Abstract
A 29-year old male with recurrent respiratory and skin infections, anaemia and neutropaenia during childhood required immunoglobulin replacement for antibody deficiency from age 16. He remained relatively well until age 28 when he presented with a two-week history of fatigue, sore throat, fever and productive cough. He was found to have EBV viraemia and splenomegaly and a diagnosis of EBV-driven lymphoproliferative disease was made following bone marrow trephine. Family history was notable with three siblings: a healthy sister and two brothers with anaemia and neutropaenia; one who succumbed to septicaemia secondary to neutropaenic enterocolitis age 5 and another who developed intestinal vasculitis and antibody deficiency and had a successful haemopoetic stem cell transplant.
The proband's DNA underwent targeted sequencing of 279 genes associated with immunodeficiency (GRID panel). The best candidates were two ADA2 variants, p.Arg169Gln (R169Q) and p.Asn370Lys (N370K). Sanger sequencing and co-segregation of variants in the parents, unaffected sister and all three affected brothers was fully consistent with compound heterozygous inheritance. Subsequent whole genome sequencing of the proband identified no other potential causal variants. ADA2 activity was consistent with a diagnosis of ADA2 deficiency in affected family members.
This is the first description of EBV-driven lymphoproliferative disease in ADA2 deficiency. ADA2 deficiency may cause susceptibility to severe EBV-induced disease and we would recommend that EBV status and viral load is monitored in patients with this diagnosis and allogeneic SCT is considered at an early stage for patients whose ADA2 deficiency is associated with significant complications.
Keywords: ADA2, EBV, Lymphoproliferative disease, Immunodeficiency, Antibody deficiency, Viraemia
Abbreviations: ADA, Adenosine DeAminase; A2AR, Adenosine A2A receptor; CMV, Cytomegalovirus; CT, Computed Tomography; EBV, Epstein Barr Virus; GRID, Genomics of Rare Immune Disorders; PET, Positron Emission Tomography; RSV, Respiratory Syncytial Virus; SCID, Severe Combined ImmunoDeficiency; SCT, Stem Cell Transplant
Highlights
-
•
We report a patient with ADA2 deficiency and EBV-driven lymphoproliferative disease.
-
•
ADA2 deficiency may predispose to severe EBV-induced disease.
-
•
We would recommend that EBV status and viral load is monitored in patients with ADA2 deficiency.
To the Editor,
We report a 29-year old male, third-born child of healthy, non-consanguineous parents. At three months of age he presented with impetigo, microcytic anaemia (haemoglobin 8.5 g/dL) and splenomegaly. Further infections in infancy included skin infections (Staphylococcus aureus and Streptococcus pneumoniae), respiratory tract infection (RSV, Haemophilus influenzae and S. pneumoniae) and herpetic mouth ulcer. Neutropaenia was noted at 17 months. Bone marrow examination showed left-shifted leucopoiesis and maturation arrest. Serial neutrophil counts did not support a diagnosis of cyclic neutropaenia, anti-neutrophil antibodies were not detected and NitroBlue Tetrazolium test of the neutrophil oxidative burst was normal. He had chickenpox aged 4 and molluscum contagiosum age 6, both without sequelae.
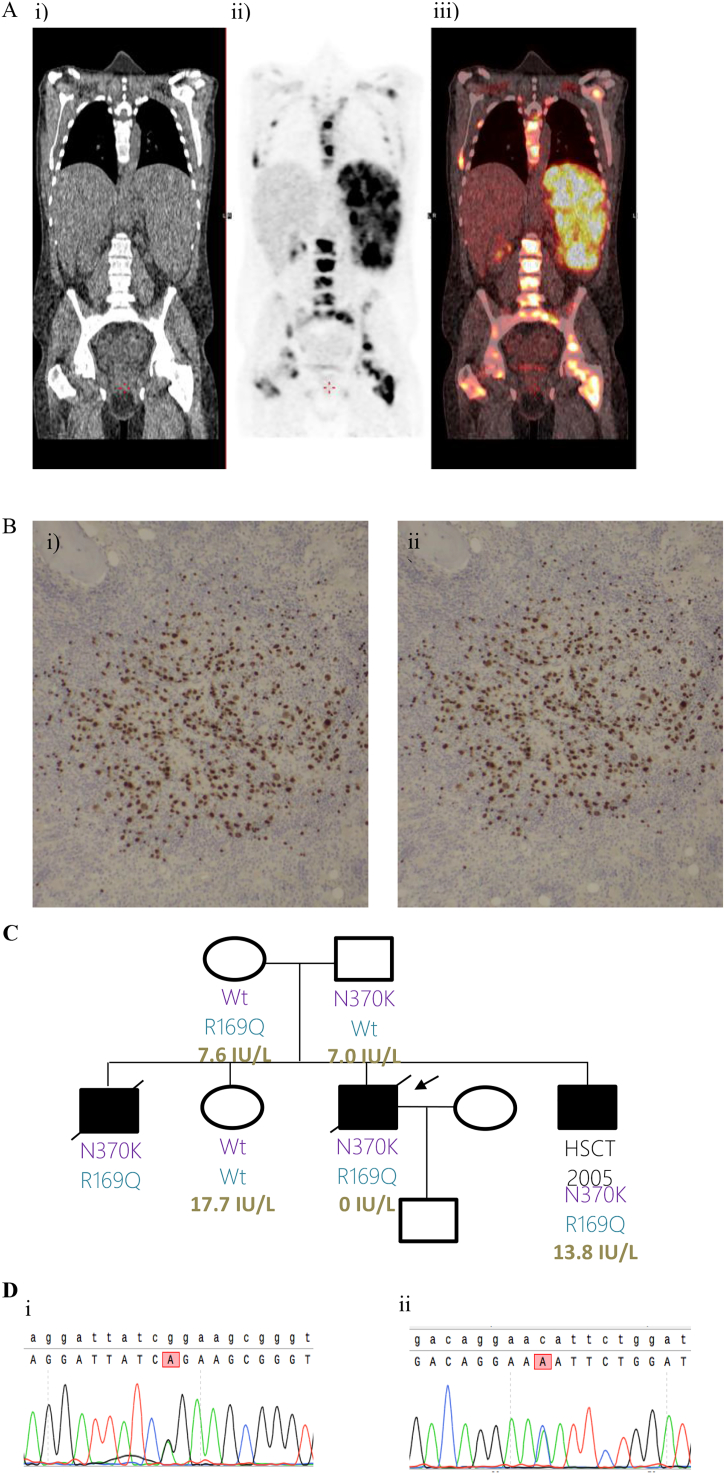
Assessment of his immune system at age 16 showed low IgG and IgM with low B-cell numbers (Supplementary Table 1). Immunoglobulin replacement was commenced and he remained relatively well until age 28 when he presented with a two-week history of fatigue, sore throat, fever and productive cough. He had increased splenomegaly and EBV viraemia (2.3 × 106 IU/mL). CT imaging of his chest, abdomen and pelvis was initially felt to be consistent with acute EBV infection, but he continued to deteriorate with high fevers and cytopaenias. CT-PET scan was performed. In addition to the changes expected with acute EBV infection, this showed patchy areas of increased uptake in the spleen, porta hepatis and bone marrow. A bone marrow trephine biopsy confirmed the diagnosis of EBV-driven lymphoproliferative disease (Fig. 1A, B). He was treated with chemotherapy, but EBV remained detectable at high level in peripheral blood (6.6 × 105 IU/mL) and he proceeded to undergo an allogeneic stem cell transplant (SCT) from an unrelated donor. Following his transplant EBV became undetectable in the peripheral blood, but the procedure was complicated by CMV reactivation, hepatic veno-occlusive disease and failure to engraft. A second allogeneic SCT engrafted successfully but recovery was complicated by multiple infections and he died age 29.
Fig. 1.
A: PET-CT scan demonstrating active disease on both sides of the diaphragm and marrow involvement.
i) Single slice coronal low dose CT image.
ii) Single slice coronal attenuation corrected PET image.
iii) Fused CT and PET images (i and ii).
B: Bone marrow trephine biopsy immunostains: i) PAX5 ii) EBER.
C: Pedigree diagram, showing co-segregation of ADA2 variants (N370K and R169Q) and ADA2 activity (measured at Viapath, Purine Research Laboratory, St Thomas' Hospital; in brief sera incubated with adenosine and ADA1 inhibitor for 3 h, products (inosine and hypoxanthine) separated and measured by high performance liquid chromatography; adult normal range in plasma 8.7–30.0 IU/L). Arrow indicates proband.
D: Sanger confirmation in i) Mother ii) Father.
i Mother: Top row shows wild-type sequence. Lower row shows change from G to A at position 506 in the mother changing the codon from CGG (Arginine, R) to CAG (Glutamine, Q): c.506G > A, p.R169Q.
ii Father: Top row shows wild-type sequence. Lower row shows change from C to A at position 1110 in the father changing the codon from AAC (Asparagine, N) to AAA (Lysine, K): c.1110C > A, p.N370K.
His older brother required regular red cell transfusions, developed neutropaenia in early childhood and had recurrent HSV and warts. Aged 5 years after 2 days of vomiting and diarrhoea he had a sudden hypotensive collapse and died. Post-mortem findings included necrotic caecum and ascending colon with submucosal oedema and gas bubbles. Abundant Gram-positive bacilli were identified as Clostridium septicum by specific immunofluorescence and cause of death given as septicaemia secondary to neutropaenic enterocolitis.
His sister is healthy and well.
His younger brother was anaemic at birth (8.6 g/dL) and had intermittent neutropaenia. He commenced immunoglobulin replacement for hypogammaglobulinaemia and was diagnosed with intestinal vasculitis on angiogram age 12. He was treated with methylprednisolone and infliximab with some improvement. He had an unrelated donor haemopoietic SCT at 14 years of age complicated by significant graft vs host disease (EBV-negative prior to transplant). He had good immune reconstitution and is fit and well.
Targeted sequencing of 279 IUIS 2015 genes [1] associated with immunodeficiency (GRID panel) [2] identified rare heterozygous variants in 3 genes associated with autosomal recessive immunodeficiency: (single variants in CR2 and STXBP2, two in ADA2). Sanger sequencing of the parents, unaffected sister and all three affected brothers confirmed compound heterozygosity of the two ADA2 variants in the proband and his affected siblings, whilst the unaffected sister did not inherit either of the two variants (Fig. 1C). The c.506G>A, p.Arg169Gln (R169Q) inherited from the mother is the commonest ADA2 mutation found in European Caucasians and has been identified in multiple homozygous and compound heterozygous cases of ADA2 deficiency [3]. The c.1110C>A, p.Asn370Lys (N370K) inherited from the father was previously reported as N328K (different transcript) in two siblings with predominantly cutaneous features [4]. Whole genome sequencing of the proband and analysis of the coding regions according to ACMG guidelines [5] did not identify other potentially causal autosomal recessive or X-linked genes, including those described more recently as associated with EBV susceptibility [6,7] or the additional recessive genes in the IUIS 2019 list [8].
ADA2 activity was consistent with a diagnosis of ADA2 deficiency in affected family members (Fig. 1C).
ADA2 deficiency was first reported in two parallel publications in 2014 in association with polyarteritis nodosa, vasculopathy and early-onset stroke (reviewed in [3]). It has since been reported in association with antibody deficiency without vasculitis, enteropathy, red cell aplasia, lymphadenopathy, splenomegaly and hepatomegaly in addition to the cytopaenias, neurological, cutaneous and vasculitic features initially described [9].
ADA2 is predominantly expressed by monocytes and other cells of the myeloid lineage. It converts adenosine to inosine and deoxyadenosine to deoxyinosine. It has approximately 100-fold lower affinity for its substrates than ADA1 (deficiency of which causes a SCID phenotype). It also differs from ADA1 in being predominantly secreted rather than intracellular. ADA2 is most active at acid pH, at sites of inflammation and tissue hypoxia [10]. Three patients homozygous for R169Q with lymphoproliferation have recently been reported: 2 with T-cell large granular lymphocytic infiltration of the bone marrow at 17 and 31 years of age and a further patient with lymphadenopathy and T-cell hyperplasia in the bone marrow at 4 years of age [11].
High serum ADA2 activity has been detected in a number of infectious, inflammatory and malignant diseases but was particularly increased in infectious mononucleosis [12]. This is the first description of EBV-driven lymphoproliferative disease in ADA2 deficiency. The prototypical primary immunodeficiency associated with unique susceptibility to EBV-induced disease is X-linked lymphoproliferative disease (XLP1). Other primary immunodeficiencies manifesting as severe EBV-induced disease are associated with susceptibility to a broader spectrum of viruses and are associated with mutations in genes including XIAP, ITK, MAGT1, CORO1A, CD27, CD70, NFKB1 and RASGRP1 which code for proteins involved in the interaction between CD8+ T-cells and B-cells and/or intrinsic T-cell signalling pathways [6]. EBV susceptibility is also a feature of monogenic NK cell disorders including CD16 deficiency, MCM4 deficiency and GATA2 deficiency.
Two unrelated patients with missense mutations in 4-1BB, persistent EBV viraemia and EBV-induced lymphoproliferation were recently described. The CD8+ T cells of these patients had reduced proliferation, impaired expression of interferon-γ and perforin and diminished cytotoxicity against EBV-infected B-cells [7]. Deficiency of ADA2 may lead to increased adenosine binding at A2A receptors. This also leads to reduced T-cell activation, TCR-signalling and reduced cytotoxic activity of CD8+ T-cells and NK cells [13]. There are four Adenosine Receptors (AR) which belong to the superfamily of G protein-coupled receptors (A1AR and A3AR are inhibitory; A2AAR and A2BAR are stimulatory). A recent study found that deficiency of ADA2 triggers adenosine-mediated formation of neutrophil extracellular traps and TNF production. This could be inhibited by recombinant ADA2, A1AR or A3AR antagonists, or by an A2AR agonist [14].
This is the first report of EBV-driven lymphoproliferative disease in ADA2 deficiency. The clinical phenotype of ADA2 deficiency is broad and expanding. The variability in severity and heterogeneity of presentation is striking, even in patients with the same ADA2 variants or within the same family. Whole genome sequencing of the proband did not identify other potentially pathogenic recessive variants in the proband. Additional environmental or genetic factors may have played a role in the development of EBV-driven lymphoproliferative disease in this patient but this case raises the possibility that ADA2 deficiency is another primary immunodeficiency that causes susceptibility to severe EBV-induced disease. We would recommend that EBV status and viral load is monitored in patients with this diagnosis and allogeneic SCT is considered at an early stage for patients whose ADA2 deficiency is associated with significant complications.
Declaration of funding sources
The patient was sequenced as part of the NIHR BioResource, funding for which was provided by the UK National Institute for Health Research (NIHR, grant number RG65966). We gratefully acknowledge the participation of all NIHR BioResource volunteers, and thank the NIHR BioResource centre and staff for their contribution.
J.E.D.T. is supported by the MRC (RG95376 and MR/L006197/1). The NIHR Cambridge Biomedical Research Centre (BRC) is a partnership between Cambridge University Hospitals NHS Foundation Trust and the University of Cambridge, funded by the National Institute for Health Research (NIHR). This research was co-funded by the support listed above and the National Institute for Health Research (NIHR) Cambridge Biomedical Research Centre (BRC). E.G.D. was supported by NIHR and Great Ormond Street Hospital BRC.
Acknowledgements
We would like to thank Lynette Fairbanks at Viapath, Purine Research Laboratory St Thomas' Hospital for determining ADA2 activity.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.clim.2020.108443.
Contributor Information
Emily Staples, Email: es656@mrc-tox.cam.ac.uk.
James E.D. Thaventhiran, Email: jedt2@mrc-tox.cam.ac.uk.
Appendix A. Supplementary data
Supplementary Table 1. Results of standard immunological investigations at different ages.
References
- 1.Bousfiha A., Jeddane L., Al-Herz W. The 2015 IUIS phenotypic classification for primary Immunodeficiencies. J. Clin. Immunol. 2015;35:727–738. doi: 10.1007/s10875-015-0198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simeoni I., Shamardina O., Deevi S.V.V. GRID - Genomics of Rare Immune Disorders: a highly sensitive and specific diagnostic gene panel for patients with primary immunodeficiencies. bioRxiv. 2018:431544. [Google Scholar]
- 3.Caorsi R., Penco F., Schena F., Gattorno M. Monogenic polyarteritis: the lesson of ADA2 deficiency. Pediatr. Rheumatol. 2016 doi: 10.1186/s12969-016-0111-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Santiago T.M.G., Zavialov A., Saarela J., Seppanen M., Reed A.M., Abraham R.S., Gibson L.E. Dermatologic features of ADA2 deficiency in cutaneous polyarteritis nodosa. JAMA Dermatol. 2015 doi: 10.1001/jamadermatol.2015.1635. [DOI] [PubMed] [Google Scholar]
- 5.Richards S., Aziz N., Bale S. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015;17:405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tangye S.G., Palendira U., Edwards E.S.J. Human immunity against EBV—lessons from the clinic. J. Exp. Med. 2017;214:269–283. doi: 10.1084/jem.20161846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alosaimi M., Hoenig M., Jaber F. Immunodeficiency and Epstein Barr virus induced lymphoproliferation caused by 4-1BB deficiency. J. Allergy Clin. Immunol. 2019 doi: 10.1016/j.jaci.2019.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tangye S.G., Al-Herz W., Bousfiha A. Human inborn errors of immunity: 2019 update on the classification from the International Union of Immunological Societies Expert Committee. J. Clin. Immunol. 2020:24–64. doi: 10.1007/s10875-019-00737-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schepp J., Proietti M., Frede N. Screening of 181 patients with antibody deficiency for deficiency of adenosine deaminase 2 sheds new light on the disease in adulthood. Arthritis Rheum. 2017;69:1689–1700. doi: 10.1002/art.40147. [DOI] [PubMed] [Google Scholar]
- 10.Zavialov A.V., Engström Å. Human ADA2 belongs to a new family of growth factors with adenosine deaminase activity. Biochem. J. 2005;391:51–57. doi: 10.1042/BJ20050683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trotta L., Martelius T., Siitonen T. ADA2 deficiency: clonal lymphoproliferation in a subset of patients. J. Allergy Clin. Immunol. 2018;141 doi: 10.1016/j.jaci.2018.01.012. 1534–1537.e8. [DOI] [PubMed] [Google Scholar]
- 12.Ungerer J.P.J., Vermaak W.J.H. Serum adenosine deaminase: isoenzymes and diagnostic application. Clin. Chem. 1992;38:1322–1326. [PubMed] [Google Scholar]
- 13.Cekic C., Linden J. Purinergic regulation of the immune system. Nat. Rev. Immunol. 2016;16:177–192. doi: 10.1038/nri.2016.4. [DOI] [PubMed] [Google Scholar]
- 14.Carmona-Rivera C., Khaznadar S.S., Shwin K.W. Deficiency of adenosine deaminase 2 triggers adenosine-mediated NETosis and TNF production in patients with DADA2. Blood. 2019;134:395–406. doi: 10.1182/blood.2018892752. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Results of standard immunological investigations at different ages.