Abstract
Introduction
WHO recommends influenza vaccination for pregnant women and health providers (HPs), yet global uptake for both is persistently low. Research suggests that HPs greatly influence uptake of influenza vaccine in pregnant women. Our review studies HPs’ recommendation of influenza vaccine to pregnant women, determinants and barriers to recommendation, and the role that HPs may play in global influenza vaccine coverage.
Methods
We undertook a comprehensive global review of literature relating to HPs’ recommendation of seasonal influenza vaccines to pregnant women and the determinants and barriers to recommendation and how this may vary by country and context. We evaluated data from each study including frequency of HP recommendation, vaccine coverage, determinants and barriers to recommendation, and the odds of recommending. We tracked the frequency of determinants and barriers to recommendation in heat maps and organized data by world regions and income classifications.
Results
From 32 studies in 15 countries, we identified 68 determinants or barriers to HPs’ recommendation. Recommendation rates were highest (77%) in the Americas and lowest in South East Asia (18%). A HP’s own influenza vaccine status was a main determinant of recommendation in multiple country contexts and from different provider types. Financial barriers to recommendation were present in higher-income countries and policy-related barriers were highlighted in lower-income countries. HP perceptions of safety, efficacy, and the utility of vaccine were the most frequently cited barriers, relevant in almost every context.
Conclusions
HP recommendation is important to influenza vaccine implementation in pregnant women. A HP’s own status is an important recommendation determinant in multiple contexts. Vaccine program implementation plans should consider the impact of HPs' knowledge, awareness and vaccine confidence on their own uptake and recommendation practices, as well as on the uptake among pregnant women. Addressing safety and efficacy concerns is relevant in all contexts for HPs and pregnant women.
Abbreviations: HP(s), Health Providers(s); ObGyn(s), Obstetrician Gynecologist(s); PP(s), Prenatal Provider(s); GP(s), General Practitioner(s); AMR, The Americas Region; EUR, The European Region; SEAR, The South East-Asian Region; WPR, The Western Pacific Region; OR, Odds Ratio; PR, Prevalence Ratio; CI, Confidence Interval; MIC, Middle-income countries; LIC, Low-income countries
Keywords: Influenza, Vaccine, Recommendation, Health provider, Pregnancy, Determinants, Health worker
1. Introduction
Pregnant women seem to be at a higher risk than the general population for influenza-related complications, including higher rates of hospitalization and fetal and maternal death [1], [2], [3], [4], [5], [5] In 2012, the World Health Organization’s (WHO) Strategic Advisory Group of Experts on Immunization (SAGE) confirmed this risk by declaring that pregnant women be given the highest priority for seasonal influenza vaccination [6]. This position was taken further in the Global Influenza Strategy of 2019–2030 [7] through WHO’s commitment to develop strong, national capacities to implement seasonal influenza vaccine programs in all high-risk groups as part of the wider global strengthening of preparedness and response. Health providers (HPs) are an explicit part of this broader strategy to serve and ‘protect the individual, maintain critical health services during influenza outbreaks, and to reduce transmission to other vulnerable groups.’[7].
Despite these recommendations, less than 50% of the 194 WHO member states report having a policy for influenza vaccination in pregnant women in 2018 and only 56% report having a policy in HPs [8]. The majority of countries reporting policies are from high-income countries [8], illustrating the global disparity in influenza policies and vaccine access [9]. Achieving and maintaining high influenza vaccine coverage in pregnant women and in HPs is an ongoing challenge [10], [11], [12], even in countries with long-standing and prioritized influenza vaccination programs, such as in the United States [13].
Low vaccine uptake in pregnant women has been partly attributed to vaccine hesitancy, which is complex and multidimensional and can be influenced by individual, logistical, or cultural and sociologic factors [11], [14], among others. An added consideration to this is the importance of a HP’s recommendation of influenza vaccine, which research now suggests may be the strongest determinant of uptake in pregnant women [12], [15], [16], [17], even those with existing vaccine hesitancy. This trend is consistent across diverse country contexts [11], [18], patient populations [19], [20], [21], and vaccines [22].
Pregnancy provides HPs with a unique opportunity for repeat clinical encounters to educate pregnant women on the benefits of influenza vaccination, to advocate for vaccination, and to vaccinate. Despite the WHO’s recommendation to prioritize pregnant women for influenza vaccination and the role that HPs have in implementing this recommendation, they do not consistently recommend influenza vaccines [18], [23], [24], [25], [26] to pregnant women. Even though most HPs endorse vaccines as a saving measure for their patients, negative attitudes towards vaccination [10], [27] are not uncommon, and lower confidence in vaccine safety or vaccine benefit has also been found in HPs with lower willingness to recommend vaccines [10] or receive vaccines themselves [28].
To date, most studies on influenza vaccine uptake in pregnant women focus strictly on pregnant women and not on HPs and their contribution to vaccine acceptance in pregnant women [22], [29]. Additionally, no studies to our knowledge have evaluated the breadth of determinants or barriers to HPs’ influenza vaccine recommendation practices in pregnant women, globally. We used a scoping review [30] approach to identify studies across the globe on HPs’ recommendation practices in pregnant women in multiple contexts and the determinants or barriers to their recommendation. A better understanding of vaccine recommendation practices in HPs, and the determinants and barriers to recommendation in different contexts may facilitate the development of improved strategies to increase influenza vaccine coverage in pregnant women worldwide.
2. Methods
2.1. Literature search strategy
We developed and used a scoping review [30] approach to systematically, yet iteratively review evidence from clinical and non-clinical, qualitative and quantitative, cross-sectional, intervention, or systematic and non-systematic reviews relating to HPs’ recommendation of seasonal influenza vaccine to pregnant women. The search had no date range limitation and included foreign language publications with at least the abstract in the English language. In a pilot search to identify the most appropriate search terms, we queried multiple iterations of terms and combined the results. From this, we refined our search strategy and the respective terms to identify the most appropriate publications (see appendix for full search protocol and associated search terms). Part of the search focused on ‘HPs’ as the population, ‘influenza vaccine recommendation’ from the provider as the intervention, the ‘lack of an influenza vaccine recommendation’ as a comparator, and ‘influenza vaccine uptake’ or ‘influenza vaccine coverage’ in pregnant women as the outcome. We also searched more broadly for determinants and barriers to recommendation. The same search was performed by KM and PL separately using PubMed, The Cochrane Library Databases, WHO Global Health Medicus (which includes EMBASE) and Google Scholar. Results were then pooled and any disagreements were settled through discussions and using a checklist of inclusion and exclusion criteria. Multiple iterations were executed in the same database with and without the following MESH terms: “health personnel”, “health providers”, “allied health personnel”, “influenza vaccines”, “pregnancy”, “pregnant women”, “attitudes of health personnel”, “health knowledge, attitudes and practice”. Non-MESH terms included “recommend*”, “willingness to recommend“, “recommendation rates”, ”promote“, ”advocate“, ”vaccination rates“, ”coverage”, “determinants”, and “factors”.
2.2. Inclusion and exclusion criteria
We included studies on the determinants or barriers of HP recommendation or HP offer of influenza vaccines to pregnant women; and/or studies about their behaviors, knowledge, attitudes, beliefs, or practices of recommending influenza vaccine to pregnant women. Studies were also included on influenza vaccination coverage in pregnant women if the component of recommendation practices from health providers was included as part of the study analysis or objectives.
Commentaries, policy documents, non-research studies, and individual studies that did not focus on influenza vaccination in pregnant women were all excluded. Additionally, studies focusing on influenza vaccination in pregnant woman were excluded if they did not include HP recommendation practices as part of their objective or study results. Reviews that captured various study designs and objectives were excluded if not evaluating some studies on HPs and their recommendation practices of influenza vaccination in pregnant women.
2.3. Data presentation and analysis
Literature was organized by country, WHO world region, and topic of interest. Long-format tables captured further details including author, year, influenza vaccine (pandemic or seasonal or both), study design, number/type of participants, settings, outcomes, and conclusions. Data was then summarized into a succinct table (see Table 2) of all studies and analyzed in Excel as needed. When available, data was captured on HPs’ recommendation rate of influenza vaccine to pregnant women, all identified determinants and barriers to recommendation, influenza vaccine coverage in pregnant women, and odds ratios/prevalence ratios and confidence intervals of significant determinants or barriers.
Table 2.
All included studies, their reported provider recommendation rates with other details including average recommendation rates by region (AMR- The Americas; EUR- European Region; SEAR-South-East Asian Region; WPR-Western Pacific Region) and by high or middle-income status.
 |
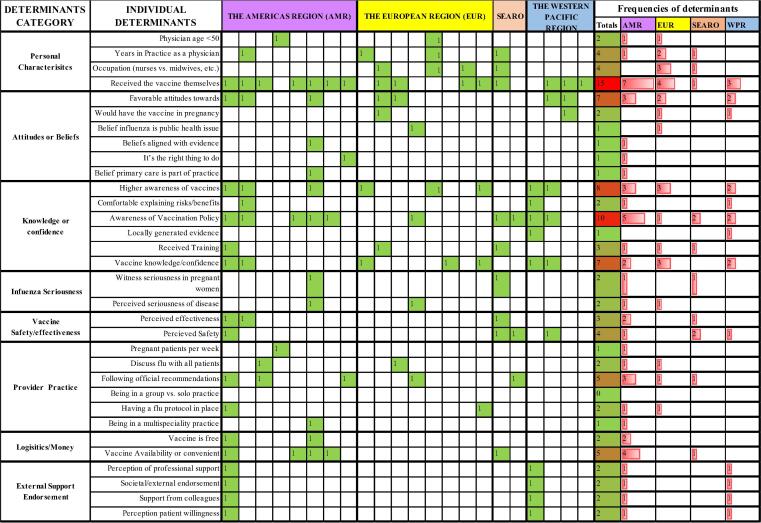
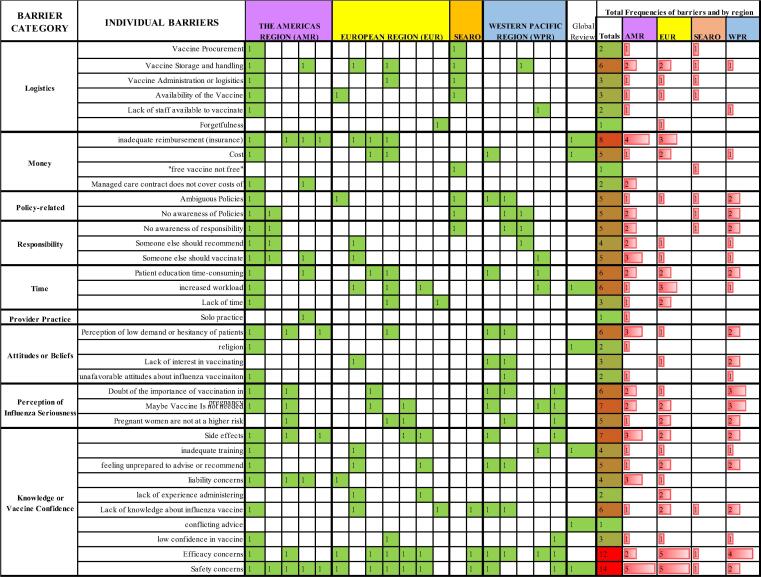
The frequency of individual determinants and barriers to recommendation practices were captured in heat maps and organized into categories (see Fig. 3, Fig. 5). The categories were loosely based on examples from the included publications and were intended to provide a non-fixed structure to the long list of determinants and barriers. Some determinants or barriers may fit into more than one category. The heat maps were color coded with red indicating the highest frequency and green the lowest.
Fig. 3.
Heat Map of the frequency of all identified determinants from 22 studies organized into suggested categories; studies organized by the WHO Region from which the study was executed and frequencies of determinants by region.
Fig. 5.
Heat Map of identified barriers in suggested categories from 20 studies and their total frequencies and the frequencies by WHO region.
2.4. Definitions used in this study
We used the following terms in our review with the following underlying assumptions.
Health Providers (HPs) are any and all health workers, care providers, midwives, nurses or personnel who were recommending influenza vaccine to pregnant women and vaccinating pregnant women, or recommending and referring women to be vaccinated.
Obstetrician/gynecologists (ObGyns) include obstetricians, gynecologists, or obstetrician/gynecologists.
Prenatal Providers (PPs) are all health personnel mentioned in a study to have contributed to the care of pregnant women including general practitioners, gynecologists, obstetricians, antenatal care specialists, obstetrician/gynecologists (ObGyns), nurses, midwives, pharmacists, physician assistants, or nurse practitioners.
General Practitioners (GPs) include family health practitioners/family physicians/family doctors/primary care physicians.
Obstetrics Health Workers are any or all staff working in obstetric practices.
Recommendation Rate is the percentage of all providers per study who would or did recommend the influenza vaccine to pregnant women OR the percentage of pregnant women who say they were recommended the vaccine by their health provider (a distinction was made between these two rates, they were not grouped together).
Determinants by at least one study to have positively influenced a health provider’s recommendation/proposal/offer of the influenza vaccine to a pregnant patient population.
Barriers are all things confirmed by at least one study to have negatively influenced or prohibited a recommendation of influenza vaccine in a pregnant woman.
World Regions are according to the WHO World regions classification [31] and the income classification is according to the World Bank Income classifications
3. Main findings
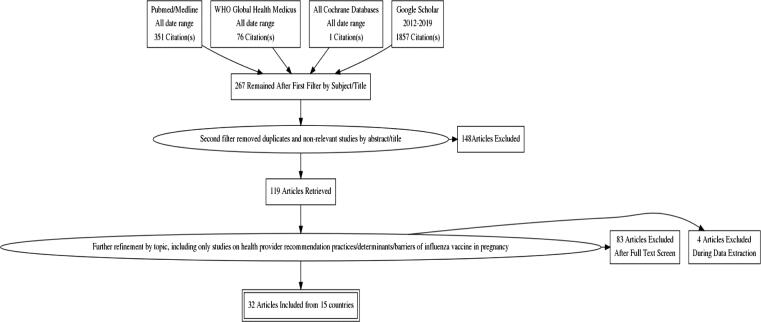
The General Literature Search from all databases resulted in a total of 2285 manuscripts. After multiple stages of filtering and applying the inclusion and exclusion criteria (see Fig. 1), 32 full-text articles remained. The included studies came from 15 countries in 4 WHO World regions [31], including 10 high-income countries (HICs) and 5 middle-income countries (MICs) – Nicaragua [32], Georgia [24], Thailand [18], [33], China [34], and India [35], [36] (see Table 1). Most studies were from HICs (26/32) and only 6 studies came from MICs; no studies were from LICs. The HIC studies came from the Americas (AMR), Europe (EUR) and the Western Pacific (WPR) (see Table 1, Table 2).
Fig. 1.
Scoping Literature Search flow diagram.
Table 1.
Total included studies and references by countries, WHO World regions. [31]and World Bank Income levels[36]
| WHO Regions and income levels of included studies | High Income | Upper Middle-Income | Low Middle-Income | Region Totals | Included Countries | References |
|---|---|---|---|---|---|---|
| The Americas Region (AMR) | 10 | – | 1 | 11 | Nicaragua | [32] |
| Canada | [37], [38] | |||||
| United States | [23], [26], [39], [40], [41], [42], [43], [44] | |||||
| The European Region (EUR) | 10 | 1 | – | 11 | United Kingdom | [45], [46] |
| Germany | [47], [48], [49] | |||||
| Spain | [50], [51] | |||||
| Israel | [25] | |||||
| Belgium | [52] | |||||
| France | [53] | |||||
| Georgia | [24] | |||||
| South-East Asian Region (SEARO) | – | 2 | 1 | 3 | India | [35] |
| Thailand | [18], [33] | |||||
| Western Pacific Region (WPR) | 5 | 1 | – | 6 | China | [34] |
| Australia | [54], [55], [56], [57] | |||||
| Republic of Korea | [58] | |||||
| Global | 1 | Global Review | [11] | |||
| TOTAL Number of Studies | 25 | 3 | 3 | 32 |
Of the 32 studies, 2 were reviews, 1 focused on the United States [44] and 1 was a global review [11]; 27 were cross-sectional surveys; 1 was a survey which included an intervention [41], and 2 included face-to-face structured interviews [34], [55]. The maximum number of participants included in a study was 3441 and the lowest number of participants was 17 (structured interviews). Thirty studies included a reported HP recommendation rate. Twenty-two studies included determinants of recommendation [18], [24], [25], [26], [33], [34], [35], [37], [38], [39], [40], [41], [43], [44], [45], [46], [47], [51], [52], [53], [54], [56], [57], 20 studies included barriers to recommendation [11], [24], [25], [26], [33], [34], [35], [37], [40], [41], [44], [46], [47], [48], [51], [53], [54], [55], [57], [58], and 16 studies included both determinants and barriers. Twenty-three studies included ObGyns or obstetricians and 19 studies included midwives, nurses, or GPs. One study included pharmacists (see Table 2).
3.1. Recommendation practices (seeTable 2andFig. 2)
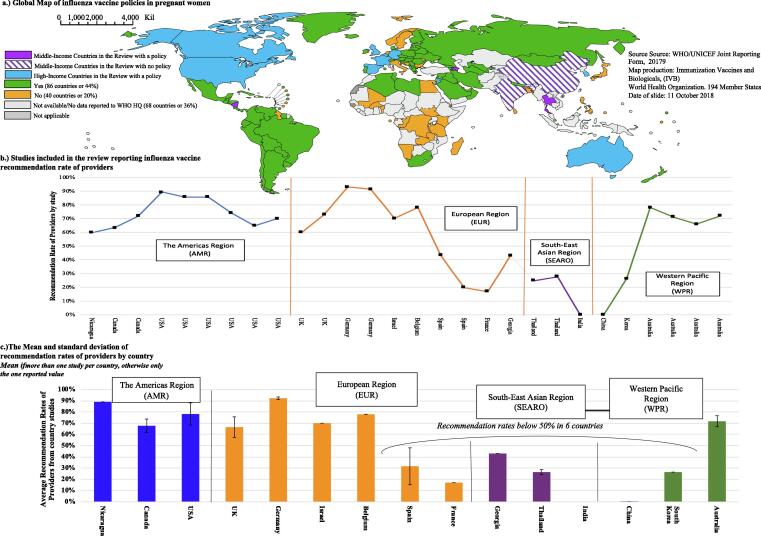
Fig. 2.
A Global map of influenza policies in pregnant women (countries with or without/ and those without data) with the countries included in the review marked; the corresponding recommendation rates of health providers reported from 28 studies and their country and region.
Twenty-eight studies reported HPs’ recall of recommendation including 21 studies that also included data on influenza vaccine coverage in the pregnant women participants of their study. Close to 1/3rd of all 21 studies reported HP recommendation rates < 50%, including Spain (20%, 43%) [50], [51], France (17%) [53], and the Republic of Korea (26.5%) [58] in the HICs and Georgia (43%) [24], Thailand (26.5%) [18], [33], China (0%) [34], and India (0%) [35] in the MICs. The highest reported coverage in pregnant women was 51% [43] and the lowest coverage < 1% [33], [34], [35]. Low HP recommendation rates of <50% were consistently reported with low coverage rates (<20%), if studies reported both outcomes. (see Table 2) Low recommendation rates and/or low coverage was reported in HIC and MICs countries and in countries with or without an official influenza vaccine policy in pregnant women. All countries included in our review reported having an influenza policy in pregnant women in either 2018 or 2014 through the WHO/UNICEF joint reporting form [8], [59], except for India. However, China’s policy appears to be unofficial and solely for the private sector as of 2016 [60], [61].
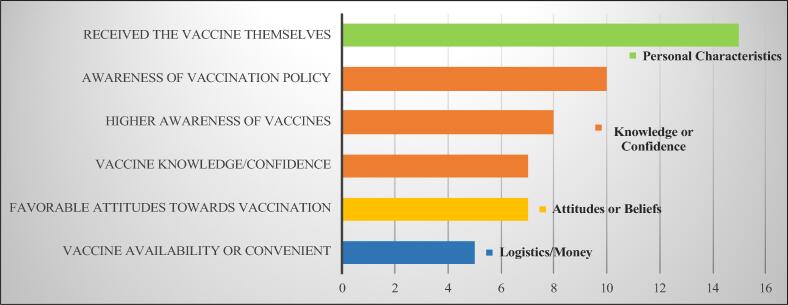
3.2. Determinants of influenza vaccine recommendation to pregnant women (seeFig. 3, Fig. 4)
Fig. 4.
Frequency of Determinants of Included Studies - Bar Chart of Individual Determinants with the highest frequency and their associated categories indicated by color.
We identified 32 individual determinants from 22 studies, which we organized into 7 categories. The most frequently cited determinant of recommendation was a HP’s own influenza vaccine uptake, which was cited in 65% of all studies representing all 4 WHO world regions included in our review. Only 1 MIC study from Thailand [33] cited own uptake as a determinant (prevalence ratio-PR: 1.7; confidence interval-CI: 1.1–2.7) and then reported other determinants such as being in practice for >3 years (PR 1.9, CI 1.2–2.3) and HP perceived safety and effectiveness of the vaccine (PR 1.9, CI 1.3–2.9). Seventy-eight percent of the studies in HICs cited own uptake as a determinant with prevalence rates as low as 1.2 in the United States [26] and odds ratios as high as 14 in Germany (OR: 14.32; CI: 7.94–29.82) [47]. In both a French [53] and Spanish study [51], odds of recommending in vaccinated HPs were higher (Range of OR: 3.7–6.6) even though recommendation rates were extremely low (17% and 43% respectively) and HP own vaccine uptake was also low (31.% and 39% respectively).
The next three determinants cited with the highest frequency from all studies were (1.) awareness of the vaccination policy; (2.) awareness of vaccines; (3.) vaccine knowledge/confidence. Awareness of the vaccination policy was cited in all 4 MIC studies which reported determinants and in several HIC studies as well. Awareness of vaccines in general was also cited in MICs, as was knowledge and vaccine confidence. Knowledge was cited in several studies including a French study [53] of midwives that showed that midwives with the highest levels of knowledge about influenza vaccine were almost 60 times more likely to recommend the vaccine to their pregnant patients (OR = 59.4; CI = 12–295.7). Provider type was cited as a determinant in studies from Canada, Europe, and Thailand [33], [46], [51], [52], with more frequent recommendations coming from ObGyns, gynecologists or nurses [37] than midwives, except in one study where it was the opposite [50]. Several other studies found that recommendation rates did not vary by provider type [26], [38].
Data for determinants in MICs was limited to 4 countries - Thailand, Georgia, China and India; however, consistent determinants from those countries were higher awareness of the vaccination policy, and perceived safety [18], [33], [34]. Studies in HICs most frequently cited own uptake of the providers as the main determinant of recommendation in pregnant women. Other determinants from high-income countries included a higher awareness of vaccines, and favorable attitudes or higher vaccine confidence.
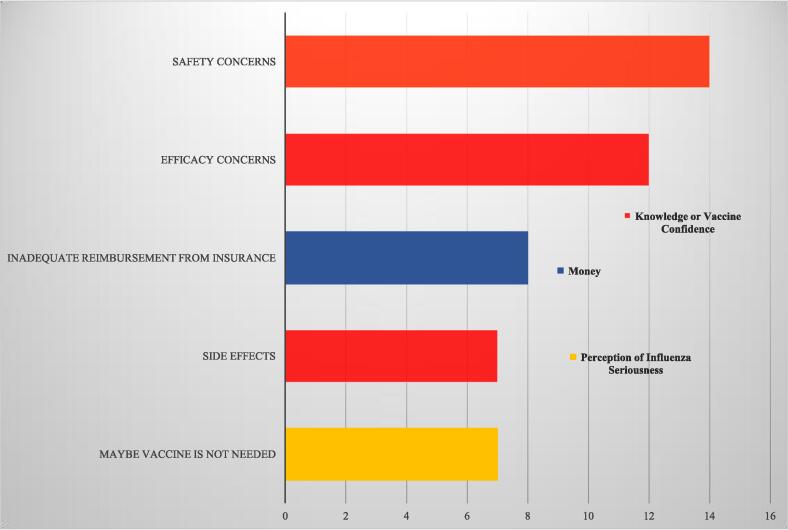
3.3. Barriers of influenza vaccine recommendation to pregnant women (see Figs. 5 and 6)
We identified 20 studies which included HP barriers to recommendation of influenza vaccine to pregnant women. Collectively, there were a total of 36 individual barriers, which we organized into 9 categories (see Fig. 5). The most frequently cited individual barriers to recommendation (see Fig. 6) were concerns about safety or efficacy, cited in 75% of studies, including those with very high reported recommendation rates, such as Germany [47], [48], and in settings with very low or non-existent rates, such as India and China [34], [35]. Even in the presence of a long-standing influenza vaccine policy in pregnant women, such as in the US, concerns over safety and efficacy persisted [26], [40], [41], [44]. Studies in Israel [25] and the Republic of Korea [58] illustrated that safety and efficacy concerns also persisted when providers had a high level of knowledge. However, studies in Canada [37] and France [53] suggested that although safety concerns may persist, higher levels of knowledge or better attitudes resulted in higher recommendation rates.
Fig. 6.
Frequency of Barriers of Included Studies - Bar Chart of Individual Barriers with the highest frequency and their category indicated by color.
Following the concerns over safety and efficacy, MICs also cited concerns over ambiguous policies, vaccine logistics, and a lack of need for the vaccine, among others (see Fig. 5 and Fig. 6). Another barrier most frequently cited was financial reimbursement, although this barrier was unique to the US [26], [40], [41], Germany [47], [48], and the UK [46]. A US review included in our study from 2018 of the barriers and determinants of maternal immunization in pregnant women and health providers also found that financial concerns and insurance reimbursement were the most frequently cited barriers of 24 identified barriers [44]. A global review included in our review, with 65% of its 113 studies on influenza in the United States [11] found the key barrier for HPs was inadequate reimbursement from insurance companies to the providers themselves followed by inadequate training.
Other frequent individual barriers included several which fit into the category of perception of influenza seriousness. Studies found that HP’s had low risk perception for the seriousness of influenza and doubted whether influenza vaccines were needed at all in pregnant women [25], [41], [44], [47], [54], [55], [58]. This was especially relevant in the Western Pacific Region where 4 out of 5 studies cited a barrier in the category of perception of Influenza Seriousness, including the study of mostly well-informed gynecologists in the republic of Korea [58]. The studies in Israel [25] and the country of Georgia [24] also questioned the necessity of the influenza vaccine along with major concerns over safety. Although HPs in India had little knowledge about influenza vaccines, they strongly doubted its safety, efficacy, and necessity, and also alluded that vaccines and other vaccine policies are driven by profit [35].
The top 11 individual barriers fit mostly under the category of Knowledge/Vaccine confidence (confidence in the vaccine). If we removed concerns over safety, efficacy, and side effects from the list of barriers, the category of Knowledge/Vaccine confidence was still the most frequently cited category. The other frequently cited top barriers fit into the categories of money (costs to doctors or a prohibitive cost to patients), perception of seriousness, time, and logistics.
4. Discussion
The review attempted to evaluate health providers’ influenza vaccine recommendation practices in pregnant women and the determinants and barriers to recommendation in different country-contexts. However, not surprisingly [11], 80% of the studies included in our 15 country review came from HICs, which grossly limits the generalizability of our findings to low and middle-income countries (LMICs). Even still, our review does initiate a needed dialogue on HP influenza vaccine recommendation practices as a critical part of vaccine implementation in pregnant women that may shed light on ways to improve critically low influenza vaccine coverage in pregnant women.
We confirmed that HP recommendation of influenza vaccines to pregnant women is inconsistent and that the highest rates of recommendation originate from HICs (see Table 2) and the lowest from MICs characterized by ambiguous or non-existent influenza policies, such as India and China [60], [61]. Inconsistent rates are not surprising as global adoption of influenza policies in pregnant women and the HPs who vaccinate has been slow to catch on, especially in LMICs [8], [31]. We found that studies with HP recommendation rates <50% also consistently reported coverage of <20% in their patient population. However, some studies reporting very high recommendation rates (91–93%) also reported very low coverage in pregnant women (11–23%) [47], [48], indicating that HP recommendation rate is not a reliable indicator of coverage or a conclusive endpoint on its own but rather a gauge of individual HP recommendation practices - a crucial part of the larger vaccine program implementation in each context [12], [62], [9].
The determinants and barriers to recommendation varied across countries, studies, and regions; however, there were some marked consistencies. We found that a HP’s own influenza vaccine uptake was the most frequently cited determinant to recommendation overall, and also relevant in high and some MICs. A German study highlighted that the strength of this association depended on the frequency of vaccination of the providers [47] and a very large review of 185 articles found that own uptake was important around the world in different types of HPs, with different vaccines, and in different environments including Israel, Nigeria, Iran, Canada, and the US [10]. In a study of US pediatric health workers working in emergency rooms with critical patients, own uptake was the only determinant of universal recommendation of influenza vaccine to their young patients (OR = 15, 95% CI 6.1–41.4, p < 0.001) [63]. This finding highlights that HP uptake should be considered as part of any vaccine program implementation. A study evaluating influenza vaccine coverage in the European Member States of the EUR warned that alarmingly low levels of influenza vaccine uptake in HPs should be a major concern, as this also influences their role as vaccinators [64].
If HPs who are vaccinated are more likely to recommend vaccine to their patients, it is important to understand what leads them to be vaccinated. A comprehensive review on influenza vaccine uptake and acceptance of HPs found that HPs are motivated to protect oneself and their family members [14], [28] and that vaccine confidence was a critical [14], [65] part of a HPs decision to be vaccinated. SAGE defines vaccine confidence as ‘trust in the effectiveness and safety of vaccines and in the system that delivers them, including the reliability and competence of the health services and health professionals and having trust in the motivations of the policy makers who decide which vaccines are needed and when they are needed’ [66]. A comprehensive Finnish study explored the relationship of HPs’ own vaccine uptake and their recommendation practices [67] and found that lower vaccine confidence was associated with a lower willingness to be vaccinated, to vaccinate their children, and to recommend vaccine to vaccine-hesitant patients.
The study frequently identified determinants relating to HPs’ knowledge and awareness. Several studies highlighted [37], [68], [69], [70] that the strength and magnitude of a HP’s knowledge directly impacted recommendation practices. A recent French study evaluated recommendation practices and vaccine uptake in midwives in Paris [53] and found that a high (high, average, low) level of influenza vaccine-specific knowledge was the greatest determinant of recommendation (OR 59.4; CI 12–295.7) of influenza vaccine to pregnant patients. The same study also found that midwives’ own vaccine uptake was associated with higher levels of knowledge and more frequent recommendation (OR 15; CI 4.8–16.8) to pregnant women. A review by Herzog et al. illustrated the link between the willingness to recommend and higher vaccine awareness, favorable attitudes, and beliefs that aligned with scientific evidence [71]. A Canadian study of ObGyns and family primary care providers found a linear relationship with rising levels of knowledge (low, medium, high) or attitude (poor attitude, neutral, positive) about influenza and recommendation in both healthy and high-risk patients [37]. Two reviews in nurses also showed that higher knowledge and a positive attitude regarding influenza vaccines was directly related to their own uptake and their recommendation of vaccine to patients [69], [70].
We found that concerns over safety and efficacy were the most frequently cited barriers to recommendation, and relevant in almost every country and region and in every provider type. In China and India [34], [72] several misperceptions about safety and efficacy of influenza vaccines persisted, yet in the Republic of Korea and Israel, higher levels of knowledge did not defray ObGyns’ concerns over safety, efficacy, side effects, or their perception of risk. We found small variations in this concern for safety and efficacy by provider type, although these differences were inconsistent across studies. Midwives or community nurses had more concerns about safety than physicians [46], [51], [57] in some studies, but other studies found that midwives recommended the vaccine just as much or more than physicians [50], [57]. Provider-specific studies would be useful to measure barriers or determinants of recommendation in the HPs deemed responsible for vaccinating, such as midwives in some countries and GPs in others [53].
Financial barriers for doctors or concerns about insurance coverage were specific specito the US and Europe; whereas, vaccinating responsibility were barriers to recommendation in Canada, Australia, and Belgium. Studies including different maternal health providers found that most believed the primary responsibility for vaccination was with general practitioners [37], [52], [57], including 86% of ObGyns who felt it was the family physician’s responsibility to provide the vaccine. This presents a problem for recommendation practices since the same study [37] also found family physicians more hesitant to recommend vaccines to pregnant women than ObGyns (36% vs. 65%).
Our review evaluated multiple studies with different study designs, survey instruments, HP populations and contexts, which limits the ability to make firm or generalizable conclusions. However, our findings illustrate how recommendation practices may vary due to context-specific factors such as policy communications, costs, logistics, or vaccine availability combined with knowledge, awareness (vaccine and policy), and confidence in the vaccine. Our findings suggest that knowledge, awareness, and vaccine confidence of HPs are critical to vaccine implementation and are only as strong as the influenza program with which they reside. We found that vaccine confidence is not only related to belief in the safety and effectiveness of the vaccine or the trust in the health system, but also to the knowledge, belief, and perception of real influenza risk in the local context and the extent to which influenza vaccine reduces this risk, while not adding undue harm.
It is clear that major barriers to influenza vaccine implementation exist which influence HPs recommendation practices, such as financial or insurance-related barriers in some HICs and vague policies and vaccine supply barriers in MICs. An important first step would be to develop or improve influenza policies to be sure they are clear, consistent, persistent and well-communicated from the highest level to the HPs, their patients, and the community. Simultaneously, implementation and vaccine-supply logistics must be guaranteed. When resources are limited, it seems justified to focus on HP vaccination to establish a strong foundation for implementation of influenza vaccination, which will also improve future influenza vaccine coverage in pregnant women. In HICs it is still critical to solve barriers relating to insurance reimbursement, as even providers with high levels of confidence, available vaccine, and a willing patient population will not recommend vaccine if they will incur costs and lose money.
4.1. Limitations
-
•
The heterogeneity of study designs, settings, and populations in addition to the limitations inherent in cross-sectional studies greatly reduces the strength of our findings.
-
•
Our studies were predominantly from HICs (25/32) representing only 3 of the 6 WHO World Regions.
-
•
The identification of determinants and barriers to recommendation was dependent on the questions posed in each study. This may lead to selection bias of the list of determinants and barriers or an over or underestimation of the frequencies of included determinants and barriers.
-
•
Recommendation recall from health providers served as the basis for a number of study’s findings which is susceptible to recall bias and may lead to inaccurate conclusions
-
•
Although we tried to be consistent with our inclusion, exclusion and search criteria, it is possible we missed important studies due to publication bias
-
•
Although we included all foreign language studies with at least an abstract in English, we may have missed several studies in foreign language search engines resulting in selection bias
5. Conclusion
Health provider recommendation of influenza vaccination to pregnant women plays a critical role in global influenza vaccine coverage and should be considered as part of any influenza vaccine program or pandemic preparedness plan. Strategies to increase global influenza vaccine coverage in pregnant women should aim to improve HP vaccine uptake and always target HPs in any communication plans to roll-out influenza vaccine to pregnant women. Barriers to recommendation due to concerns over safety and efficacy or the perception of risk persisted across countries; however, limiting factors such as vaccine availability, proper communication, and other context-specific factors may supersede these vaccine-related barriers. When resources are limited, it is justified to solely focus on HP influenza vaccination to establish a solid foundation, sound policies, and the platform for reliable implementation and future expansion to other high-risk groups or in response to a possible pandemic.
Taken together, increasing HP vaccine uptake of influenza vaccine globally may indirectly contribute to higher influenza vaccine coverage in pregnant women. By addressing local, contextual barriers first and any vaccine-related concerns, it may be easier to enhance vaccine confidence of HPs and improve global coverage of both vaccinators and their patients.
Future directions
-
•
More research is needed in LMICs on HP recommendation practices and determinants and barriers to recommendation
-
•
Countries without an official influenza policy should focus first on the development of a clear, consistent, and well communicated policy which is advocated from the top down.
-
•
Policies should consider elucidating the vaccinating responsibility of HPs and systematizing the process of vaccination, tracking, and reporting to limit confusion from HPs and patients
-
•
HP recommendation practices should be considered as a valuable part of any larger policy or contextual analyses to improve global influenza vaccine coverage in pregnant women.
-
•
Research is needed in HICs to identify and address any financial, health-systems or in-practice logistical barriers and drivers to health providers’ recommendation of influenza vaccine
-
•
Vaccine implementation strategies should use locally-generate evidence if possible to identify barriers and determinants to recommendation to improve coverage.
-
•
Future studies should evaluate the connection between knowledge, confidence, uptake in HPs and recommendation behavior and the combined impact on vaccine coverage in pregnant women
-
•
NITAGs should consider HP associations or equivalent groups in the vaccine introduction process given their influential role in translating vaccine policies into practice
Disclaimer
The authors have no interests to declare. Lambach P and Menning L work for the World Health Organization. Morales KF is a partner at Sierra Strategy Group. The authors alone are responsible for the views expressed in this publication and they do not necessarily represent the decisions, policy or views of the WHO.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This work was funded by a grant from the WHO department of Immunization, Vaccines and Biologicals. The authors would like to acknowledge the contributions of the US CDC, which provides financial support to the WHO IVR (U50CK000431).
Contributor Information
Kathleen F. Morales, Email: kmorales@sierrastrategygroup.com.
Philipp Lambach, Email: lambachp@who.int.
References
- 1.Siston A.M., Rasmussen S.A., Honein M.A., Fry A.M., Seib K., Callaghan W.M. Pandemic 2009 influenza A(H1N1) virus illness among pregnant women in the United States. JAMA. 2010;303:1517–1525. doi: 10.1001/jama.2010.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chacon R., Mirza S., Rodriguez D., Paredes A., Guzman G., Moreno L. Demographic and clinical characteristics of deaths associated with influenza A(H1N1) pdm09 in Central America and Dominican Republic 2009–2010. BMC Public Health. 2015;15:734. doi: 10.1186/s12889-015-2064-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gunnes N., Gjessing H.K., Bakken I.J., Ghaderi S., Gran J.M., Hungnes O. Seasonal and pandemic influenza during pregnancy and risk of fetal death: a Norwegian registry-based cohort study. Eur J Epidemiol. 2020 doi: 10.1007/s10654-020-00600-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Somerville L.K., Basile K., Dwyer D.E., Kok J. The impact of influenza virus infection in pregnancy. Future Microbiol. 2018;13:263–274. doi: 10.2217/fmb-2017-0096. [DOI] [PubMed] [Google Scholar]
- 5.Bhalerao-Gandhi A., Chhabra P., Arya S., Simmerman J.M. Influenza and pregnancy: a review of the literature from India. Infect Dis Obstet Gynecol. 2015;2015 doi: 10.1155/2015/867587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vaccines against influenza WHO position paper - November 2012. Wkly Epidemiol Rec 2012; 87:461–76. [PubMed]
- 7.Organization WH. Organization WH. Global influenza strategy 2019-2030. World Health Organization http://wwwwhoint/iris/handle/10665/311184 Lizenz: CC BY-NC-SA 30 IGO; 2019.
- 8.Organization WH. WHO/UNICEF Joint Reporting Form; 2018.
- 9.Palache A., Abelin A., Hollingsworth R., Cracknell W., Jacobs C., Tsai T. Survey of distribution of seasonal influenza vaccine doses in 201 countries (2004–2015): The 2003 World Health Assembly resolution on seasonal influenza vaccination coverage and the 2009 influenza pandemic have had very little impact on improving influenza control and pandemic preparedness. Vaccine. 2017;35:4681–4686. doi: 10.1016/j.vaccine.2017.07.053. [DOI] [PubMed] [Google Scholar]
- 10.Paterson P., Meurice F., Stanberry L.R., Glismann S., Rosenthal S.L., Larson H.J. Vaccine hesitancy and healthcare providers. Vaccine. 2016;34:6700–6706. doi: 10.1016/j.vaccine.2016.10.042. [DOI] [PubMed] [Google Scholar]
- 11.Wilson R.J., Paterson P., Jarrett C., Larson H.J. Understanding factors influencing vaccination acceptance during pregnancy globally: a literature review. Vaccine. 2015;33:6420–6429. doi: 10.1016/j.vaccine.2015.08.046. [DOI] [PubMed] [Google Scholar]
- 12.Yuen C.Y.S., Tarrant M. Determinants of uptake of influenza vaccination among pregnant women - a systematic review. Vaccine. 2014;32:4602–4613. doi: 10.1016/j.vaccine.2014.06.067. [DOI] [PubMed] [Google Scholar]
- 13.Shavell V.I., Moniz M.H., Gonik B., Beigi R.H. Influenza immunization in pregnancy: overcoming patient and health care provider barriers. Am J Obstet Gynecol. 2012;207:S67–S74. doi: 10.1016/j.ajog.2012.06.077. [DOI] [PubMed] [Google Scholar]
- 14.Schmid P., Rauber D., Betsch C., Lidolt G., Denker M.L. Barriers of influenza vaccination intention and behavior – a systematic review of influenza vaccine hesitancy, 2005–2016. PLoS ONE. 2017;12 doi: 10.1371/journal.pone.0170550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Healy C.M., Rench M.A., Montesinos D.P., Ng N., Swaim L.S. Knowledge and attitiudes of pregnant women and their providers towards recommendations for immunization during pregnancy. Vaccine. 2015;33:5445–5451. doi: 10.1016/j.vaccine.2015.08.028. [DOI] [PubMed] [Google Scholar]
- 16.Jung E.J., Noh J.Y., Choi W.S., Seo Y.B., Lee J., Song J.Y. Perceptions of influenza vaccination during pregnancy in Korean women of childbearing age. Hum Vaccines Immunotherap. 2016;12:1997–2002. doi: 10.1080/21645515.2015.1119347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henninger M.L., Irving S.A., Thompson M., Avalos L.A., Ball S.W., Shifflett P. Factors associated with seasonal influenza vaccination in pregnant women. J Women's Health. 2002;2015(24):394–402. doi: 10.1089/jwh.2014.5105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaoiean S., Kittikraisak W., Suntarattiwong P., Ditsungnoen D., Phadungkiatwatana P., Srisantiroj N. Predictors for influenza vaccination among Thai pregnant woman: the role of physicians in increasing vaccine uptake. Influenza Other Respir Viruses. 2019;13:582–592. doi: 10.1111/irv.12674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ye L., Chen J., Fang T., Cui J., Li H., Ma R. Determinants of healthcare workers' willingness to recommend the seasonal influenza vaccine to diabetic patients: A cross-sectional survey in Ningbo, China. Hum Vaccines Immunotherap. 2018:1–8. doi: 10.1080/21645515.2018.1496767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pandolfi E., Marino M.G., Carloni E., Romano M., Gesualdo F., Borgia P. The effect of physician's recommendation on seasonal influenza immunization in children with chronic diseases. BMC Public Health. 2012;12:984. doi: 10.1186/1471-2458-12-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lim P.L., Tan J., Yusoff Y., Win M.K., Chow A. Rates and predictors for influenza vaccine prescriptions among HIV-infected clinic patients in Singapore. Ann Acad Med Singapore. 2013;42:173–177. [PubMed] [Google Scholar]
- 22.Myers K.L. Predictors of maternal vaccination in the United States: an integrative review of the literature. Vaccine. 2016;34:3942–3949. doi: 10.1016/j.vaccine.2016.06.042. [DOI] [PubMed] [Google Scholar]
- 23.Broughton D.E., Beigi R.H., Switzer G.E., Raker C.A., Anderson B.L. Obstetric health care workers' attitudes and beliefs regarding influenza vaccination in pregnancy. Obstet Gynecol. 2009;114:981–987. doi: 10.1097/AOG.0b013e3181bd89c2. [DOI] [PubMed] [Google Scholar]
- 24.Dvalishvili M., Mesxishvili D., Butsashvili M., Kamkamidze G., McFarland D., Bednarczyk R.A. Knowledge, attitudes, and practices of healthcare providers in the country of Georgia regarding influenza vaccinations for pregnant women. Vaccine. 2016;34:5907–5911. doi: 10.1016/j.vaccine.2016.10.033. [DOI] [PubMed] [Google Scholar]
- 25.Gesser-Edelsburg A., Shir-Raz Y., Hayek S., Aassaraf S., Lowenstein L. Despite awareness of recommendations, why do health care workers not immunize pregnant women? Am J Infect Control. 2017;45:436–439. doi: 10.1016/j.ajic.2016.11.025. [DOI] [PubMed] [Google Scholar]
- 26.Kissin D.M., Power M.L., Kahn E.B., Williams J.L., Jamieson D.J., MacFarlane K. Attitudes and practices of obstetrician-gynecologists regarding influenza vaccination in pregnancy. Obstet Gynecol. 2011;118:1074–1080. doi: 10.1097/AOG.0b013e3182329681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dube E., Laberge C., Guay M., Bramadat P., Roy R., Bettinger J. Vaccine hesitancy: an overview. Hum Vaccines Immunother. 2013;9:1763–1773. doi: 10.4161/hv.24657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dini G., Toletone A., Sticchi L., Orsi A., Bragazzi N.L., Durando P. Influenza vaccination in healthcare workers: A comprehensive critical appraisal of the literature. Hum Vaccin Immunother. 2018;14:772–789. doi: 10.1080/21645515.2017.1348442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ellingson M.K., Dudley M.Z., Limaye R.J., Salmon D.A., O'Leary S.T., Omer S.B. Enhancing uptake of influenza maternal vaccine. Expert Rev Vaccines. 2019;18:191–204. doi: 10.1080/14760584.2019.1562907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peters M.D., Godfrey C.M., Khalil H., McInerney P., Parker D., Soares C.B. Guidance for conducting systematic scoping reviews. Int J Evid Based Healthc. 2015;13:141–146. doi: 10.1097/XEB.0000000000000050. [DOI] [PubMed] [Google Scholar]
- 31.Organization WH. Health statistics and information systems; 2019.
- 32.Arriola C.S., Vasconez N., Bresee J., Ropero A.M. Knowledge, attitudes and practices about influenza vaccination among pregnant women and healthcare providers serving pregnant women in Managua, Nicaragua. Vaccine. 2018;36:3686–3693. doi: 10.1016/j.vaccine.2018.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Praphasiri P., Ditsungneon D., Greenbaum A., Dawood F.S., Yoocharoen P., Stone D.M. Do Thai physicians recommend seasonal influenza vaccines to pregnant women? a cross-sectional survey of physicians' perspectives and practices in Thailand. PLoS ONE. 2017;12 doi: 10.1371/journal.pone.0169221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li R., Xie R., Yang C., Rainey J., Song Y., Greene C. Identifying ways to increase seasonal influenza vaccine uptake among pregnant women in China: a qualitative investigation of pregnant women and their obstetricians. Vaccine. 2018;36:3315–3322. doi: 10.1016/j.vaccine.2018.04.060. [DOI] [PubMed] [Google Scholar]
- 35.Koul P.A., Bali N.K., Ali S., Ahmad S.J., Bhat M.A., Mir H. Poor uptake of influenza vaccination in pregnancy in northern India. Int J Gynaecol Obstet. 2014;127:234–237. doi: 10.1016/j.ijgo.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 36.Bank W. World Bank Country and Lending Groups; 2019.
- 37.Tong A., Biringer A., Ofner-Agostini M., Upshur R., McGeer A. A cross-sectional study of maternity care providers' and women's knowledge, attitudes, and behaviours towards influenza vaccination during pregnancy. J Obstet Gynaecol Can. 2008;30:404–410. doi: 10.1016/s1701-2163(16)32825-0. [DOI] [PubMed] [Google Scholar]
- 38.Dube E., Gagnon D., Kaminsky K., Green C.R., Ouakki M., Bettinger J.A. Vaccination against influenza in pregnancy: a survey of Canadian maternity care providers. J Obstet Gynaecol Can. 2018 doi: 10.1016/j.jogc.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 39.Silverman N.S., Greif A. Influenza vaccination during pregnancy. Patients' and physicians' attitudes. J Reprod. Med. 2001;46:989–994. [PubMed] [Google Scholar]
- 40.Dolan S.M., Cox S., Tepper N., Ruddy D., Rasmussen S.A., MacFarlane K. Pharmacists' knowledge, attitudes, and practices regarding influenza vaccination and treatment of pregnant women. J Am Pharm Assoc: JAPhA. 2012;52:43–51. doi: 10.1331/JAPhA.2012.10141. [DOI] [PubMed] [Google Scholar]
- 41.Panda B., Stiller R., Panda A. Influenza vaccination during pregnancy and factors for lacking compliance with current CDC guidelines. J Maternal-fetal Neonatal Med: Off J Eur Assoc Perinatal Med Federation Asia Oceania Perinatal Soc Int Soc Perinatal Obstet. 2011;24:402–406. doi: 10.3109/14767058.2010.497882. [DOI] [PubMed] [Google Scholar]
- 42.Kahn K.E., Black C.L., Ding H., Williams W.W., Lu P.J., Fiebelkorn A.P. Influenza and Tdap vaccination coverage among pregnant women – United States, April 2018. MMWR Morb Mortal Wkly Rep. 2018;67:1055–1059. doi: 10.15585/mmwr.mm6738a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arao R.F., Rosenberg K.D., McWeeney S., Hedberg K. Influenza vaccination of pregnant women: attitudes and behaviors of Oregon physician prenatal care providers. Matern Child Health J. 2015;19:783–789. doi: 10.1007/s10995-014-1569-x. [DOI] [PubMed] [Google Scholar]
- 44.Lutz C.S., Carr W., Cohn A., Rodriguez L. Understanding barriers and predictors of maternal immunization: Identifying gaps through an exploratory literature review. Vaccine. 2018;36:7445–7455. doi: 10.1016/j.vaccine.2018.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilcox C.R., Calvert A., Metz J., Kilich E., MacLeod R., Beadon K. Determinants of influenza and pertussis vaccination uptake in Pregnancy: a multicenter questionnaire study of pregnant women and healthcare professionals. Pediatr Infect Dis J. 2019;38:625–630. doi: 10.1097/INF.0000000000002242. [DOI] [PubMed] [Google Scholar]
- 46.Vishram B., Letley L., Jan Van Hoek A., Silverton L., Donovan H., Adams C. Vaccination in pregnancy: Attitudes of nurses, midwives and health visitors in England. Hum Vaccines Immunother. 2018;14:179–188. doi: 10.1080/21645515.2017.1382789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bohm S., Robl-Mathieu M., Scheele B., Wojcinski M., Wichmann O., Hellenbrand W. Influenza and pertussis vaccination during pregnancy - attitudes, practices and barriers in gynaecological practices in Germany. BMC Health Services Res. 2019;19:616. doi: 10.1186/s12913-019-4437-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bodeker B., Seefeld L., Buck S., Ommen O., Wichmann O. Implementation of seasonal influenza and human papillomavirus vaccination recommendations in gynecological practices in Germany. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2016;59:396–404. doi: 10.1007/s00103-015-2303-6. [DOI] [PubMed] [Google Scholar]
- 49.Baum S., Hitschold T., Becker A., Smola S., Solomayer E., Rody A. Implementation of the recommendation to vaccinate pregnant women against seasonal influenza - vaccination Rates and Acceptance. Geburtshilfe Frauenheilkd. 2017;77:340–351. doi: 10.1055/s-0043-103970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vilca Yengle LM, Campins Marti M, Cabero Roura L, Rodrigo Pendas JA, Martinez Gomez X, Hermosilla Perez E, et al. [Influenza vaccination in pregnant women. Coverage, practices and knowledge among obstetricians]. Medicina clinica. 2010; 134:146–51. [DOI] [PubMed]
- 51.Vilca L.M., Martinez C., Burballa M., Campins M. Maternal Care Providers' Barriers Regarding Influenza and Pertussis Vaccination During Pregnancy in Catalonia, Spain. Maternal Child Health J. 2018;22:1016–1024. doi: 10.1007/s10995-018-2481-6. [DOI] [PubMed] [Google Scholar]
- 52.Maertens K., Braeckman T., Top G., Van Damme P., Leuridan E. Maternal pertussis and influenza immunization coverage and attitude of health care workers towards these recommendations in Flanders, Belgium. Vaccine. 2016;34:5785–5791. doi: 10.1016/j.vaccine.2016.09.055. [DOI] [PubMed] [Google Scholar]
- 53.Loubet P., Nguyen C., Burnet E., Launay O. Influenza vaccination of pregnant women in Paris, France: Knowledge, attitudes and practices among midwives. PLoS ONE. 2019;14 doi: 10.1371/journal.pone.0215251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Regan A.K., Hauck Y., Nicolaou L., Engelbrecht D., Butt J., Mak D.B. Midwives' knowledge, attitudes and learning needs regarding antenatal vaccination. Midwifery. 2018;62:199–204. doi: 10.1016/j.midw.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 55.Maher L., Dawson A., Wiley K., Hope K., Torvaldsen S., Lawrence G. Influenza vaccination during pregnancy: a qualitative study of the knowledge, attitudes, beliefs, and practices of general practitioners in Central and South-Western Sydney. BMC Family Pract. 2014;15:102. doi: 10.1186/1471-2296-15-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lu A.B., Halim A.A., Dendle C., Kotsanas D., Giles M.L., Wallace E.M. Influenza vaccination uptake amongst pregnant women and maternal care providers is suboptimal. Vaccine. 2012;30:4055–4059. doi: 10.1016/j.vaccine.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 57.Krishnaswamy S., Wallace E.M., Buttery J., Giles M.L. A study comparing the practice of Australian maternity care providers in relation to maternal immunisation. Aust N Z J Obstet Gynaecol. 2018 doi: 10.1111/ajo.12888. [DOI] [PubMed] [Google Scholar]
- 58.Noh J.Y., Seo Y.B., Song J.Y., Choi W.S., Lee J., Jung E. Perception and attitudes of Korean obstetricians about maternal influenza vaccination. J Korean Med Sci. 2016;31:1063–1068. doi: 10.3346/jkms.2016.31.7.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ortiz J.R., Perut M., Dumolard L., Wijesinghe P.R., Jorgensen P., Ropero A.M. A global review of national influenza immunization policies: Analysis of the 2014 WHO/UNICEF Joint Reporting Form on immunization. Vaccine. 2016;34:5400–5405. doi: 10.1016/j.vaccine.2016.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Feng L.Z., Peng Z.B., Wang D.Y., Yang P., Yang J., Zhang Y.Y. Technical guidelines for seasonal influenza vaccination in China, 2018–2019. Zhonghua liu xing bing xue za zhi = Zhonghua liuxingbingxue zazhi. 2018;39:1413–1425. doi: 10.3760/cma.j.issn.0254-6450.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 61.Zheng Y., Rodewald L., Yang J., Qin Y., Pang M., Feng L. The landscape of vaccines in China: history, classification, supply, and price. BMC Infect Dis. 2018;18:502. doi: 10.1186/s12879-018-3422-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sherman M.J., Raker C.A., Phipps M.G. Improving influenza vaccination rates in pregnant women. J Reprod Med. 2012;57:371–376. [PubMed] [Google Scholar]
- 63.Grossman Z., Berkovitch M., Braunstein R., Cohen H.A., Mirons D. Influenza vaccination of pediatric staff as a predictor for recommendations to children. Harefuah. 2012;151(342–5):78. [PubMed] [Google Scholar]
- 64.Jorgensen P., Mereckiene J., Cotter S., Johansen K., Tsolova S., Brown C. How close are countries of the WHO European Region to achieving the goal of vaccinating 75% of key risk groups against influenza? Results from national surveys on seasonal influenza vaccination programmes, 2008/2009 to 2014/2015. Vaccine. 2018;36:442–452. doi: 10.1016/j.vaccine.2017.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dube E., Gilca V., Sauvageau C., Boulianne N., Boucher F.D., Bettinger J.A. Canadian family physicians' and paediatricians' knowledge, attitudes and practices regarding A(H1N1) pandemic vaccine. BMC Res Notes. 2010;3:102. doi: 10.1186/1756-0500-3-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Group, TSVHW . WHO; Geneva, Switzerland: 2013. What influences vaccine acceptance: a model of determinants of vaccine hesitancy. [Google Scholar]
- 67.Karlsson L.C., Lewandowsky S., Antfolk J., Salo P., Lindfelt M., Oksanen T. The association between vaccination confidence, vaccination behavior, and willingness to recommend vaccines among Finnish healthcare workers. PLoS ONE. 2019;14 doi: 10.1371/journal.pone.0224330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Loubet P., Guerrisi C., Turbelin C., Blondel B., Launay O., Bardou M. Influenza during pregnancy: incidence, vaccination coverage and attitudes toward vaccination in the French web-based cohort G-GrippeNet. Vaccine. 2016;34:2390–2396. doi: 10.1016/j.vaccine.2016.03.034. [DOI] [PubMed] [Google Scholar]
- 69.Smith S., Sim J., Halcomb E. Nurses' knowledge, attitudes and practices regarding influenza vaccination: an integrative review. J Clin Nurs. 2016;25:2730–2744. doi: 10.1111/jocn.13243. [DOI] [PubMed] [Google Scholar]
- 70.Zhang J., While A.E., Norman I.J. Knowledge and attitudes regarding influenza vaccination among nurses: a research review. Vaccine. 2010;28:7207–7214. doi: 10.1016/j.vaccine.2010.08.065. [DOI] [PubMed] [Google Scholar]
- 71.Herzog R., Alvarez-Pasquin M.J., Diaz C., Del Barrio J.L., Estrada J.M., Gil A. Are healthcare workers' intentions to vaccinate related to their knowledge, beliefs and attitudes? A systematic review. BMC Public Health. 2013;13:154. doi: 10.1186/1471-2458-13-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Koul P.A., Bali N.K., Mir H., Jabeen F., Ahmad A. Influenza illness in pregnant Indian women: a cross-sectional study. Infect Dis Obstet Gynecol. 2016;2016:1248470. doi: 10.1155/2016/1248470. [DOI] [PMC free article] [PubMed] [Google Scholar]