Highlights
-
•
We found rVSVΔG-ZEBOV-GP to be immunogenic at 28- and 180-days post vaccination.
-
•
At 28 days post-vaccination, seroresponse rate was higher in the high-risk group.
-
•
There is a significant pairwise correlation at 28 days post-vaccination between assays.
-
•
One dose of rVSVΔG-ZEBOV-GP induces a cellular response that increased with time.
Keywords: Ebola vaccine, Immunogenicity, Humoral response, Cellular response, Frontline workers
Abstract
Background
As part of a Phase III trial with the Ebola vaccine rVSVΔG-ZEBOV-GP in Guinea, we invited frontline workers (FLWs) to participate in a sub-study to provide additional information on the immunogenicity of the vaccine.
Methods
We conducted an open‐label, non‐randomized, single-arm immunogenicity evaluation of one dose of rVSVΔG-ZEBOV-GP among healthy FLWs in Guinea. FLWs who refused vaccination were offered to participate as a control group. We followed participants for 84 days with a subset followed-up for 180 days. The primary endpoint was immune response, as measured by ELISA for ZEBOV-glycoprotein–specific antibodies (ELISA-GP) at 28 days. We also conducted neutralization, whole virion ELISA and enzyme-linked immunospot (ELISPOT) assay for cellular response.
Results
A total of 1172 participants received one dose of vaccine and were followed-up for 84 days, among them 114 participants were followed-up for 180 days. Additionally, 99 participants were included in the control group and followed up for 180 days. Overall, 86.4% (95% CI 84.1–88.4) of vaccinated participants seroresponded at 28 days post-vaccination (ELISA- GP) with 65% of these seroresponding at 14 days post-vaccination. Among those who seroresponded at 28 days, 90.7% (95% CI 82.0–95.4) were still seropositive at 180 days. The proportion of seropositivity in the unvaccinated group was 0.0% (95% CI 0.0–3.8) at 28 days and 5.4% (95% CI 2.1–13.1) at 180 days post-vaccination. We found weak correlation between ELISA-GP and neutralization at baseline but significant pairwise correlation at 28 days post-vaccination. Among samples analysed for cellular response, only 1 (2.2%) exhibited responses towards the Zaire Ebola glycoprotein (Ebola GP ≥ 10) at baseline, 10 (13.5%) at day 28 post-vaccination and 27 (48.2%) at Day 180.
Conclusions
We found one dose of rVSVΔG-ZEBOV-GP to be highly immunogenic at 28- and 180-days post vaccination among frontline workers in Guinea. We also found a cellular response that increased with time.
1. Introduction
During the 2013–2016 outbreak of Ebola Virus Disease (EVD) in West Africa, the Ministry of Health of Guinea, the World Health Organization (WHO), Médecins Sans Frontières (MSF), Epicentre and the Norwegian Institute of Public Health among other partners collaborated to conduct an Ebola vaccine trial with rVSVΔG-ZEBOV-GP in Guinea. The “Ebola ça Suffit!” trial aimed to leverage contact tracing as a means to implement an efficacy trial during the ongoing outbreak, enabling a randomized clinical trial with a novel study design. Participants (contacts and contacts of contacts of a laboratory-confirmed EVD case) were enrolled after detection of a laboratory confirmed EVD case and were randomized to receive rVSVΔG-ZEBOV-GP at a nominal dose of 2 × 107 plaque-forming units (PFU) either immediately or 21 days after enrolment as a control group. The period of observation for risk of infection was set as the 21-day period from 10 to 30 days post enrolment, regardless of when the vaccine was administered. The comparison of EVD incidence beginning 10 days after vaccination amongst 2108 participants vaccinated immediately and 3075 eligible and allocated to delayed vaccination, led to an estimated vaccine efficacy of 100% (95% CI 68.9–100.0, p = 0.0045) [1].
In parallel to the ring vaccination trial, a sub-study among front line workers (FLW) was implemented to evaluate the immunogenicity and safety of rVSVΔG-ZEBOV-GP [2]. Early phase trials in Europe, North America, and Africa have described the immune response to this vaccine, which at a dose of 2 × 107 PFU induces strong IgG response within 7 days post-vaccination [3], [4], [5], [6]. The cellular response to the vaccine is less well-described, but there is evidence that a CD8 + T-cell response predominates in the immediate period post-vaccination [7], and analysis of cytokine response suggests the important role of monocytes in the immediate response [8]. Ongoing “omics” analyses aims at further refining understanding of the immune response, analysing samples collected in a variety of early-phase trials [9]. Safety results of the sub-study among FLW in Guinea have been previously published [2]. Here we describe the humoral and cellular immune responses to rVSVΔG-ZEBOV-GP among frontline workers in Guinea.
2. Methods
2.1. Study design and participants
We conducted an open‐label, non‐randomized, single arm safety and immunogenicity evaluation of one dose of rVSVΔG-ZEBOV-GP in the city of Conakry, Guinea. Between March 2015 and July 2016, FLWs which included any personnel working in Ebola or non-Ebola health facilities and services were invited to participate. At the time of initiation of the sub-study, the outbreak was waning with the end declared in June 2016 [10].
Information and discussion sessions were held with personnel working in organizations or structures caring for Ebola patients or providing non-Ebola related health care. Volunteers wishing to participate were referred to the study site (Donka Hospital). After providing written informed consent, FLW were enrolled in the study if they were working in health services (including Ebola treatment centres, Ebola outreach and non-Ebola related health services) and agreed to follow study procedures. Exclusion criteria included known previous Ebola infection or recent exposure, previous receipt of an investigational Ebola therapeutic, current participation in a clinical trial, self-reported clinically important immunodeficiency, history of anaphylaxis to a vaccine or a vaccine component, severe illness, known pregnancy, breastfeeding and current fever. Therefore, participants enrolled in the rVSVΔG-ZEBOV-GP efficacy study were not eligible for the sub-study among FLWs.
The study included the possibility to participate without receiving the vaccine. Unvaccinated participants followed the same study procedures as other participants and were followed for safety and immunogenicity outcomes. Study visits were scheduled at days 3, 14, 28 and 84 after enrolment, and a subset of participants had a visit at day 180 after enrolment. On the day of enrolment, vaccinated participants received one dose of 2 × 107 PFU of rVSVΔG-ZEBOV-GP by intramuscular injection.
We hypothesized that FLW with different prior risk of being exposed to Ebola virus (either in the form of live or inactivated virus particles) could show different immunological response to the vaccine dependent on their pre-existing levels of antibodies against the Zaire Ebola virus glycoprotein). We defined EVD exposure risk according to the individuals’ profession and work location. Personnel who could have been directly in contact with EVD patients (doctors, nurses, lab technicians, cleaning personnel, surveillance teams, inhumation teams, and ambulance personnel) were classified as high-risk regardless of work location. Administrative personnel and security personnel were classified as high risk if they worked in Ebola related services. The low risk group consisted in administrative, security personnel and other support staff working in non-Ebola services.
We calculated that enrolling 519 participants in each exposure group would provide 90% power to detect a difference in mean anti-GP IgG antibody concentration of 0.10 Arbitrary enzyme-linked immunosorbent assays Units (AEU)/mL between the groups at 28 days post-vaccination, at a 5% significance level. Allowing for 10% missing data and loss to follow-up, we aimed to enrol 1200 participants. Based on a secondary exploratory outcome, we included a subset of 100 participants to be characterized in depth for immunological responses. There was no a priori sample size calculation for those wishing to participate but not to be vaccinated. The targeted 100 participants providing additional blood samples at 180 days post-vaccination were recruited on random days after the first 800 participants were enrolled. This exploratory sample represented an added burden on participants due to increased blood sample volume and on laboratory procedures due to strict processing and storage conditions necessary for analyses.
2.2. Assessment of immunogenicity
Study participants (including the unvaccinated cohort) were asked to provide 8 ml of blood prior to vaccination and at 14, 28 and 84 days post-vaccination to assess total IgG antibody levels against the Zaire Ebola virus (ZEBOV) glycoprotein (GP). The subset of participants included in the in-depth immunological analysis provided 25 ml of blood prior to vaccination and at 14, 28, 84 and 180 days post-vaccination to assess antibody response against the whole virion and specific cellular responses.
Samples were collected in SST vacutainer tubes tubes for antibody response and EDTA vacutainer tubes to prepare Peripheral Blood Mononuclear Cells (PBMC) to assess the cellular response. They were processed in compliance with Good Clinical Laboratory Practices (GCLP) that were established by Public Health England and Donka Hospital staff within a two week deadline to allow the initiation of the study during the outbreak. After storage at −80 °C in Guinea, samples were shipped on dry ice to Public Health England’s Laboratories at Porton Down and subsequently to Institute for Virology, Marburg, Germany and Imperial College, London, UK for PBMC testing and to Q2 laboratories San Francisco, California for antibody testing.
Total IgG antibody levels against ZEBOV-GP were measured by two assays. The first assay was an enzyme-linked immunosorbent assays (ELISA) that used the homologous Zaire-Kikwit strain glycoprotein as antigen. This assay was developed by the US Army Medical Research Institute of Infectious Diseases (USAMRIID), has been validated by Filovirus Animal Nonclinical Group (FANG) [11], [31] following US Food and Drug Administration (FDA) guidelines and used in previous evaluations [3], [5], [7], [12].
The second assay, plaque reduction neutralization (NAb) assay based on a VSV backbone, also developed by USAMRIID and validated by FANG, used ZEBOV as the antigen [14]. Both assays were performed by Q2 Solutions (California, USA). The results of the ELISA assay are reported as anti-GP IgG per milliliter and seropositivity was defined as IgG against ZEBOV-GP concentration ≥ 200 AEU/ml. For the NAb, seropositivity was defined as geometric mean titre > 20 against ZEBOV-GP. For these assays, we defined seroresponse as a > 4 fold increase in the concentration or titer from baseline. Prior to shipment from Guinea for analysis, FLW specimens were gamma irradiated for safety reasons. Grant-Klein et al observed that Gamma irradiation was associated with slightly higher antibody concentrations in pre-vaccination samples and slightly lower concentrations post-vaccination. However they concluded that Gamma irradiation remains a viable method for treating samples from regions where filoviruses are endemic because of their minor effects on antibody titers [13].
Additional analyses for the subset of participants for additional analyses were performed at the Institute for Virology, Marburg, Germany and Imperial College, London, UK. These laboratories were selected to ensure comparability with previous studies. Antibody response against the whole virion was assessed by ELISA and neutralization assays at the Institute for Virology, Marburg, Germany [4], [15]. For these assays, seropositivity was defined as ELISA IgG > 500 AEU/ml against ZEBOV whole virion and NAb > 8 against the ZEBOV whole virion. We defined seroresponse as a ≥ 4 fold increase in the concentration or titer.
PBMC samples were isolated from EDTA-blood and frozen following standard operating procedures. PBMC were characterized on site by flow cytometry to allow identification of the different cell populations [16]. Specific cellular responses were tested by enzyme-linked immunospot (ELISpot) assay using methods previously described [17], [18]. Samples passed quality control if their mock stimulus ELISpot responses were ≤ 50 SFU and PHA positive control stimulus ≥ 500 SFU per million PBMC. An ELISpot response to Ebola GP was considered positive where mean spot forming units (SFU) per million PBMC in quadruplicate wells with GP stimulus were ≥ 10 with the mean SFU of mock stimulus wells subtracted and mean SFU for GP stimulus was ≥ twice or fourth mock stimulus. Cellular immune responses were analysed at the International AIDS Vaccine Initiative (IAVI), Imperial College, London, United Kingdom.
2.3. Statistical analysis
Seropositivity and seroresponse rates are reported as proportion with 95% confidence interval (CI) calculated using the Wilson score method [19]. Antibody responses are reported as the Geometric Mean Concentration (GMC) or Geometric Mean Titer (GMT) with 95% CI. Change in antibody response over time is assessed by comparing GMCs or GMTs at each time point with baseline using the Wilcoxon signed-rank test for paired data.
A chi-square test was used to assess differences in seroresponse among vaccinated individuals at 14, 28, and 180 days by the following baseline variables: sex, age (≤26, 26–30, 30–37, and > 37), risk category and vaccination status. Fisher’s exact test was used when cell counts were < 5. Log binomial regression was used to assess the association between these variables and seroresponse at 28 days according to IgG concentration.
The different assays’ results were compared to each other using Spearman’s rank correlation coefficient at day 0 and day 28. The proportion of individuals tested with all five tests who tested positive for each one is reported. In addition, correlations between whole virion ELISA and NAb using live virus at day 0, 14 and 28 post-vaccination are reported. We also assessed the correlation between cellular and humoral response by comparing the mock-adjusted Zaire Ebola GP and whole virion concentration, and NAb.
The analysis is based on the intention-to-treat principle and includes all participants that provided at least one blood sample. Additionally, we assessed immunogenicity outcomes in the per-protocol population of participants who gave a blood sample at each time point within the window specified in the protocol. Per-protocol results are presented in the Supplementary Appendix.
Data analysis was conducted using SAS® software, Version 9.4 of the SAS System for Unix (SAS Institute Inc., Cary, NC, USA).
2.4. Ethical considerations
The trial was conducted in accordance with Good Clinical Practice guidelines and was registered in the Pan African Clinical Trials Registry (Reference: PACTR201503001057193). The study protocol was approved by the ethics committee of the World Health Organization (Switzerland), the Norwegian Regional Ethics Committee, the Comité National d’Ethique pour la Recherche en Santé (Guinea), and the Médecins Sans Frontières Ethics Review Board. A data safety monitoring board regularly reviewed study data. All authors vouch for the accuracy and completeness of the data and analyses reported.
3. Results
3.1. Study participants and assays
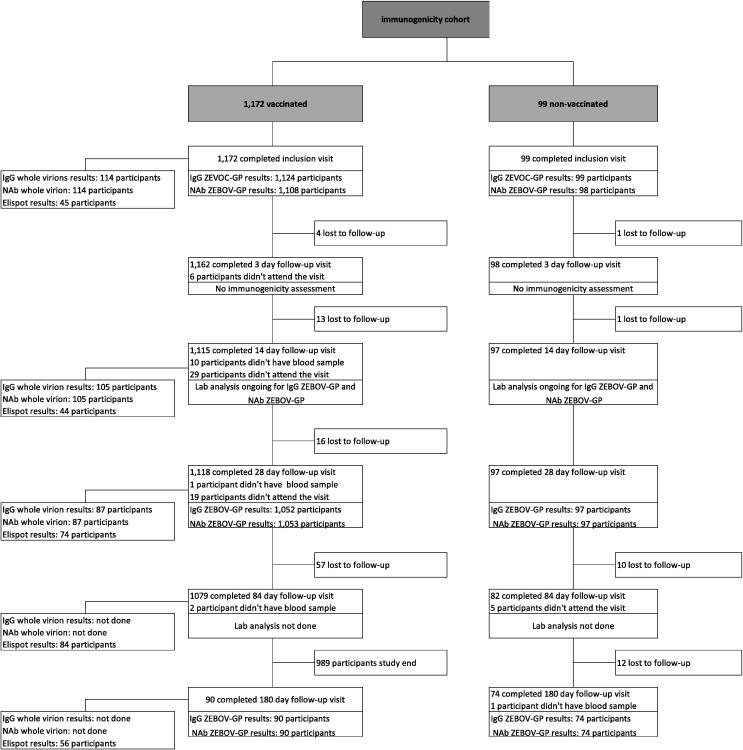
From March 25th to August 7th, 2015, a total of 1172 participants were screened and received one dose of vaccine. Amongst these, 114 participants consented to participate in the subset and were follow-up for 180 days post-vaccination. Additionally, 99 participants not wishing to be vaccinated were recruited for comparisons (Fig. 1). The median age of all participants was 30 years (SD 10.97, range 18–75) and 72.5% were male (Table 1). Among vaccinated individuals, 609 (52%) were in high-risk professions, and 564 (48%) were in low-risk professions. Compared to vaccinated individuals, unvaccinated individuals were younger, more likely to be female, and more likely to be in a high-risk profession (p < 0.05).
Fig. 1.
Flowchart of trial participants.
Table 1.
Characteristics of trial participants.
| Vaccinated | Non-vaccinated | |
|---|---|---|
| Participants | 1172 | 99 |
| Age (years) | 34.5 (11.10) | 28.31 (7.04) |
| Female | 312 (26.62) | 37 (37.37) |
| Male | 860 (73.38) | 62 (62.63) |
| Workplace | ||
| Ebola Treatment Center | 173 (14.76) | 0 (0) |
| Ebola outreach services | 219 (18.69) | 19 (19.19) |
| Hospital | 445 (37.97) | 11 (11.11) |
| Health Centre | 251 (21.42) | 64 (64.65) |
| Clinic | 6 (0.51) | 0 (0) |
| Other | 78 (6.66) | 5 (5.05) |
| Risk category | ||
| High-risk | 609 (51.96) | 68 (68.69) |
| Low-risk | 563 (48.04) | 31 (31.31) |
*Data are means (SD) or numbers (%).
The number of participants with results for the different assays at the different time points is provided in the participants’ flow chart (Fig. 1). A total of 1118 (94%) of vaccinated participants completed the 28-day follow-up visit. ZEBOV-GP IgG assay results were available for 1052 and NAb assay results were available for 1053 participants. The 28 day follow-up visit occurred within a window of ±3 days for 91.4% of participants. In total, 97 (98.0%) of non-vaccinated participants completed the 28 day follow-up visit, with the majority (93.8%) completing it within a window of ±3 days. The 180 day follow-up visit exhibited a wider range of times, with 82.2% of participants completing the visit within a window of ±16 days.
3.2. Humoral response
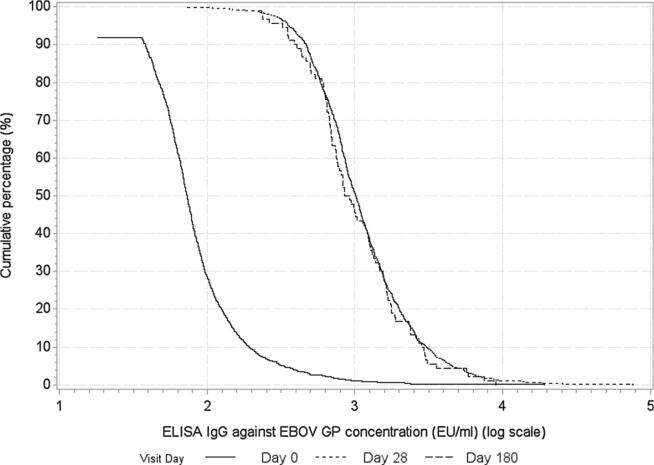
At baseline, 9.4% (n = 106) of vaccinated participants were seropositive for ZEBOV-GP IgG (Table 2). The GMC of IgG ZEBOV-GP specific antibodies was significantly higher at each later time point after vaccination compared to baseline as shown in Table 2 and Fig. 2. Overall, 86.4% (95% CI 84.1–88.4) of vaccinated participants seroresponded at 28 days post-vaccination. Among those with results 180 days post-vaccination (n = 90), the seroresponse persisted at 180 days post-vaccination for 90.7% (95% CI 82.0–95.4). In addition, an antibody response was seen at 14 days post-vaccination, with 65.1% (95% CI 62.1–68.1) seroresponding. Among participants who were seronegative at baseline, GMCs and seropositivity rates at 28 and 180 days post-vaccination were similar to GMCs and seropositivity rates among the whole study population (Supplementary Appendix).
Table 2.
Humoral responses to rVSV ZEBOV vaccination.
| Assay | Day 0 | Day 14 | Day 28 | Day 180 |
|---|---|---|---|---|
| IgG ELISA ZEBOV-GP | ||||
|
N° participants Geometric mean concentration – AEU/ml (95%CI) Participants seropositive - % (95%CI) Participants with seroresponse - % (95%CI) |
1124 78.6 (75.0,82.4) 9.4 (7.9,11.3) - |
1072 545.8+ (515.0,578.5) 88.2 (86.2,90.0) 65.1 (62.1,68.1) |
1052 1105.4+ (1053.0,1160.3) 98.9 (98.0,99.3) 86.4 (84.1,88.4) |
90 1017.6+ (866.1,1195.7) 100.0 (95.9, 100.0) 79.8 (70.3,86.8) |
| NAb against ZEBOV-GP | ||||
|
N° participants Geometric mean titer – titer (95%CI) Participants seropositive - % (95%CI) Participants with seroresponse - % (95%CI) |
1108 17.7 (17.5,17.9) 0.5 (0.1,1.1) - |
1060 99.1+ (93.8,104.7) 93.4 (91.7,94.7) 63.7 (60.6,66.7) |
1053 159.9+ (151.5,168.7) 98.0 (97.0,98.7) 83.0 (80.5,85.3) |
90 118.9+ (99.3,142.4) 95.6 (89.1,98.3) 82.0 (72.8,88.6) |
| IgG ELISA ZEBOVwhole virion | ||||
|
N° participants Geometric mean concentration –AEU/ml (95%CI) Participants seropositive - % (95%CI) Participants with seroresponse - % (95%CI) |
114 850.7 (709.4,1020.2) 27.2 (19.9,36.0) - |
110 2244.3+ (1810.8,2781.5) 71.8 (62.8,79.4) 31.8 (23.9,41.0) |
108 1828.7+ (1496.6,2234.6) 70.4 (61.2,78.2) 26.9 (19.4,35.9) |
|
| NAb against whole virion | ||||
|
N° participants Geometric mean titer – titer (95%CI) Participants seropositive - % (95%CI) Participants with seroresponse - % (95%CI) |
114 5.7 (5.3,6.1) 10.5 (6.1,17.5) - |
110 13.1+ (11.6,14.8) 71.8 (62.8,79.4) 27.3 (19.8,36.3) |
108 9.6+ (8.4,10.9) 47.2 (38.1,56.6) 15.7 (10.1,23.8) |
Seropositivity is defined as ELISA IgG against ZEBOV-GP concentration > 200 AEU/ml; NAb titer > 20; ELISA IgG against ZEBOV whole virion concentration > 500; NAb against whole virion titer > 8.
Seroresponse is defined as an increase in titer ≥ 4-fold from baseline.
p-value < 0.05 for difference from baseline, estimated from Wilcoxon signed-rank test on the difference in concentration from baseline.
Fig. 2.
Reverse cumulative distribution curve for IgG ZEBOV-GP ELISA in AEU/ml, vaccinated participants.
GMCs of ZEBOV-GP IgG among non-vaccinated participants remained low over time, with no differences compared to baseline. Seroresponse rates in the unvaccinated group were 0.0% (95% CI 0.0–4.8) at 28 days and 5.4% (95% CI 2.1–13.1) at 180 days post-vaccination. 12 non-vaccinated individuals were seropositive at baseline, of whom 11 remained seropositive at 28 days post-vaccination. The GMC of ZEBOV-GP IgG declined over time in these individuals.
In the Supplementary Appendix we report ZEBOV-GP IgG assay results as titer.
In relation to risk, there was no significant difference in baseline ZEBOV-GP IgG GMCs between risk groups. At 28 days post-vaccination, GMCs were slightly higher in the high-risk group compared to the low-risk group. Seroresponse was higher in the high-risk group (88.0%, 95% CI 85.4–90.2) compared to the low-risk group (82.0%, 95% CI 76.9–86.3, p = 0.02). This difference remained after controlling for age and sex but was not seen at the 180 day follow-up visit. There were no differences in IgG ZEBOV-GP seroresponse rates by age group or sex (Supplementary Appendix).
Neutralizing antibodies against ZEBOV-GP were detectable at baseline in 0.5% (95% CI 0.1–1.1) of participants and titers were higher at each time point after vaccination compared to baseline (p < 0.05) (Table 2). At 28 days post-vaccination, 98.0% (95% CI 97.0–98.7) of vaccinated participants were seropositive for NAb and 95.6% (95% CI 89.1–98.3) at 180 days post-vaccination. A total of 83.0% (95% CI 80.5–85.3) of participants had NAb seroresponse at 28 days; this seroresponse persisted at 180 days post-vaccination among 84.2% (95% CI 74.4–90.7) of vaccinated participants who seroresponded at 28 days post-vaccination. At 14 days post-vaccination, 93.4% (95% CI 91.7–94.7) of vaccinated participants were seropositive for NAb and 63.7% (95% CI 60.6–66.7) had NAb seroresponse. Neutralizing antibodies remained undetectable in the unvaccinated group, with 0% seroresponse at 28 and 180 days post-vaccination (Supplementary Appendix).
3.3. Antibody responses against the whole virion
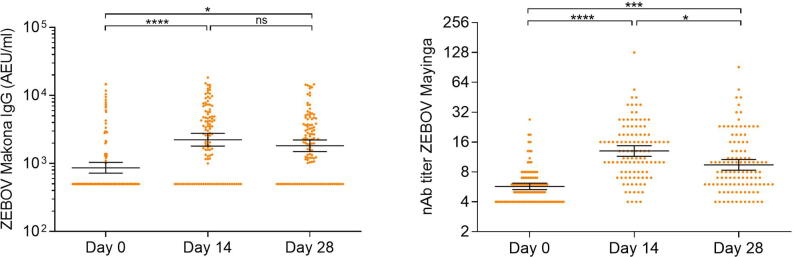
Among the subset of 114 participants, 27.2% (95% CI 19.9–36.0) were seropositive at baseline by ELISA IgG against ZEBOV-whole virion, with GMC > 500 AEU/ml. Compared to baseline, anti- ZEBOV-whole virion IgG GMCs were higher at 14 and 28 days after vaccination (p < 0.05). Seroresponse rates by ELISA were 31.8% (95% CI 23.9–41.0) at 14 day follow-up and 26.9% (95% CI 19.4–35.9) at 28 day follow-up.
At baseline, 10.5% (95% CI 6.1–17.5) of individuals were seropositive to neutralizing antibodies against the live ZEBOV-whole virion. As with the ELISA, NAb whole virion GMTs were higher at 14 and 28 days after vaccination compared to baseline (p < 0.05). Seropositivity rates were higher 14 days post-vaccination and showed a considerable decline at 28 days post-vaccination, going from 71.8% (95% CI 62.8–79.4) to 47.2% (95% CI 38.1–56.6) . The highest seroresponse rate for the NAb against the whole virion was seen in the subset tested 14 days post-vaccination with 27.3% (95% CI 19.8–36.3) of individuals seroresponding.
We found weak or no correlation between the assay results before vaccination (Table 4). None of the 112 participants that have results for all the tests at baseline were seropositive for all of them. However, at day 28 there is significant pairwise correlation between ELISA EBOV GP and ELISA whole virion and neutralization assay (Table 4). Results of whole virion ELISA and neutralization assay using live virus is shown in Fig. 3. We also found that 45 of 105 participants (43%) that had results from all tests at day 28 were seropositive by all the assays.
Table 4.
Correlation between Assays for humoral response.
| Assay 1 | Assay 2 | Time | Correlation* |
|---|---|---|---|
| IgG against ZEBOV GP (concentration) | NAb against ZEBOV GP | 0 | 0.08 |
| IgG against ZEBOV GP (concentration) | NAb against ZEBOV GP | 28 | 0.44 |
| IgG against ZEBOV GP (concentration) | IgG against whole virion | 0 | 0.30 |
| IgG against ZEBOV GP (concentration) | IgG against whole virion | 28 | 0.52 |
| IgG against ZEBOV GP (concentration) | NAb against whole virion | 0 | 0.35 |
| IgG against ZEBOV GP (concentration) | NAb against whole virion | 28 | 0.40 |
| NAb against ZEBOV GP | IgG against whole virion | 0 | N/A |
| NAb against ZEBOV GP | IgG against whole virion | 28 | 0.16 |
| NAb against ZEBOV GP | NAb against whole virion | 0 | N/A |
| NAb against ZEBOV GP | NAb against whole virion | 28 | 0.34 |
| IgG against whole virion | NAb against whole virion | 0 | −0.02 |
| IgG against whole virion | NAb against whole virion | 28 | 0.48 |
Spearman rank correlation coefficient estimated for those whose antibody response was available for per-protocol analysis by both assays.
Fig. 3.
Antibody responses were measured by the whole virion ELISA and live neutralizing antibody assay. Statistical analysis was done by one-way ANOVA and Tukey's multiple comparison test.
3.4. Cellular response
Among the vaccinated sub-set of 114 participants, we tested 717 samples from 111 participants and five time points (day 0, 14, 28, 84 and 180 post-vaccination). Among the samples tested, 303 (42.2%) passed the quality control and were included in the ELISpot analysis. Of all samples tested, median cytomegalovirus (CMV) response was 638 and maximum 2,568 SFU per million PBMC. A large proportion (70.9%) of those had > 80% viability after thaw and overnight rest. An additional positive control stimulus of peptides matched to the CMV pp65 protein resulted in responses in 94% of samples tested, suggesting that CD8 T cell responses were not compromised in the cold chain and transport.
Among samples that passed the quality control, 50 (16.5%) exhibited Ebola responses, with only 1 (2.2%) at baseline, 10 (13.5%) at day 28 post-vaccination and 27 (48.2%) at day 180 post-vaccination (Table 3a, Table 3b). Of 105 subjects tested, 33 (31.4%) exhibited a response to GP at some point post-vaccination.
Table 3a.
Enzyme-linked immunospot assay (ELISpot) responses to rVSV ZEBOV vaccination: Distribution of samples with a background (mock stimulus) subtracted Ebola GP response ≥ 10 SFU per million PBMC and > 2x Mock response by time point.
| Time point | Samples with Ebola GP ≥ 10 (%) |
|---|---|
| D0 (n = 45) | 1(2.2) |
| D14 (n = 44) | 2 (4.5) |
| D28 (n = 74) | 10 (13.5) |
| D84 (n = 84) | 10 (11.9) |
| D180 (n = 56) | 27 (48.2) |
| Total (n = 303) Total post-vaccination (n = 258) |
50 (16.5) 49 (19.0) |
Table 3b.
Enzyme-linked immunospot assay (ELISpot) responses to rVSV ZEBOV vaccination: Distribution of samples with a background (mock stimulus) subtracted Ebola GP response ≥ 10 SFU per million PBMC and > 4x Mock response by time point.
| Time point | Samples with Ebola GP ≥ 10 (%) |
|---|---|
| D0 (n = 45) | 1(2.2) |
| D14 (n = 44) | 2 (4.5) |
| D28 (n = 74) | 9 (12.2) |
| D84 (n = 84) | 9 (10.7) |
| D180 (n = 56) | 11 (19.6) |
| Total (n = 303) Total post-vaccination (n = 258) |
32 (10.6) 31 (12.0) |
Of the 163 participants who have a humoral and cellular result for Day 0, 14 or 28, we did not find any correlation between humoral and cellular response (data not shown).
4. Discussion
In this trial among FLWs conducted in Guinea at the tail end of the West African outbreak, we found the rVSVΔG/ZEBOV vaccine is able to produce an increase in IgG and neutralizing antibodies. Antibody responses measured by ELISA and NAb assays were higher at each time point after vaccination compared to baseline. The highest rate of seroresponse was found with the ELISA IgG ZEBOV-GP assay. From the subset of participants followed up for 180 days, IgG and NAb increased 28 days post-vaccination and were detectable at 180 days post-vaccination in most participants.
T-cell responses to Ebola virus glycoprotein were detected in 13.5% of individuals after 28 days and in 48.2% at day 180 post-vaccination. We did not find a correlation between cellular and humoral response. This could imply a dual mechanism of action of the vaccine with a first response based on production of antibodies against GP and a later cellular response based on production of CD8 cells [20].
The primary analysis was a comparison of high-risk and low-risk exposure groups, defined by a combination of profession and work location. Although there was evidence for a stronger ZEBOV-GP IgG seroresponse among high-risk individuals 28 days post-vaccination, this result was not replicated with the NAb assay, and there was no difference between the risk groups at baseline. The comparison of high-risk and low-risk groups was limited using profession and work location as a proxy for exposure. This proxy likely has low sensitivity and specificity, but as this was a prospective study, misclassification is non-differential with respect to seroresponse and any bias would be towards the null. Other analyses could have provided more evidence of differences in seroresponse between the two groups, such as seroresponse among individuals seronegative and seropositive at baseline. However, such analyses lack power with the available data.
Although the different assays, targeting different components of the immune response, show an increase in titers compared to baseline, the rates of seropositivity and seroresponse are different. It is important to note that in EBOV-endemic settings the ELISA ZEBOV whole virion assay may be a less sensitive method to detect vaccine-induced antibody responses targeting GP [3], [18]. Similarly, we observed a higher immune response by neutralizing antibodies against live virus than the whole virion assay. These differences are not well understood but may stem from different techniques and assay platforms that are being used to measure serological responses [21].
It is also important to note that compared with other studies assessing the immune response of one dose of 2 × 107 PFU rVSVΔG-ZEBOV-GP prior or during the West Africa outbreak, our study corroborates a good immune response despite the difference in assays and/or threshold used in different studies [3], [5], [7]. The study conducted in Liberia, measured IgG antibody levels against ZEBOV-GP at baseline, 1 week and 1, 6 and 12 months post-vaccination using the FANG assay. The study found that antibody response peaked at 1 month post-vaccination with 83.7% of participants seroresponding [22]. This was a randomized trial including a control non-vaccinated group which remained with significantly lower response than the vaccinated group up to 12 months post-vaccination [22].
Although over 95% of participants developed IgG specific antibodies 28 days after vaccination, and 65% at 14 days post-vaccination, interpretation in the context of a lack of correlate of protection is difficult. This trial was conducted in parallel to the ring vaccination trial. The parent trial and this sub-study targeted different populations, with regards to the timing of potential exposure to EVD. None of the FLWs followed during this study reported developing EVD or were admitted as a laboratory confirmed case. This study was conducted when transmission had slowed and when infection and control precautions were correctly applied. Further, both asymptomatic and pauci-symptomatic infections have been reported and learning concerning the natural history of infection and clinical symptoms continues [23].
The vaccine was not assigned at random, and as a result there were differences in demographic characteristics between the vaccinated and unvaccinated at baseline that could introduce unmeasured confounding into comparisons. However, as no unvaccinated individual seroresponded by 28 days post-vaccination, compared with 86.4% of the vaccinated group, as measured by ZEBOV-GP IgG, unmeasured confounding alone is very unlikely to account for this difference. It is also important to note that three-quarters of participants were male. Although no apparent differences between male and female participants were found in this study, a multivariate analysis indicated that female sex had a higher risk for the development of arthritis post vaccination [24] though this was not found in all vaccine trials [25].
To assess cellular immunity, frozen PBMC samples are usually run on site or maintained in vapor phase liquid nitrogen from point of freezing, through shipping and storage until the point of thawing and test. However, the Ebola PBMC collected in Conakry could not be run on site. Samples were stored for up to 3 months at −80 °C due to the need to quarantine in accordance with international health and biological sample regulations before shipment. This may explain the large proportion of PBMC samples with < 80% viability. Though high CMV values suggest that CD8 T cell were not compromised in the cold chain, storage and shipment, the Ebola responsive CD4 T cells may have not survived nor remained functional.
It is important to note the importance of providing sufficient and clear information on infection control and prevention measures to those electing to receive the vaccine remains essential until additional information is acquired. Qualitative analyses conducted at the same time as this study also highlight the importance of accompanying any use of the vaccine with ongoing information and awareness strategies [26].
Since the West African outbreak, rVSVΔG-ZEBOV-GP has been used in an expanded use framework [27] in the response to the two EVD outbreaks in the Democratic Republic of Congo in 2018 and 2019 and has recently received market authorization by the EMA and FDA [11], [28] The first of these outbreaks, in Equator province was declared on May 8th, 2018 and vaccination activities targeting FLW and contacts and contacts of contacts started on May 21st, 2018. In over one month a total of 3,481 people were vaccinated [29]. The last laboratory confirmed EVD case developed symptoms on June 2nd, 2018. No cases of EVD were detected amongst the vaccinated, consequently this experience did not provide additional effectiveness data. At present, rVSVΔG-ZEBOV-GP vaccine is being used as a part of the ongoing EVD outbreak in North Kivu, Democratic Republic of Congo. As of January 2020, over 260,000 person have been vaccinated in the DRC since the outbreak was declared in August 2018 with now over 3500 confirmed cases [30].
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
We are grateful to frontline workers in Guinea for their participation, and all the field, laboratory, and data management staff who worked extremely hard and under difficult conditions to successfully implement this study. Merck Sharp & Dohme provided the vaccine used in the trial. We would like to acknowledge the support of the following organizations: Guinean Ministry of Health, Wellcome Trust, UK Department of International Development, Norwegian Ministry of Foreign Affairs, Research Council of Norway, US Department of Defense, Public Health Agency of Canada, Swiss Agency for Therapeutic Products, the Bill & Melinda Gates Foundation, Health Canada, and the European Commission. We also thank all members of our scientific advisory group, our data and safety monitoring board, and the Guinea vaccine trial working group.
We also want to acknowledge Kolié Ouo-Ouo, Sarah Katharina Fehling, Verena Krähling, Mamadouba Conté, Cristina Leggio , Babak Afrough, Debbie Bishop, Catherine Pratt, Didier Ngabo, Phill Brown, Alphonse Foromo Kpakpavogui, Ola Miloszewska, Ross Fothergill, Mel Clifford, Kim Steeds, Kathryn Ryan, and Sonal Shah.
Sources of support
Funding for this sub-study was provided by MSF Operational Center Belgium, the World Health Organization, and Research Council of Norway. Vaccine was provided in kind. Epicentre receives core funding from Médecins Sans Frontières.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2020.04.066.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Henao-Restrepo A.M., Camacho A., Longini I.M., Watson C.H., Edmunds W.J., Egger M. Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: final results from the Guinea ring vaccination, open-label, cluster-randomised trial (Ebola Ça Suffit!) Lancet. 2017;389(10068):505–518. doi: 10.1016/S0140-6736(16)32621-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Juan-Giner A., Tchaton M., Jemmy J.P., Soumah A., Boum Y., Faga E.M. Safety of the rVSV ZEBOV vaccine against Ebola Zaire among frontline workers in Guinea. Vaccine. 2019;37(48):7171–7177. doi: 10.1016/j.vaccine.2018.09.009. [DOI] [PubMed] [Google Scholar]
- 3.Agnandji S.T., Fernandes J.F., Bache E.B., Obiang Mba R.M., Brosnahan J.S., Kabwende L. Safety and immunogenicity of rVSVΔG-ZEBOV-GP Ebola vaccine in adults and children in Lambaréné, Gabon: a phase I randomised trial. von Seidlein L, editor. PLOS Med. 2017;14(10) doi: 10.1371/journal.pmed.1002402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agnandji S.T., Huttner A., Zinser M.E., Njuguna P., Dahlke C., Fernandes J.F. Phase 1 trials of rVSV Ebola vaccine in Africa and Europe. N Engl J Med. 2015;374(17):1647–1660. doi: 10.1056/NEJMoa1502924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Regules J.A., Beigel J.H., Paolino K.M., Voell J., Castellano A.R., Hu Z. A recombinant vesicular stomatitis virus ebola vaccine. N Engl J Med. 2017;376(4):330–341. doi: 10.1056/NEJMoa1414216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huttner A., Dayer J.-A., Yerly S., Combescure C., Auderset F., Desmeules J. The effect of dose on the safety and immunogenicity of the VSV Ebola candidate vaccine: a randomised double-blind, placebo-controlled phase 1/2 trial. Lancet Infect Dis. 2015;15(10):1156–1166. doi: 10.1016/S1473-3099(15)00154-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dahlke Christine, Kasonta Rahel, Lunemann Sebastian, Krähling Verena, Zinser Madeleine E., Biedenkopf Nadine, Fehling Sarah K., Ly My L., Rechtien Anne, Stubbe Hans C., Olearo Flaminia, Borregaard Saskia, Jambrecina Alen, Stahl Felix, Strecker Thomas, Eickmann Markus, Lütgehetmann Marc, Spohn Michael, Schmiedel Stefan, Lohse Ansgar W., Becker Stephan, Addo Marylyn M., Addo Marylyn M., Becker Stephan, Krähling Verena, Agnandji Selidji Todagbe, Krishna Sanjeev, Kremsner Peter G., Brosnahan Jessica S., Bejon Philip, Njuguna Patricia, Siegrist Claire-Anne, Huttner Angela, Kieny Marie-Paule, Modjarrad Kayvon, Moorthy Vasee, Fast Patricia, Savarese Barbara, Lapujade Olivier. Dose-dependent T-cell dynamics and cytokine cascade following rVSV-ZEBOV immunization. EBioMedicine. 2017;19:107–118. doi: 10.1016/j.ebiom.2017.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huttner A., Combescure C., Grillet S., Haks M.C., Quinten E., Modoux C. A dose-dependent plasma signature of the safety and immunogenicity of the rVSV-Ebola vaccine in Europe and Africa. Sci Transl Med. 2017;9(385) doi: 10.1126/scitranslmed.aaj1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Medaglini Donata, Siegrist Claire-Anne. Immunomonitoring of human responses to the rVSV-ZEBOV Ebola vaccine. Curr Opin Virol. 2017;23:88–94. doi: 10.1016/j.coviro.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 10.WHO. Ebola Situation Report - 4 November 2015; 2015.
- 11.Rudge T.L., Sankovich K.A., Niemuth N.A., Anderson M.S., Badorrek C.S., Skomrock N.D. Development, qualification, and validation of the Filovirus Animal Nonclinical Group anti-Ebola virus glycoprotein immunoglobulin G enzyme-linked immunosorbent assay for human serum samples. PLoS ONE. 2019;14(4):1–28. doi: 10.1371/journal.pone.0215457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanchez-Lockhart M., Reyes D.S., Gonzalez J.C., Garcia K.Y., Villa E.C., Pfeffer B.P. Qualitative profiling of the humoral immune response elicited by rVSV-ΔG-EBOV-GP using a systems serology assay, domain programmable arrays. Cell Rep. 2018;24(4):1050–1059.e5. doi: 10.1016/j.celrep.2018.06.077. [DOI] [PubMed] [Google Scholar]
- 13.Grant-Klein R.J., Antonello J., Nichols R., Dubey S., Simon J. Effect of gamma irradiation on the antibody response measured in human serum from subjects vaccinated with recombinant vesicular stomatitis virus-zaire ebola virus envelope glycoprotein vaccine. Am J Trop Med Hyg. 2019;101(1):207–213. doi: 10.4269/ajtmh.19-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kwilas S., Kishimori J.M., Josleyn M., Jerke K., Ballantyne J., Royals M. A hantavirus pulmonary syndrome (HPS) DNA vaccine delivered using a spring-powered jet injector elicits a potent neutralizing antibody response in rabbits and nonhuman primates. Curr Gene Ther. 2014;14(3):200–210. doi: 10.2174/1566523214666140522122633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ewer K., Rampling T., Venkatraman N., Bowyer G., Wright D., Lambe T. A monovalent chimpanzee adenovirus ebola vaccine boosted with MVA. N Engl J Med. 2016 doi: 10.1056/NEJMoa1411627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bull M., Lee D., Stucky J., Chiu Y.L., Rubin A., Horton H. Defining blood processing parameters for optimal detection of cryopreserved antigen-specific responses for HIV vaccine trials. J Immunol Methods. 2007;322(1–2):57–69. doi: 10.1016/j.jim.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gill D.K., Huang Y., Levine G.L., Sambor A., Carter D.K., Sato A. Equivalence of ELISpot assays demonstrated between major HIV network laboratories. PLoS ONE. 2010;5(12) doi: 10.1371/journal.pone.0014330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dahlke C., Lunemann S., Kasonta R., Kreuels B., Schmiedel S., Ly M.L. Comprehensive characterization of cellular immune responses following ebola virus infection. J Infect Dis. 2017;215(2):287–292. doi: 10.1093/infdis/jiw508. [DOI] [PubMed] [Google Scholar]
- 19.DasGupta Anirban, Cai T. Tony, Brown Lawrence D. Interval estimation for a binomial proportion. Statist Sci. 2001;16(2):101–133. doi: 10.1214/ss/1009213286. [DOI] [Google Scholar]
- 20.Menicucci Andrea R., Jankeel Allen, Feldmann Heinz, Marzi Andrea, Messaoudi Ilhem, Kobinger Gary P., Biron Christine A. Antiviral innate responses induced by VSV-EBOV vaccination contribute to rapid protection. mBio. 2019;10(3) doi: 10.1128/mBio.00597-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilkinson D, Page M, Mattituzzo G, Hassall M, Dougall TPR, et al. Comparison of platform technologies for assaying antibody to Ebola virus. Vaccine. 2017;35(9):1347–52. [DOI] [PMC free article] [PubMed]
- 22.Kennedy S.B., Bolay F., Kieh M., Grandits G., Badio M., Ballou R. Phase 2 placebo-controlled trial of two vaccines to prevent ebola in Liberia. N Engl J Med. 2017;377(15):1438–1447. doi: 10.1056/NEJMoa1614067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saliou M., Diallo K., Rabilloud M., Ayouba A., Touré A., Thaurignac G. Prevalence of infection among asymptomatic and paucisymptomatic contact persons exposed to Ebola virus in Guinea: a retrospective, cross-sectional observational study. Lancet Infect Dis. 2019;19(March):308–316. doi: 10.1016/S1473-3099(18)30649-2. [DOI] [PubMed] [Google Scholar]
- 24.Lévy Y., Lane C., Piot P., Beavogui A.H., Kieh M., Leigh B. Prevention of Ebola virus disease through vaccination: where we are in 2018. Lancet. 2018;392(10149):787–790. doi: 10.1016/S0140-6736(18)31710-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jr DGH, Kemp TL, Martin BK, Ramsey WJ, Nichols R, Dasen EJ, et al. Safety and immunogenicity of the rVSV Δ G-ZEBOV-GP Ebola virus vaccine candidate in healthy adults: a phase 1b dose-response study. 2017;17(August). [DOI] [PubMed]
- 26.Grantz Kyra H., Claudot Pauline, Kambala Micky, Kouyaté Mariama, Soumah Aboubacar, Boum Yap, Juan-Giner Aitana, Jemmy Jean-Paul, Cummings Derek A.T., Grais Rebecca F. Factors influencing participation in an Ebola vaccine trial among front-line workers in Guinea. Vaccine. 2019;37(48):7165–7170. doi: 10.1016/j.vaccine.2019.09.094. [DOI] [PubMed] [Google Scholar]
- 27.Meeting of the Strategic Advisory Group of Experts on immunization, April 2017 – conclusions and recommendations. Wkly Epidemiol Rec. 2017;22(92):301–20. [PubMed]
- 28.FDA. First FDA-approved vaccine for the prevention of Ebola virus disease, marking a critical milestone in public health preparedness and response [Internet]. FDA NEWS RELEASE. 2019 [cited 2020 Jan 8]. Available from: https://www.fda.gov/news-events/press-announcements/first-fda-approved-vaccine-prevention-ebola-virus-disease-marking-critical-milestone-public-health.
- 29.WHO Health Emergency Program. Ebola Virus Disease. Democratic Republic of the Congo. External Situation Report 17. Declaration of the end of the outbreak; 2018. p. 10.
- 30.World Health Organisation. Ebola Virus disease: Democratic Republic of the Congo. 2020;1–9. Available from: https://apps.who.int/iris/bitstream/handle/10665/324996/SITREP_EVD_DRC_20190528-eng.pdf?ua=1%0Ahttps://www.afro.who.int/health-topics/ebola-virus-disease.
- 31.Heppner DG, Jr., Kemp TL, Martin BK, Ramsey WJ, Nichols R. Safety and immunogenicity of the rVSV∆G-ZEBOV-GP Ebola virus vaccine candidate in healthy adults: a phase 1b randomised, multicentre, double-blind, placebo-controlled, dose-response study. Lancet Infectious Disease. 2017;17(8):854–866. doi: 10.1016/S1473-3099(17)30313-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.