefly, animals were fasted for 5 h in the morning, sedated with telazol (4 mg/kg), and intubated, and an intravenous catheter was placed in each arm. With an open biopsy technique, a baseline sample (∼30 mg) was dissected from the soleus. Insulin (Humulin R; Eli Lily) was injected intravenously at 0.05 units/kg, and a second muscle biopsy was taken 10 min later. Biopsy samples were snap frozen in liquid nitrogen and stored at −80°C.
Fetal and Juvenile Tissue Collection and Ex Vivo 2-Deoxyglucose Uptake
After euthanasia, fetal g130 and 14-month-old offspring skeletal muscles including gastrocnemius, soleus, vastus lateralis, and rectus femoris were removed, flash frozen in liquid nitrogen, and stored at −80°C. The contralateral soleus and rectus femoris in the fetus and the soleus and gastrocnemius in the juvenile were clamped with paired hemostats at a fixed distance prior to dissection, and clamped muscle was placed in ice-cold oxygenated (95% O2, 5% CO2) flasks of Krebs-Henseleit buffer (KHB). Clamped muscle was transported from necropsy to the adjacent laboratory for ex vivo analysis of muscle glucose uptake using a modified protocol from previous publications (22,23). Thin muscle strips (1 or 0.5 inches in length) were rapidly isolated from the clamped muscle, fastened with two-prong clips, and excised. A total of three to nine muscle strips were isolated from one gastrocnemius, soleus, and/or rectus femoris muscle of each animal as indicated. Clipped muscle strips were preincubated at 35°C for 30 min in vials with oxygenated KHB containing 0.1% BSA, 2 mmol/L Na-pyruvate, and 6 mmol/L mannitol and either no insulin or insulin (Humulin R) at 0.3 nmol/L or 12 nmol/L. After 30 min, muscles were transferred to a second vial and incubated at 35°C for 20 min in KHB plus 0.1% BSA, 9 mmol/L [14C]-mannitol (0.025 mCi/mmol; PerkinElmer, Boston, MA), and 1 mmol/L [3H]-2-deoxyglucose (2DG) (2 mCi/mmol; PerkinElmer, Boston, MA) with the same insulin concentration. After 20 min, muscles were trimmed from clips, blotted on ice-cold filter paper, and snap frozen in liquid nitrogen. Muscles were weighed and then homogenized in 0.5 mL cell lysis buffer (20 mmol/L Tris-HCl, pH 7.4; 150 mmol/L NaCl; 20 mmol/L NaF; 2 mmol/L EDTA, pH 8.0; 2.5 mmol/L Na4P2O7; 20 mmol/L β-glycerophosphate; 1% NP-40; and 10% glycerol) with protease and phosphatase inhibitor cocktails using glass-on-glass tissue grinders (Kontes). Samples were solubilized (1 h, 4°C, with end-over-end rotation) and centrifuged (12,000g), and the supernatant was collected. Supernatant (0.1 mL) was quantified by scintillation counting. The rate of 2DG uptake was calculated as previously described (24,25). Samples with insufficient volume for duplicate counting were not included in the data summary but were used for insulin signaling studies.
Protein Analysis
Protein concentration of muscle homogenates was determined by Pierce BCA Assay according to manufacturer’s instructions. Abundance and activation of insulin signaling proteins in rectus femoris of fetal samples and gastrocnemius of juveniles were measured by Simple Western (Wes; ProteinSimple, San Jose, CA). Plates were loaded according to manufacturer’s instructions with samples at a final concentration of 0.2 mg/mL total protein. Primary antibody dilutions used are reported in Supplementary Table 1. Proteins were separate using the 12–230 kDa capillary separation module (cat. no. SM-W003; ProteinSimple); data were quantified using Compass software, version 4.0.
Statistical Analysis
Data were analyzed as indicated in figure legends using GraphPad Prism, version 8.2.
Data and Resource Availability
The authors declare that the main data supporting the findings of this study are available within the article and its Supplementary Material. All other data supporting the findings of this study are available from the corresponding author upon request.
Results
Dam Phenotype and Pregnancy Characteristics
The characteristics of a larger cohort of dams on WSD have previously been described (16). Females on the WSD were slightly older, but reproductively healthy, and subsequently had higher parity compared with females on the CON diet (Table 1). Additionally, WSD-fed dams compared with CON had increased body weight and body fat percent and elevated fasting insulin prior to pregnancy and during the 3rd trimester (Table 1). During an IVGTT, IAUC was significantly elevated prior to pregnancy and during the 3rd trimester; however, the glucose AUC was only significantly increased during the 3rd trimester. There was no difference in fetal weight at gestational day 130. Interestingly, gestational length in this cohort was significantly increased in the WSD-fed females (Table 1). Together, these findings indicate greater insulin resistance and obesity in the adult females on the WSD compared with CON.
Table 1.
Maternal phenotype and pregnancy characteristics
| CON average | SEM | n | WSD average | SEM | n | P | |
|---|---|---|---|---|---|---|---|
| Maternal age (years) | 9.0 | 0.6 | 13 | 11.0 | 0.3 | 19 | 0.0003 |
| Parity | 3.7 | 0.5 | 13 | 5.6 | 0.3 | 19 | 0.003 |
| Body fat % | 13.5 | 1.5 | 13 | 34.0 | 2.2 | 19 | <0.0001 |
| Prepregnancy weight (kg) | 8.7 | 0.5 | 13 | 13.3 | 0.7 | 19 | <0.0001 |
| 3rd-trimester weight (kg) | 9.6 | 0.6 | 12 | 13.5 | 0.5 | 15 | <0.0001 |
| Prepregnancy fasting insulin (mU/L) | 12.9 | 2.6 | 13 | 25.9 | 4.4 | 18 | 0.03 |
| 3rd-trimester fasting insulin (mU/L) | 14.1 | 3.1 | 12 | 35.0 | 7.7 | 14 | 0.03 |
| Prepregnancy fasting glucose (mg/dL) | 56.9 | 3.1 | 13 | 53.6 | 2.5 | 19 | 0.4 |
| 3rd-trimester fasting glucose (mg/dL) | 46.6 | 1.8 | 12 | 45.3 | 2.0 | 12 | 0.6 |
| Prepregnancy IAUC | 1,890 | 388 | 12 | 6,365 | 692 | 17 | 0.01 |
| 3rd-trimester IAUC | 3,653 | 519 | 11 | 5,899 | 738 | 13 | 0.02 |
| Prepregnancy glucose AUC | 4,932 | 778 | 12 | 6,487 | 575 | 19 | 0.1 |
| 3rd-trimester glucose AUC | 4,020 | 539 | 12 | 6,430 | 751 | 13 | 0.01 |
| Fetal weight at g130 (g) | 348 | 15 | 5 | 351 | 8 | 11 | 0.5 |
| Gestational length (days) | 165 | 3 | 8 | 175 | 1.7 | 8 | 0.01 |
Maternal Obesity and WSD Reduce Insulin-Stimulated Glucose Uptake in Fetal Skeletal Muscle
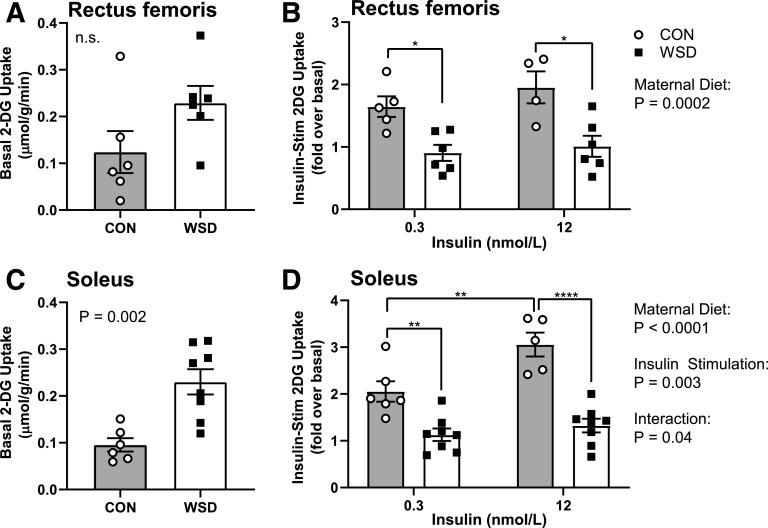
To test the effect of maternal WSD on insulin-stimulated glucose uptake, we measured [H3]-labeled 2DG uptake into skeletal muscle in the presence of low (0.3 nmol/L) insulin or high (12 nmol/L) insulin or in the absence of insulin (basal). In rectus femoris, there was no significant difference in basal glucose uptake between maternal CON and WSD fetuses (Fig. 2A). In the soleus, there was a significant increase in basal 2DG uptake with maternal WSD (Fig. 2C). Insulin-stimulated 2DG uptake, expressed as a fold increase over basal, was significantly reduced in rectus femoris and soleus from WSD compared with CON at both insulin concentrations (Fig. 2B and D).
Figure 2.
Fetal skeletal muscle ex vivo glucose uptake. Muscle strips from male and female fetal offspring at g130 from dams on CON or WSD diet were assayed for ex vivo glucose uptake. 2DG uptake was measured in basal (A and C) and insulin-stimulated (Insulin-Stim) (B and D) rectus femoris (A and B) and soleus (C and D) muscles at 0.3 nmol/L and 12 nmol/L insulin doses. Data are expressed as mean ± SEM, with individual data points shown. Basal glucose uptake was analyzed by unpaired t test. Insulin-stimulated glucose uptake was analyzed by two-way ANOVA with Tukey multiple comparisons test. Brackets indicate group comparisons (*P ≤ 0.05, **P ≤ 0.01, ****P ≤ 0.0001, n = 4–5 in CON and 6–8 in WSD).
Reduced Ex Vivo Insulin-Stimulated Glucose Uptake in Fetal Skeletal Muscle Exposed to Maternal WSD Is Associated With Attenuated Insulin Signaling
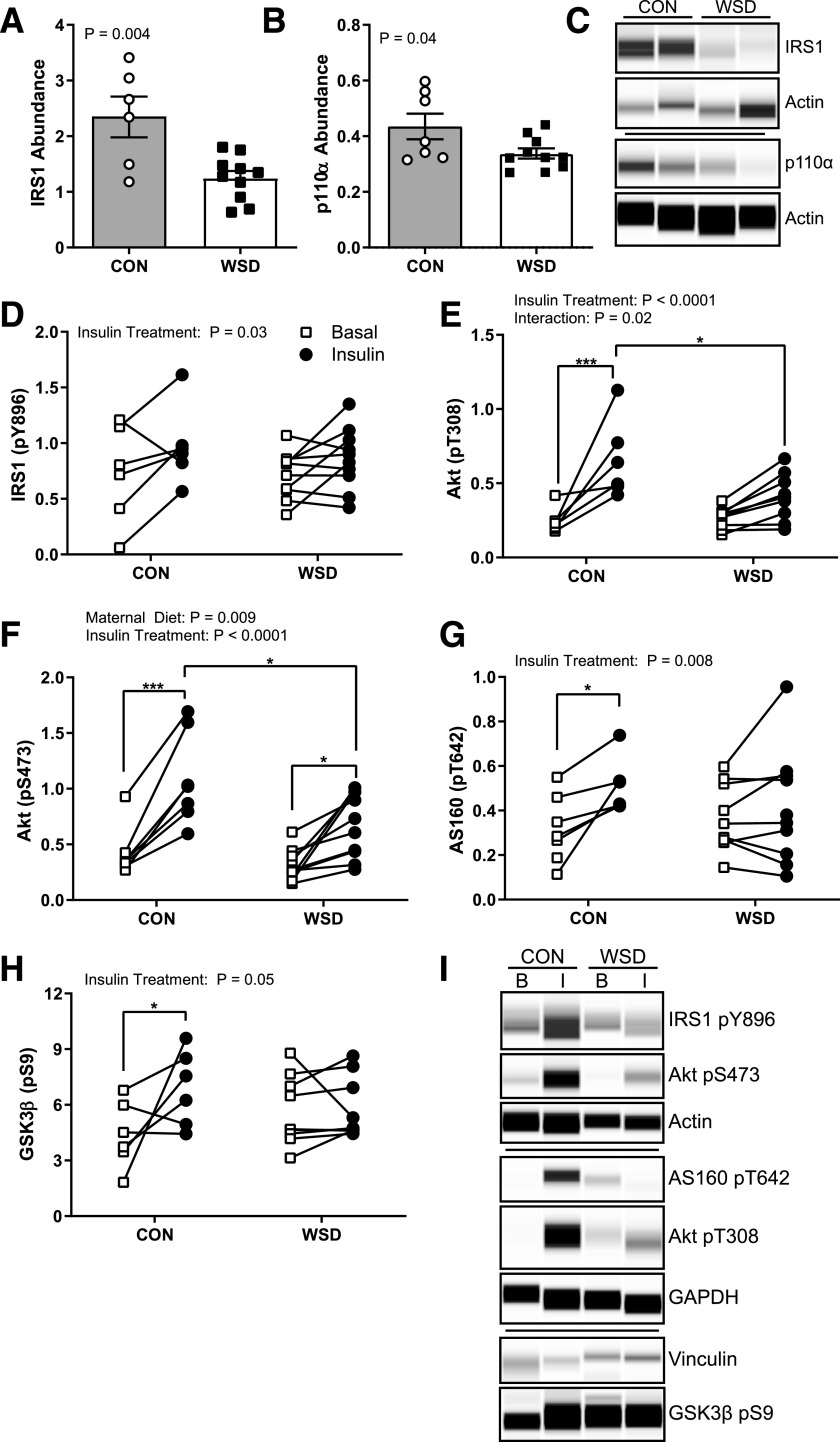
In rectus femoris muscle, IRS1 and p110α abundance were significantly decreased in the maternal WSD group (Fig. 3A–C), while insulin receptor, p85α, Akt, GSK3β, and GLUT1 abundance were not different between groups (Supplementary Fig. 1). While insulin-stimulated IRS1 phosphorylation at Y896 in fetal muscle was not affected by maternal diet, Akt phosphorylation at T308 and S473 was significantly lower in WSD compared with CON (Fig. 3D–F and I). Consistent with reduced insulin-stimulated Akt activation, AS160 and GSK3β phosphorylation showed no insulin-stimulated increase over basal in the WSD group (Fig. 3G–I). These data suggest that developmental exposure to maternal obesity and a WSD reduces insulin action in fetal muscle ex vivo even in the absence of systemic factors.
Figure 3.
Fetal skeletal muscle ex vivo insulin response. The abundance of total IRS1 (A) and total p110α (B) measured in homogenate of soleus muscle in the basal state expressed as the peak area relative to the control protein Actin. C: Example of Simple Western probing for IRS1, p110α, and Actin. D–H: Quantification of Simple Western probing of soleus muscle homogenates for insulin-responsive phosphorylation of IRS1 Y896 (D), Akt T308 (E), Akt S473 (F), AS160 T642 (G), and GSK3β S9 (H) expressed as the peak area relative to control proteins Actin, GAPDH, or Vinculin in basal and insulin-stimulated samples ex vivo. I: Example Simple Western probing for insulin-responsive protein phosphorylation. Data are expressed as mean ± SEM, with individual data points shown. Total protein abundance was analyzed by unpaired t test. Phosphorylated (p) Akt abundance was analyzed by two-way ANOVA with Tukey multiple comparisons test. All other phosphorylated protein abundance was analyzed by a mixed-effects model with Sidak correction. Brackets indicate group comparisons (*P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, n = 6–7 in CON and 8–10 in WSD).
Juvenile Phenotype
A separate cohort of offspring was studied at approximately 7 months of age, prior to weaning, and again at ∼14 months of age. At ∼8 months of age, offspring were weaned to new group housing and either maintained on the same diet as their maternal group or switched to the opposite diet, creating four diet groups (maternal diet/offspring diet: CON/CON, CON/WSD, WSD/CON, and WSD/WSD [Fig. 1]). Offspring exposed to maternal WSD had increased body weight relative to the offspring from the maternal CON group (Table 2). Postweaning WSD also resulted in increased body weight. Neither maternal nor postweaning WSD resulted in increases in body fat percentage, fasting insulin, fasting glucose, or increased IAUC during an IVGTT in juvenile offspring. Glucose AUC was lower in offspring weaned to the WSD compared with CON. The phenotype of a larger cohort of juvenile offspring has previously been reported (15,26). In the larger cohort, fasting insulin and IAUC were increased in the WSD/WSD group. In our subset, fasting insulin trended toward being increased in the WSD/WSD animals but did not reach significance.
Table 2.
Juvenile phenotype at 14 months of age
| CON/CON | CON/WSD | WSD/CON | WSD/WSD | ANOVA | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SEM | n (F/M) | Mean | SEM | n (F/M) | Mean | SEM | n (F/M) | Mean | SEM | n (F/M) | P (M) | P (PW) | ||
| Weight (kg) | 2.4a | 0.04 | 1/3 | 2.8ab | 0.2 | 2/2 | 3.0ab | 0.07 | 1/3 | 3.2b | 0.2 | 1/3 | 0.005 | 0.03 | |
| Body fat (%) | 1.7 | 0.4 | 1/3 | 3.0 | 0.8 | 1/1^ | 2.9 | 1.2 | 1/3 | 1.7 | 0.7 | 1/3 | 0.9 | 1.0 | |
| Insulin (mU/L)* | 2.1 | 0.5 | 1/3 | 4.0 | 1.1 | 1/2^ | 3.7 | 1.0 | 1/3 | 4.3 | 1.1 | 1/3 | 0.3 | 0.2 | |
| Glucose (mg/dL)* | 56.3 | 2.1 | 1/3 | 55.0 | 3.6 | 1/2 | 64.0 | 6.2 | 1/3 | 54.8 | 5.4 | 1/3 | 0.5 | 0.3 | |
| Glucose AUC | 5,681 | 285 | 1/3 | 4681 | 269 | 2/2 | 5,751 | 444 | 1/3 | 4,802 | 377 | 1/3 | 0.8 | 0.02 | |
| IAUC | 1,259 | 187 | 1/3 | 1071 | 239 | 2/2 | 1,026 | 164 | 1/3 | 1,154 | 142 | 1/3 | 0.7 | 0.9 | |
F, female; Int, interaction; M, male; PW, postweaning diet.
Different letters represent differences between groups (P < 0.05).
Taken at necropsy.
Missing data points.
Reduced Insulin-Stimulated Skeletal Muscle Glucose Uptake in Offspring Exposed to Maternal WSD Persists in 14-Month-Old Juveniles
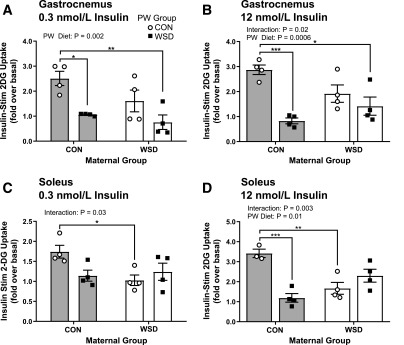
To determine whether decreases in insulin-stimulated glucose uptake in fetal skeletal muscle of WSD-fed mothers persists and influences the effects of postweaning WSD, we measured 2DG uptake in gastrocnemius and soleus muscles of juvenile offspring. In the gastrocnemii, at both insulin concentrations, postweaning WSD reduced 2DG uptake within the maternal CON groups (Fig. 4A and B). Postweaning WSD reduced 2DG uptake within the maternal WSD groups as compared with CON/CON but was not different from that in offspring exposed only to maternal WSD or only to postweaning WSD. In the soleus muscle, maternal WSD resulted in reduced insulin-stimulated 2DG uptake within the postweaning CON groups at both insulin concentrations (Fig. 4C and D). There was no difference between postweaning diets within each maternal group at the lower insulin dose. At the higher insulin concentration, postweaning WSD led to significantly reduced insulin-stimulated 2DG uptake within the maternal CON group. Again, there was no difference between postweaning WSD and CON in offspring exposed to maternal WSD. Taken together, these data show that maternal WSD exposure results in defects in insulin-stimulated 2DG uptake that persist in skeletal muscle postweaning at 1 year of age prior to increases in offspring adiposity or clinical measures of impaired glucose tolerance. The magnitude of suppression is similar to that of a postweaning WSD alone.
Figure 4.
Juvenile skeletal muscle ex vivo glucose uptake. Insulin-stimulated (Insulin-Stim) 2DG uptake measured ex vivo in 14-month-old juvenile gastrocnemius at 0.3 nmol/L (A) or 12 nmol/L (B) insulin concentration. Insulin-stimulated 2DG uptake measured in 14-month-old soleus ex vivo at 0.3 nmol/L (C) or 12 nmol/L (D) insulin concentration. Insulin-stimulated 2DG uptake is reported as fold increase over basal (unstimulated) glucose uptake. Data are expressed as mean ± SEM, with individual data points shown, and analyzed by two-way ANOVA with Tukey multiple comparisons test. Brackets indicate group comparisons (*P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, n = 3–4 in maternal CON and 4 in maternal WSD). PW, postweaning diet.
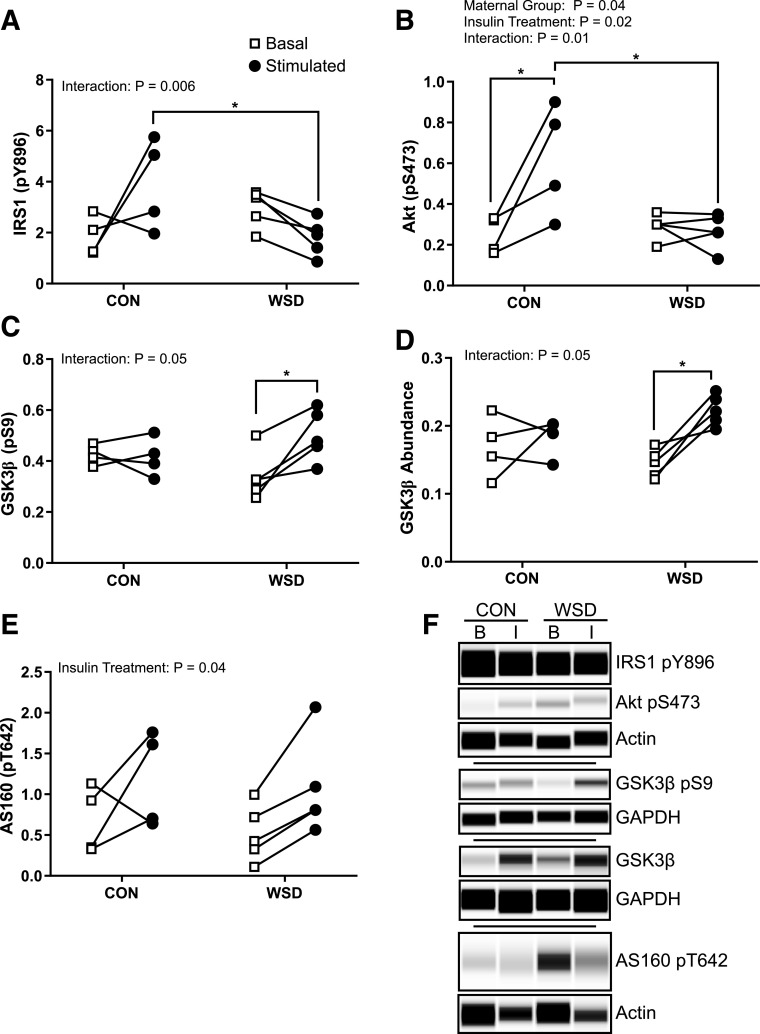
Ex Vivo Activation of Insulin Signaling Is Modestly Impaired in Skeletal Muscle of 14-Month-Old Juvenile Offspring Exposed to Maternal WSD
For determination of the mechanisms for reduced insulin-stimulated 2DG uptake, the abundance and activation state of key insulin signaling intermediates were measured in basal and insulin-stimulated (12 nmol/L) gastrocnemius. There was no difference in the total abundance of IRS1 or p110α as previously observed in the fetal muscle or in Akt by maternal or postweaning diet (Supplementary Fig. 2). We observed a significant effect of diet group on IRS1 phosphorylation by two-way ANOVA; however, there was not a specific difference in insulin effect among groups (Fig. 5A and E). There was, however, a significant increase in Akt phosphorylation at S473, but not T308, with insulin stimulation in the CON/CON group that was not observed in any other diet group, indicating a modest reduction in insulin signaling pathway activation by either maternal or postweaning diet (Fig. 5B, C, and E). Insulin stimulation of AS160 phosphorylation at T642 also showed a significant effect by insulin treatment but not by group (Fig. 5D and E). Together, we find that insulin signaling activation was modestly reduced by either maternal or postweaning WSD, but overall insulin signaling pathway activation was low in this ex vivo assay.
Figure 5.
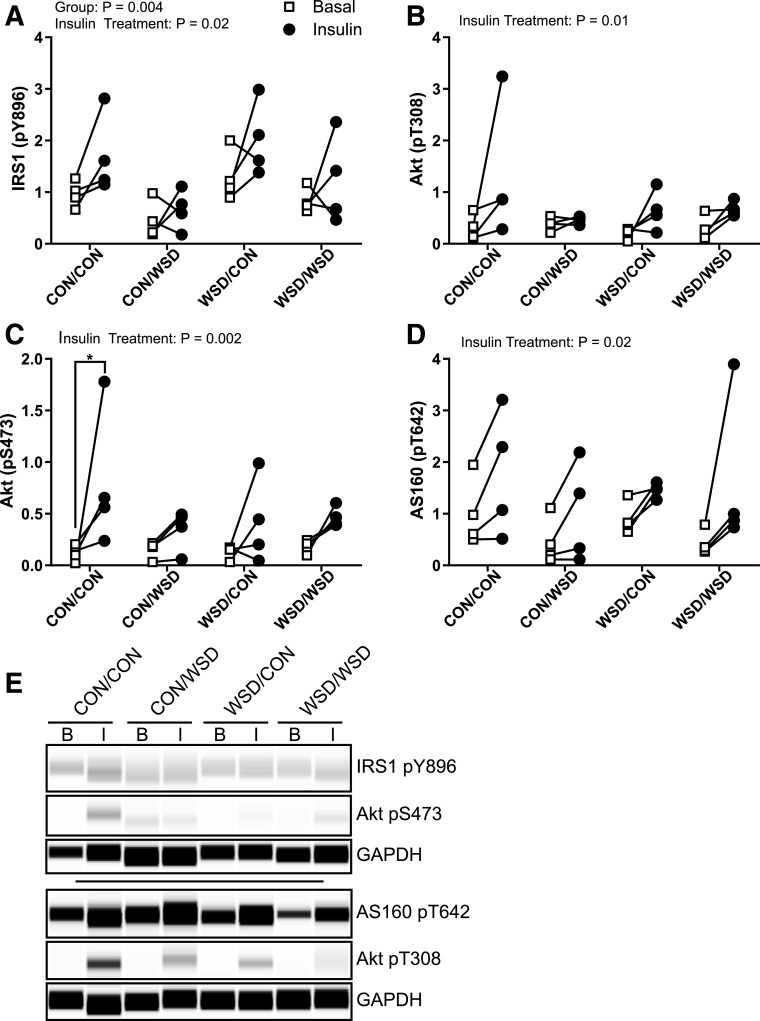
Juvenile skeletal muscle ex vivo insulin response. Insulin-responsive protein phosphorylation (p) measured by Simple Western in ex vivo basal and insulin-stimulated soleus. IRS1 pY896 (A), Akt pT308 (B), Akt pS473 (C), and AS160 pT642 (D) peak area expressed as a ratio to the control protein GAPDH. E: Example Simple Western of phosphorylated protein staining in basal (B) or insulin-stimulated (I) samples. CON/CON, maternal CON diet, postweaning CON diet group; CON/WSD, maternal control diet, postweaning WSD group; WSD/CON, maternal WSD, postweaning CON diet group; WSD/WSD, maternal WSD, postweaning WSD group. Data were analyzed by two-way ANOVA with Tukey multiple comparisons test. Brackets indicate group comparisons (*P ≤ 0.05, n = 4 in all groups).
In Vivo Activation of the Skeletal Muscle Insulin Signaling Cascade Is Reduced in 7-Month-Old Offspring Exposed to a Maternal WSD
To complement our ex vivo measures of skeletal muscle insulin signaling, we also measured activation of the insulin signaling cascade in soleus muscle biopsies from 7-month-old animals before and after an intravenous insulin bolus (Fig. 1). For reference, offspring start eating independently by 4 months of age (27); therefore, data collected in 7-month-old offspring reflect a combination of maternal exposures during fetal development through lactation and WSD exposure through food introduction during this preweaning developmental period. In offspring muscle, phosphorylation of both IRS1 and Akt was significantly reduced in insulin-stimulated muscle of animals from the maternal WSD group (Fig. 6A, B, and F). Insulin stimulation increased GSK3β phosphorylation in the WSD but not CON group (Fig. 6C and F). The increase in GSK3β activation corresponded to a significant increase in the total abundance of GSK3β after insulin stimulation, indicating that the difference in activation between CON and WSD is not due solely to an increase in the fraction of GSK3β that is phosphorylated (Fig. 6D and F). Despite differences in Akt phosphorylation, insulin treatment resulted in a significant increase in AS160 phosphorylation that was not different by maternal diet group (Fig. 6E and F). Consistent with the ex vivo juvenile signaling data, but in contrast to the fetal insulin signaling data, we observed no difference in total abundance of IR, IRS1, p110α, or Akt (Supplementary Fig. 3).
Figure 6.
Juvenile skeletal muscle in vivo insulin response. Insulin-responsive protein phosphorylation (p) measured by Simple Western in gastrocnemius biopsies collected before (basal) and 10 min after (stimulated) intravenous insulin injection; IRS1 pY896 (A), Akt pS473 (B), GSK3β pS9 (C), total GSK3β (D), and AS160 pT642 (E). Abundance expressed as a ratio of protein peak area relative to Actin or GAPDH. F: Example Simple Western probing of protein abundance. Data were analyzed by two-way ANOVA with Tukey multiple comparisons test. Brackets indicate group comparisons (*P ≤ 0.05, n = 4 in CON and 5 in WSD).
Discussion
Maternal obesity is strongly associated with greater infant adiposity, insulin resistance (5), and an earlier risk for developing metabolic diseases like type 2 diabetes and nonalcoholic fatty liver disease (28–30); however, few studies have directly examined the effect of maternal obesity and WSD on activation of insulin signaling pathways in offspring tissues regulating glucose uptake and utilization, such as skeletal muscle. Skeletal muscle insulin resistance with impaired glucose uptake is a common metabolic disorder in obese individuals and a primary contributor to the etiology of type 2 diabetes (11,12). Here, we investigated insulin stimulation of glucose uptake in skeletal muscle of offspring exposed to maternal obesity and WSD during early developmental periods (i.e., fetal and preweaning) and shortly after weaning in Japanese macaques. We show for the first time that intrauterine exposure to maternal WSD-induced obesity reduces insulin-stimulated glucose uptake and activation of insulin signaling in fetal skeletal muscle in association with a reduction in IRS1 and p110α.
Fetal exposure to maternal WSD resulted in reduced fetal skeletal muscle glucose uptake in response to insulin stimulation at both low and high insulin concentrations in soleus and to a lesser extent in the rectus femoris. Interestingly, basal glucose uptake was increased in the soleus. Reduced glucose uptake in fetal rectus femoris muscle was accompanied by attenuated expression of IRS1 and the p110α subunit of PI3K, potentially resulting in reduced capacity to respond to insulin or reduced sensitivity. Consistent with reduced insulin signaling activation, insulin-stimulated phosphorylation of Akt and substrates of Akt was reduced by maternal obesity and WSD. Surprisingly, we saw no difference by maternal diet in insulin-stimulated phosphorylation of IRS1 in the fetal muscle. Impaired signaling at the level of IRS1 activation is a common marker of insulin resistance with high-fat diet or obesity in adults and is often linked to elevated activation of proinflammatory cytokine or stress signaling by JNK or atypical PKCs (31). Our observations in fetal samples suggest that glucose transport is attenuated by maternal obesity through reduced signal transduction prior to Akt activation, potentially through reduced PI3K activity.
In agreement with our fetal data, maternal obesogenic diet during pregnancy and lactation resulted in reduced abundance of both IRS1 and the p110β subunit of PI3K in adult rodent offspring skeletal muscle (32) and adipose tissue (33). Further, IRS1-PI3K activity, but not IRS1 phosphorylation, was reduced in fetal muscle in offspring of obese sheep (34); although these measures in fetal sheep were made in fasted, and not insulin-stimulated, tissues, they provide additional evidence that IRS1-PI3K may be a critical node regulated by maternal obesity in the fetal muscle. Lastly, reduced insulin-stimulated glucose uptake in fetal muscle has also been found in a rat model of fetal hyperglycemia with impairments in insulin signaling occurring at the level of Akt and not IRS1 (35).
The decrease in glucose uptake may also be associated with low oxidative capacity and glucose oxidation as a fuel source by mitochondria reported previously in primary myotubes from WSD fetuses (17). Previously, we found that fetal muscle responds to maternal obesity and WSD by upregulating fatty acid oxidation, mitochondrial complex activity, and metabolic switches (CPT-1, PDK4) that promote lipid utilization over glucose oxidation (17). The mechanisms underpinning this adaption are unclear but are likely initiated by functional demands on the muscle given the primary energy-rich high-fat diet and reduced oxygen supply (36). Several studies have also demonstrated a relationship between mitochondrial dysfunction and muscle cell insulin function at the level of IRS1 abundance; therefore, the previously observed changes in mitochondrial function induced by maternal WSD may also contribute to loss of insulin response observed here (37,38).
We also observed persistent insulin resistance in skeletal muscle glucose transport of otherwise healthy juvenile offspring from obese WSD dams despite weaning offspring to a healthy control diet. Insulin-stimulated glucose uptake was reduced in the soleus, and to a lesser extent in the gastrocnemius, of offspring exposed to maternal WSD but weaned to a healthy diet. All three groups exposed to WSD exhibited a modest loss of insulin-stimulated Akt phosphorylation in gastrocnemius muscle relative to the CON/CON group. Given the modest signaling response observed ex vivo, and relatively low sample size, allowing disproportionate effects by individuals, we also assessed the activation of the insulin signaling cascade in skeletal muscle biopsies from 7-month-old offspring before and after intravenous administration of an insulin bolus. Offspring of WSD dams had reduced insulin activation of both IRS1 and Akt in agreement with our ex vivo signaling data. Downregulation in IRS1 activation may reflect exposure to postnatal WSD through a combination of independent eating and lactation. Unexpectedly, activation of GSK3β and AS160, downstream targets of Akt, was unaffected by maternal WSD. We speculate that this may be due to a compensatory increase in protein abundance in response to insulin as seen with GSK3β in WSD offspring. Together, these observations suggest that the mechanism of insulin resistance in the fetal skeletal muscle occurs downstream of IRS1 and is different from obesity or WSD-induced insulin resistance in juvenile animals.
Although increasing evidence indicates that developmental exposures to maternal obesity or diabetes or a poor-quality maternal diet increases offspring risk of obesity and metabolic diseases, the mechanisms that drive this phenomenon are poorly understood. Many of the metabolic adaptations in offspring are thought to be driven by changes in placental lipid transport (21,39,40), reduced oxygen delivery to the developing fetus (36,41), or increased fetal exposure to inflammatory cytokines (42). Fetal adaptations to this altered in utero environment are thought to persist into the postnatal period due, in part, to changes in the offspring epigenome; the activity of epigenetic regulators, which can influence the expression of molecules important to hormone signaling and metabolism; and/or changes in noncoding RNAs such as microRNAs and long noncoding RNAs (43–45).
While our study is unique in examining functional measures of insulin activation in skeletal muscle, our nonhuman primate model limits us to observational data. In the absence of loss- and gain-of-function studies, we cannot conclude that the molecular changes detected are causative of reduced insulin-stimulated glucose uptake. Additionally, in this small cohort, we cannot separate out the effects of maternal WSD from those of maternal obesity or detect differences between male and female offspring. Despite these limitations, our conclusions are based on direct measures of insulin-stimulated glucose transport and signaling pathway activation. While this study has focused on insulin activation of skeletal muscle in response to maternal obesity and WSD, it should be noted that all of the changes found in the juvenile offspring occurred in the absence of overt adiposity, hyperinsulinemia, or reduced systemic insulin sensitivity based on measures of insulin and glucose AUC during an IVGTT. Thus, the reduced skeletal muscle glucose transport and phosphorylation preceded whole-body insulin resistance. Evidence for fetal programming of adult body composition and insulin resistance in humans is derived mostly from epidemiological studies, associating poor quality nutrition and obesity during pregnancy with increased fat mass, central distribution of fat, and metabolic syndrome in the offspring (46).
Previous work in this model has shown that maternal obesity and WSD result in greater hepatic lipid accumulation and de novo lipid synthesis in fetal and postnatal offspring at 14 months of age (16,17,36) and significantly elevated β-cell–to–α-cell ratio in offspring at 14 and 36 months of age (15,47). Importantly, in viewing our outcomes in muscle in the context of this greater picture of the physiological changes, it is easy to predict that these offspring would be more susceptible to earlier development of whole-body insulin resistance and metabolic diseases when faced with challenges like a continued WSD, sedentary lifestyle, or even puberty.
Together, these data are the first to suggest that maternal obesity with WSD exposure in utero results in reduced skeletal muscle insulin response that persists at 1 year of age and is comparable with postweaning WSD exposure alone. Given that the offspring were young and lean with normal glucose tolerance, we speculate that these are primary changes, involved in the development of muscle insulin resistance, that may manifest themselves during puberty (2.5–3.0 years of age in Japanese macaques) or future metabolic challenges. While we have observed changes in insulin response and insulin-stimulated glucose uptake driven by maternal obesity and WSD, the mechanism for this altered response remains to be determined and its identification is an important goal of our future studies.
Article Information
Funding. This research was supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health (NIH), grant K12 HD057022 (to C.E.M.) and National Institute of Diabetes and Digestive and Kidney Diseases, NIH, grants R24 DK090964 (to J.E.F. and K.A.) and R01 DK089201 (to K.A.).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. C.E.M. was responsible for the conception and design of the muscle experiments. C.E.M., B.H., W.C.-B., S.S., S.R.W., D.L.T., and T.A.D. collected the samples, conducted the research, and analyzed data. J.E.F., K.A., E.L.S., P.K., D.L.T., and T.A.D. maintained and supported the animal model. B.H., W.C.-B., and C.E.M. wrote the manuscript. S.S., S.R.W., K.A., E.L.S., P.K., M.G., and J.E.F. contributed to the interpretation of the data and revision of the manuscript. All authors read and approved the final manuscript. C.E.M. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 76th Scientific Sessions of the American Diabetes Association, New Orleans, LA, 10–14 June 2016. A preprint of this paper has been deposited in BioRxiv (https://doi.org/10.1101/864082).
Footnotes
W.C.-B. and B.H. contributed equally to this work.
This article contains supplementary material online at https://doi.org/10.2337/figshare.12206021.
References
- 1.Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of Obesity Among Adults and Youth: United States, 2015–2016. Hyattsville, MD, National Center for Health Statistics, 2017 [Google Scholar]
- 2.Godfrey KM, Reynolds RM, Prescott SL, et al. Influence of maternal obesity on the long-term health of offspring. Lancet Diabetes Endocrinol 2017;5:53–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heslehurst N, Vieira R, Akhter Z, et al. The association between maternal body mass index and child obesity: a systematic review and meta-analysis. PLoS Med 2019;16:e1002817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sewell MF, Huston-Presley L, Super DM, Catalano P. Increased neonatal fat mass, not lean body mass, is associated with maternal obesity. Am J Obstet Gynecol 2006;195:1100–1103 [DOI] [PubMed] [Google Scholar]
- 5.Catalano PM, Presley L, Minium J, Hauguel-de Mouzon S. Fetuses of obese mothers develop insulin resistance in utero. Diabetes Care 2009;32:1076–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics 2005;115:e290–e296 [DOI] [PubMed] [Google Scholar]
- 7.Llewellyn CH, Trzaskowski M, Plomin R, Wardle J. Finding the missing heritability in pediatric obesity: the contribution of genome-wide complex trait analysis. Int J Obes 2013;37:1506–1509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schulz LC. The Dutch Hunger Winter and the developmental origins of health and disease. Proc Natl Acad Sci U S A 2010;107:16757–16758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyle KE, Patinkin ZW, Shapiro AL, Baker PR 2nd, Dabelea D, Friedman JE. Mesenchymal stem cells from infants born to obese mothers exhibit greater potential for adipogenesis: the Healthy Start BabyBUMP Project. Diabetes 2016;65:647–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boyle KE, Patinkin ZW, Shapiro ALB, et al. Maternal obesity alters fatty acid oxidation, AMPK activity, and associated DNA methylation in mesenchymal stem cells from human infants. Mol Metab 2017;6:1503–1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeFronzo RA, Tripathy D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care 2009;32(Suppl. 2)S157–S163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petersen KF, Dufour S, Savage DB, et al. The role of skeletal muscle insulin resistance in the pathogenesis of the metabolic syndrome. Proc Natl Acad Sci U S A 2007;104:12587–12594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nicholas LM, Morrison JL, Rattanatray L, Zhang S, Ozanne SE, McMillen IC. The early origins of obesity and insulin resistance: timing, programming and mechanisms. Int J Obes 2016;40:229–238 [DOI] [PubMed] [Google Scholar]
- 14.McCurdy CE, Bishop JM, Williams SM, et al. Maternal high-fat diet triggers lipotoxicity in the fetal livers of nonhuman primates. J Clin Invest 2009;119:323–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Comstock SM, Pound LD, Bishop JM, et al. High-fat diet consumption during pregnancy and the early post-natal period leads to decreased α cell plasticity in the nonhuman primate. Mol Metab 2012;2:10–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thorn SR, Baquero KC, Newsom SA, et al. Early life exposure to maternal insulin resistance has persistent effects on hepatic NAFLD in juvenile nonhuman primates. Diabetes 2014;63:2702–2713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCurdy CE, Schenk S, Hetrick B, et al. Maternal obesity reduces oxidative capacity in fetal skeletal muscle of Japanese macaques. JCI Insight 2016;1:e86612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frias AE, Morgan TK, Evans AE, et al. Maternal high-fat diet disturbs uteroplacental hemodynamics and increases the frequency of stillbirth in a nonhuman primate model of excess nutrition. Endocrinology 2011;152:2456–2464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thompson JR, Gustafsson HC, DeCapo M, et al. Maternal diet, metabolic state, and inflammatory response exert unique and long-lasting influences on offspring behavior in non-human primates. Front Endocrinol (Lausanne) 2018;9:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roberts VHJ, Pound LD, Thorn SR, et al. Beneficial and cautionary outcomes of resveratrol supplementation in pregnant nonhuman primates. FASEB J 2014;28:2466–2477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Tierney-Ginn P, Roberts V, Gillingham M, et al. Influence of high fat diet and resveratrol supplementation on placental fatty acid uptake in the Japanese macaque. Placenta 2015;36:903–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCurdy CE, Cartee GD. Akt2 is essential for the full effect of calorie restriction on insulin-stimulated glucose uptake in skeletal muscle. Diabetes 2005;54:1349–1356 [DOI] [PubMed] [Google Scholar]
- 23.Friedman JE, Ishizuka T, Shao J, Huston L, Highman T, Catalano P. Impaired glucose transport and insulin receptor tyrosine phosphorylation in skeletal muscle from obese women with gestational diabetes. Diabetes 1999;48:1807–1814 [DOI] [PubMed] [Google Scholar]
- 24.Cartee GD, Bohn EE. Growth hormone reduces glucose transport but not GLUT-1 or GLUT-4 in adult and old rats. Am J Physiol 1995;268:E902–E909 [DOI] [PubMed] [Google Scholar]
- 25.Young DA, Uhl JJ, Cartee GD, Holloszy JO. Activation of glucose transport in muscle by prolonged exposure to insulin. Effects of glucose and insulin concentrations. J Biol Chem 1986;261:16049–16053 [PubMed] [Google Scholar]
- 26.Fan L, Lindsley SR, Comstock SM, et al. Maternal high-fat diet impacts endothelial function in nonhuman primate offspring. Int J Obes 2013;37:254–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thompson JR, Valleau JC, Barling AN, et al. Exposure to a high-fat diet during early development programs behavior and impairs the central serotonergic system in juvenile non-human primates. Front Endocrinol (Lausanne) 2017;8:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mingrone G, Manco M, Mora MEV, et al. Influence of maternal obesity on insulin sensitivity and secretion in offspring. Diabetes Care 2008;31:1872–1876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crume TL, Shapiro AL, Brinton JT, et al. Maternal fuels and metabolic measures during pregnancy and neonatal body composition: the healthy start study. J Clin Endocrinol Metab 2015;100:1672–1680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Montazeri P, Vrijheid M, Martinez D, et al. Maternal metabolic health parameters during pregnancy in relation to early childhood BMI trajectories. Obesity (Silver Spring) 2018;26:588–596 [DOI] [PubMed] [Google Scholar]
- 31.Copps KD, White MF. Regulation of insulin sensitivity by serine/threonine phosphorylation of insulin receptor substrate proteins IRS1 and IRS2. Diabetologia 2012;55:2565–2582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shelley P, Martin-Gronert MS, Rowlerson A, et al. Altered skeletal muscle insulin signaling and mitochondrial complex II-III linked activity in adult offspring of obese mice. Am J Physiol Regul Integr Comp Physiol 2009;297:R675–R681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Almeida Faria J, de Araújo TMF, Mancuso RI, et al. Day-restricted feeding during pregnancy and lactation programs glucose intolerance and impaired insulin secretion in male rat offspring. Acta Physiol (Oxf) 2016;217:240–253 [DOI] [PubMed] [Google Scholar]
- 34.Zhu MJ, Han B, Tong J, et al. AMP-activated protein kinase signalling pathways are down regulated and skeletal muscle development impaired in fetuses of obese, over-nourished sheep. J Physiol 2008;586:2651–2664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kua KL, Hu S, Wang C, et al. Fetal hyperglycemia acutely induces persistent insulin resistance in skeletal muscle. J Endocrinol 2019;242:M1–M15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wesolowski SR, Mulligan CM, Janssen RC, et al. Switching obese mothers to a healthy diet improves fetal hypoxemia, hepatic metabolites, and lipotoxicity in non-human primates. Mol Metab 2018;18:25–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lim JH, Lee JI, Suh YH, Kim W, Song JH, Jung MH. Mitochondrial dysfunction induces aberrant insulin signalling and glucose utilisation in murine C2C12 myotube cells. Diabetologia 2006;49:1924–1936 [DOI] [PubMed] [Google Scholar]
- 38.Park SY, Choi GH, Choi HI, Ryu J, Jung CY, Lee W. Depletion of mitochondrial DNA causes impaired glucose utilization and insulin resistance in L6 GLUT4myc myocytes. J Biol Chem 2005;280:9855–9864 [DOI] [PubMed] [Google Scholar]
- 39.Heerwagen MJR, Gumina DL, Hernandez TL, et al. Placental lipoprotein lipase activity is positively associated with newborn adiposity. Placenta 2018;64:53–60 [DOI] [PubMed] [Google Scholar]
- 40.Calabuig-Navarro V, Haghiac M, Minium J, et al. Effect of maternal obesity on placental lipid metabolism. Endocrinology 2017;158:2543–2555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roberts VHJ, Frias AE, Grove KL. Impact of maternal obesity on fetal programming of cardiovascular disease. Physiology (Bethesda) 2015;30:224–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shankar K, Zhong Y, Kang P, et al. Maternal obesity promotes a proinflammatory signature in rat uterus and blastocyst. Endocrinology 2011;152:4158–4170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rando OJ, Simmons RA. I’m eating for two: parental dietary effects on offspring metabolism. Cell 2015;161:93–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aagaard-Tillery KM, Grove K, Bishop J, et al. Developmental origins of disease and determinants of chromatin structure: maternal diet modifies the primate fetal epigenome. J Mol Endocrinol 2008;41:91–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li CCY, Young PE, Maloney CA, et al. Maternal obesity and diabetes induces latent metabolic defects and widespread epigenetic changes in isogenic mice. Epigenetics 2013;8:602–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gaillard R. Maternal obesity during pregnancy and cardiovascular development and disease in the offspring. Eur J Epidemiol 2015;30:1141–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Elsakr JM, Dunn JC, Tennant K, et al. Maternal Western-style diet affects offspring islet composition and function in a non-human primate model of maternal over-nutrition. Mol Metab 2019;25:73–82 [DOI] [PMC free article] [PubMed] [Google Scholar]