Abstract
Addiction is characterized by an erosion of cognitive control toward drug taking that is accentuated by negative emotional states. Here we tested the hypothesis that enhanced interference on cognitive control reflects a loss of segregation between cognition and emotion in addiction. We analyzed Human Connectome Project data from 1206 young adults, including 89 with cannabis dependence (CD). Two composite factors, one for cognition and one for emotion, were derived using principal component (PC) analyses. Component scores for these PCs were significantly associated in the CD group, such that negative emotionality correlated with poor cognition. However, the corresponding component scores were uncorrelated in matched controls and nondependent recreational cannabis users (n = 87). In CD, but not controls or recreational users, functional magnetic resonance imaging activations to emotional stimuli (angry/fearful faces > shapes) correlated with activations to cognitive demand (working memory; 2-back > 0-back). Canonical correlation analyses linked individual differences in cognitive and emotional component scores with brain activations. In CD, there was substantial overlap between cognitive and emotional brain–behavior associations, but in controls, associations were more restricted to the cognitive domain. These findings support our hypothesis of impaired segregation between cognitive and emotional processes in CD that might contribute to poor cognitive control under conditions of increased emotional demand.
Keywords: addiction, executive function, fMRI, Human Connectome Project, marijuana
Introduction
Cannabis is the most commonly used illicit drug, with rising prevalence in parallel to increases in its legalization across most states in the United States of America (Blanco et al. 2016; Hasin et al. 2017). The addictive potential of cannabis is well known, but it remains unclear to what extent cannabis dependence (CD) interferes with the function of the human brain. Of particular interest are the effects of cannabis on 3 broad functional brain–behavior domains: reward/motivation, emotion, and cognition (Volkow et al. 2016). Current addiction models suggest a relapsing cycle in these domains such that the drug is first taken for its rewarding/motivating properties; after cessation, a state of negative emotionality related to drug withdrawal emerges; and attempts to use cognitive control to curb cravings and perform daily tasks are impaired by preoccupation with the drug of choice (Koob and Volkow 2010).
Here we hypothesize that a consequence of this repeating cycle is that the brain–behavioral domains become increasingly intertwined in addiction. As a result, emotional state, cognitive performance, and reward-driven behaviors would be linked to a higher degree in addicted than in nonaddicted individuals. While motivation, emotion, and cognition are often discussed as separate constructs, an extensive literature highlights that they are intrinsically related (Phelps 2006; Pessoa 2009; Inzlicht et al. 2015) though these studies primarily focus on acute, short-term challenges. For example, prior work illustrated how the immediate prospect of reward alters attention (Krebs et al. 2011; Boehler et al. 2014; Chiew and Braver 2016) and how viewing negative emotional images impacts working memory (WM) performance on a trial-to-trial basis (Kensinger and Corkin 2003; Dolcos and McCarthy 2006). In contrast, there is much less evidence for robust associations between trait-level measures of emotion, reward-based decision making, and cognition. Numerous studies in healthy adults found that individual differences in trait personality measures of positive and negative emotionality (extraversion and neuroticism, respectively; Costa and McCrae 1980) are associated with brain activations during various tasks, but critically, these traits were not significantly associated with behavior. Extraversion and neuroticism did not predict high- versus low-risk gambling (Cohen et al. 2005), inhibitory control (Rodrigo et al. 2015), response conflict (Yücel et al. 2007), or WM performance (Kumari et al. 2004; Gray et al. 2005). However, these factors may associate with behavior when the task itself includes emotional stimuli: extraversion correlated with emotional Stroop reaction time (Haas et al. 2006). In sum, while acute manipulations have revealed interactions between emotion, cognition, and reward, there may not be strong links between longer-term trait behaviors in these domains in healthy adults.
Emerging evidence suggests that these trait measures may be more strongly associated with behavior in adults with drug addiction than in nonaddicted individuals. Although self-reported personality measures are uncorrelated with behavioral measures of impulsivity in healthy adults (Reynolds et al. 2006), these measures are all heightened and associated in drug addiction (Chaarani et al. 2017). Trait-level emotion regulation has been associated with cognition in tobacco use disorder (Fillo et al. 2016) and with cognition and decision making in gambling disorder (Navas et al. 2016). Moreover, altered awareness of current affective state (i.e., interoception) is associated with aberrant reward and punishment processing and executive function in methamphetamine use disorder (Stewart et al. 2014). Further, trait-level neuroticism was associated with poor cognitive performance in a sample of young adults with heavy cannabis use (Huijbregts et al. 2014). While studies have demonstrated altered brain function in cannabis users related to processing of rewarding (van Hell et al. 2010; Martz et al. 2016) and emotional stimuli (Phan et al. 2008; Wesley et al. 2016) and during cognitive processing (Nader and Sanchez 2018), the interrelationships of these domains and their neurobiological underpinnings remain largely unknown.
Based on these findings, we hypothesized that emotion, cognition, and reward-based traits would be largely orthogonal to each other among generally healthy young adults, but that these domains would show heightened associations in CD. To test for this possibility, we conducted a principal component analysis (PCA) on a broad array of emotion, cognition, and reward-based measures in over 1200 young adults in the Human Connectome Project (HCP) (Van Essen et al. 2012). We expected to find separable components for each domain in the general population that would be uncorrelated in matched control subjects but significantly correlated in CD. We then sought to find parallel evidence for this phenomenon in brain responses to stimuli in each of the 3 domains. To this end, we used the emotion, cognition, and reward functional magnetic resonance imaging (fMRI) tasks collected in the HCP. Finally, we used canonical correlation analysis (CCA) to examine brain–behavior relationships that might link these 2 findings and describe individual differences in behavioral outcomes in CD.
Materials and Methods
Participants
The participants in this study provided written informed consent at Washington University in St. Louis after receiving a complete description of the study. We used all participants (n = 1206; aged 22–35) from the young adult HCP final release (https://www.humanconnectome.org/study/hcp-young-adult/document/1200-subjects-data-release; Van Essen et al. 2012). From this larger cohort, 109 individuals had imaging data and met the DSM-IV criteria for CD, based on an interview with a clinical research specialist using the semistructured assessment for the genetics of alcoholism (SSAGA) (Van Essen et al. 2013). Individuals must meet at least 3 of the following criteria: 1) tolerance to cannabis; 2) using cannabis in larger amounts or over a longer period than intended; 3) inability to cut down or reduce cannabis use; 4) spending large amounts of time to obtain, use, or recover from the effects of cannabis; 5) giving up important social, occupational, or recreational activities in favor of using cannabis; and 6) continued use of cannabis despite its adverse consequences. Although our hypothesis was not specific to cannabis and is related to addiction in general, the sample size of cannabis-dependent individuals in the HCP (without comorbid alcohol dependence) was much greater than for alcohol dependence (n = 39) or tobacco dependence (n = 35), which is why we chose to focus on CD.
After excluding individuals with comorbid alcohol dependence, or outliers on DSM levels of anxiety and depression (>3 SD from the mean of all 1206 HCP participants), the final sample was n = 89 CD and n = 562 healthy controls that self-reported ≤ 10 lifetime uses of cannabis. Recent studies have indicated that it is critical in studies of cannabis use disorders to select a well-matched control group, e.g., (Weiland et al. 2015). Therefore, we attempted to find a subset of the 562 controls that was well matched with the CD group on age, sex, education, BMI, and alcohol and tobacco usage (we calculated composite tobacco/alcohol usage the same way as in recent studies using HCP data; see Orr et al. 2016; Manza et al. 2018). To find a matched control group, we used the matchControls function in R (library e1071), which calculates a dissimilarity matrix between groups to find the closest match on multiple variables, and critically, can handle numeric, nominal, and ordinal variables in the same model (Kaufman and Rousseeuw 1990). This provided a control group (n = 89) that was well matched on all variables (P’s > 0.30) except tobacco usage, which was lower than for the CD group (P < 0.001). Subsequent analyses were performed with tobacco usage as a confound regressor to ensure results were not driven by tobacco.
Finally, to examine if any measures might be specifically related to the state of addiction rather than a general feature of individuals who are predisposed to use cannabis, we included a group who reported frequent use of recreational cannabis (REC; > 100 lifetime uses of cannabis without symptoms of dependence). Because there were fewer REC subjects in the database, we included all possible subjects without attempting to match the groups (REC n = 87 were used in behavioral analysis; a subset of n = 67 also had imaging data and were additionally used in fMRI analysis). Notably, the REC group was not matched on sex, having a significantly greater proportion of females (45%) than the CTL or CD groups (28%).
Behavioral Measures of Interest: PCA
The HCP contains a comprehensive neuropsychological battery that includes over 80 measures of cognition and emotion/personality, but unfortunately only a few on reward-based decision making. To first identify broad, aggregate measures, we z-transformed the data and reduced data dimensionality by performing probabilistic PCA on the data of all 1206 HCP participants, as implemented with the ppca function in MATLAB, with missing datapoints imputed via an expectation–maximization algorithm. We chose PCA over other similar methods, such as exploratory factor analysis, because PCA is a simpler approach for data dimensionality reduction that does not have the possibility of resulting in improper solutions based on measurement error (Anderson and Gerbing 1984) and one can calculate individual component scores directly with PCA (Brown 2015). We chose to use the entire sample of 1206 adults in the PCA analysis so that components would reflect characteristics of the general population. We included all primary measures (age-adjusted , where possible) in these domains (Supplementary Table 1). These assessments were selected for the HCP based on a comprehensive process to identify valid and reliable measures of behavior (for details, see Barch et al. 2013). These measures, including the NIH Toolbox and the NEO-FFI, have been widely used in the past and shown excellent validity/reliability in other samples, as well (McCrae and Costa 2004; Salsman et al. 2013; Heaton et al. 2014).
The first 2 components accounted for 45.3% of the variance across all measures and all 1206 participants. A third component loaded heavily on reaction time (not shown); subsequent components were heavily mixed between the domains, accounted for < 10% of the variance each, and were therefore not interpreted. Perhaps because there were very few behavioral measures of reward-based decision making—one was a reward task, which required binary choices followed by explicitly random binary win/loss outcomes, and the other was a delay discounting task, which is often considered a measure of executive function (e.g., Koffarnus et al. 2013; Schel et al. 2014)—this domain was not well represented in any of the components. Thus, we only focused on emotion and cognition in the manuscript.
We first tested whether these components were significantly different between groups using a 2-sample t-test on the subject weights of each component. Then, to test the hypothesis that behaviors in these domains may be more interrelated in a drug-dependent cohort, we tested for the group interaction of the association between PC1 and PC2 component scores. That is, we conducted a regression of PC1 on PC2 component scores, moderated by diagnosis (CD vs. controls), and tested the interaction effect. Then, to characterize the nature of the cognition–emotion associations within each group separately, we conducted a Pearson correlation between component scores of the first and second PCs in each group. Finally, we tested if the PC1–PC2 correlation was stronger among the 89 CD than among the 89 matched controls, using Fisher’s z-test.
MRI Image Acquisition and Preprocessing
Brain images were collected on a Siemens 3 T “connectome Skyra” scanner with a 32-channel coil at Washington University in St. Louis. T1- and T2-weighted anatomical scans were acquired (FOV = 224 mm, matrix = 320, 256 slices, 0.7 mm isotropic voxels). Task fMRI scans were acquired with an EPI sequence (multiband factor = 8, time repetition = 720 ms, time echo = 33.1 ms, flip angle = 52°, FOV = 208 mm, 104 × 90 matrix, 72 slices of 2 mm isotropic voxels, no gap). We used all fMRI data in the “grayordinates” fMRI pipeline, that is, both cortical surface and subcortical volume representations are included in the same image. All images were “minimally preprocessed” including gradient unwarping, motion correction, EPI distortion correction, registration to T1-weighted scans, grand-mean intensity normalization, and smoothing with a 2-mm isotropic FWHM. Images were cross-registered across subjects using the multimodal surface matching (“MSMall”) algorithm, which provides superior registration performance over legacy pipelines (Robinson et al. 2014; Glasser et al. 2016).
We utilized task fMRI scans within the 2 domains of interest: for emotion, an emotional face matching task, with the “angry/fearful faces–shapes” contrast (Hariri et al. 2002); and for cognition, a 2-back WM task with the “2-back–0-back” contrast (Drobyshevsky et al. 2006). We used the “CIFTI” files in the minimally preprocessed pipeline. We then parcellated each individual’s contrast file using the -cifti-parcellate command in Connectome Workbench version 1.2.3 (https://www.humanconnectome.org/software/connectome-workbench). For a complete description of images and preprocessing, see Barch et al. (2013) and Glasser et al. (2013).
fMRI Analyses
For each task, we parcellated each individual’s contrast file by taking the average contrast value of all surface vertices within each of 360 cortical regions in an HCP atlas (Glasser et al. 2016) and, for subcortical regions, the average value of all voxels within 19 regions in the Gordon atlas (Gordon et al. 2016), for a total of 379 parcels/individual. To examine group differences in emotion or cognitive task activation, we performed a whole-brain (parcel-wise) 2-sample t-test of these contrasts; t-tests were family-wise error corrected using 5000 permutations with PALM (Winkler et al. 2014) https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/PALM. Because the HCP data includes related family members, we maintained the family structure while permuting the data, to ensure the null distribution was valid (Winkler et al. 2015).
Then, similar to the behavioral analysis, we examined if brain responses to emotion and cognition were more interrelated in the CD group than the controls. We performed a PCA on the z-transformed brain imaging data from parcels that showed a large effect size (Cohen’s D > | ± 0.8|) for the contrasts of interest across all HCP participants with complete imaging data (n = 1005). We again examined the group interaction of the association between the “cognition fMRI” and “emotion fMRI” component scores. Finally, we tested the correlation between the cognition fMRI and emotion fMRI component scores within each group separately and tested if the slopes of these correlations were significantly different between the groups using Fisher’s z-test.
Brain–Behavior Associations
To examine comprehensive brain–behavior associations across all behavioral and imaging measures of interest, we conducted CCA using MATLAB’s canoncorr function. Each component, or mode, produced by this analysis represents a linear combination of behavioral and imaging measures that are maximally correlated across participants. In this analysis, we included the 87 behavioral measures of interest described previously and the contrast values of the 379 parcels for the 2 fMRI tasks.
Following the example of previous work (Smith et al. 2015) and extant research on confounding influences on the blood oxygen level-dependent response (Anderson et al. 2006; Gonzales et al. 2010; Murphy et al. 2013), we first determined the following measures as confounds to control for: 1) age, 2) sex, 3) height and weight, 4) systolic and diastolic blood pressure, 5) blood levels of hemoglobin A1C, 6) cube root of FreeSurfer-estimated brain volume (with and without ventricles), and 7) tobacco and alcohol composite z-scores. All continuous measures were also demeaned and squared, to help control for possible nonlinear effects, as in previous work (Smith et al. 2015). These confounds were regressed out of both the behavioral and imaging matrices. We then used PCA on the behavioral and imaging measures separately to reduce data dimensionality, because CCA is prone to overfitting (Egloff et al. 2005). We retained enough components to capture close to half of the variance in each modality: 2 components for the behavioral measures and 4 for the imaging measures (although slightly different numbers of components yielded highly similar results, see “Reliability” section in Results). Thus, the CCA included an 89 × 2 matrix of subject-weight eigenvectors of behavioral data and an 89 × 4 matrix of subject-weight eigenvectors of imaging data. To assess the significance of each brain–behavior association (mode), we used permutation testing. Rows representing the individual subject weights for the behavioral and imaging measures were permuted 10 000 times, and CCA was rerun after each permutation. The significance threshold was the number of permutations with a stronger correlation than the observed mode from the original data, divided by the total number of permutations. Again, we maintained family structure while permuting the data, to ensure the null distribution was valid (Winkler et al. 2015).
Reliability Testing
To examine if any significant CCA modes were reliable, we performed split-half analysis with train-test sets. We randomly permuted the data 5000 times, performing the CCA on the first half (training set) and applying the weights to the second half (test), as in previous work (Moser et al. 2017). In addition, although we chose a very small number of components to reduce overfitting, we also tested our CCA analysis with different numbers of inputs to ensure that our results were not unique to the chosen number of components. We reran the CCA with 4, 6, 8, 10, and 12 components each for behavior and fMRI. Then, for each of these analyses we correlated the subject weights for the behavior variate and imaging variate of the first CCA mode with our original analysis (2 behavior components and 4 fMRI components as inputs), to determine how much changing the input parameters would affect the CCA results.
Results
Demographics, Behavioral Measures, and Structural MRI Results
Demographics and lifestyle factors with descriptive statistics for each group are presented in Table 1. The CD and matched CTL groups did not significantly differ on many of the DSM-oriented scales, including depression, anxiety, attention deficit hyperactivity disorder (ADHD), aggression, panic disorder, intrusive thinking, internalizing problems, agoraphobia, and somatic problems (all P’s > 0.05), although the CD group reported higher levels of thought problems (P = 0.04), externalizing problems (P = 0.02), antisocial behavior (P = 0.03), childhood conduct problems (P = 0.003), and rule-breaking behavior (P < 1 × 10−7) than controls. (Supplementary Material). Two individuals in the CD group showed outlier levels of self-reported ADHD symptomatology (>3 SD above the population mean); when excluding those individuals, results are essentially unchanged, and therefore we only report the analyses with these individuals included.
Table 1.
Demographics and clinical characteristics for each group
| CTL | CD | REC | All other | CD versus CTL: t (P) | |
|---|---|---|---|---|---|
| Age | 28.6 ± 3.9 | 28.6 ± 3.5 | 28.3 ± 3.9 | 28.9 ± 3.7 | 0.02 (0.98) |
| Sex (% male) | 64/89 (72%) | 64/89 (72%) | 48/87 (55%) | 375/941 (40%) | |
| BMI | 27.5 ± 5.2 | 26.8 ± 4.8 | 26.6 ± 6.0 | 27.1 ± 6.0 | −0.96 (0.34) |
| Edu | 14.3 ± 1.8 | 14.3 ± 1.8 | 13.9 ± 2.0 | 15.1 ± 1.8 | −0.04 (0.97) |
| DSM depression | 53.6 ± 5.5 | 54.3 ± 7.0 | 54.3 ± 6.1 | 54.0 ± 5.7 | 0.73 (0.47) |
| DSM anxiety | 53.1 ± 5.1 | 53.7 ± 6.3 | 53.7 ± 5.4 | 53.3 ± 5.2 | 0.80 (0.43) |
| Alcohol (composite Z) | 0.17 ± 0.42 | 0.22 ± 0.46 | 0.14 ± 0.42 | −0.05 ± 0.53 | 0.80 (0.43) |
| Tobacco (composite Z) | −0.19 ± 0.56 | 0.79 ± 1.02 | 0.69 ± 1.07 | −0.12 ± 0.80 | 7.94 (<0.001) |
| Externalizing problems | 49.4 ± 7.8 | 52.7 ± 10.2 | 53.1 ± 8.5 | 48.1 ± 8.8 | 2.46 (0.02) |
| Antisocial behaviors | 53.0 ± 4.5 | 55.1 ± 7.4 | 54.7 ± 5.6 | 52.8 ± 4.5 | 2.22 (0.03) |
| Rule-breaking behavior | 53.0 ± 4.2 | 57.9 ± 7.7 | 57.3 ± 6.0 | 53.4 ± 4.7 | 5.28 (<.001) |
| Childhood conduct problems | 0.6 ± 0.7 | 1.0 ± 1.0 | 0.7 ± 0.9 | 0.5 ± 0.7 | 2.94 (0.004) |
Note: CTL means control (n = 89); CD, cannabis dependent (n = 89); REC, recreational cannabis users (n = 87); all other = all remaining participants in the HCP (n = 941). CTL and CD are the only 2 groups that are matched, so the 2-sample t-test is reported only for the comparison between these 2 groups. Note that the CTL group is well matched on all variables except for tobacco usage; therefore, tobacco usage was used as a confound regressor in the primary analyses. The externalizing problems, antisocial behaviors, and rule-breaking behaviors are reported as age- and gender-adjusted percentile scores; the childhood conduct problems are scored as part of the SSAGA.
Behavioral Analysis Linking Cognition and Emotion
We first conducted PCA across all 1206 HCP participants to get aggregate measures of cognition, emotion, and reward-based decision making. The first 2 components accounted for 45.3% of the variance and loaded rather distinctly on cognitive task performance and self-reported emotionality, respectively (Fig. 1b). Figure 1c shows the top component loadings; for a complete list of loadings see Supplementary Table 2.
Figure 1.
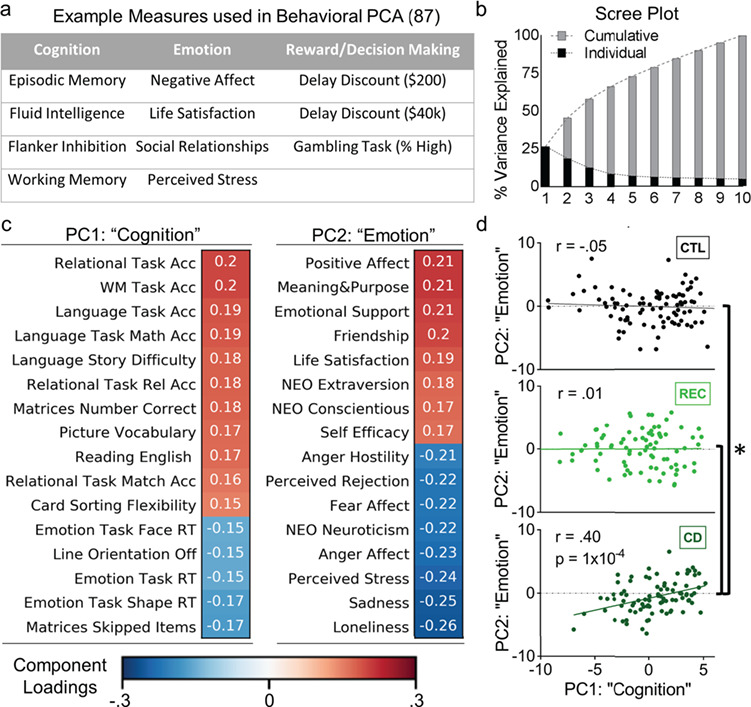
Behavioral results. (a) Example measures from a principle component analysis (PCA; n = 1206) that reduced data dimensionality across 87 measures of cognition, emotion, and reward-based decision making. (b) Scree plot showing that the top 2 components accounted for over 45% of the variance in all measures. (c) Loadings for the top 2 components. Because these measures loaded very heavily on measures of cognitive function and emotionality, respectively, we call these the “cognition” and “emotion” components. (d) Scatter plots of the PC scores for the 2 components. While a matched control group (CTL) and a group of recreational cannabis users (REC) did not show a significant correlation between the cognition and emotion PCs, the cannabis-dependent group (CD) showed a positive correlation such that strong cognitive performance was associated with higher positive emotionality. This result was highly similar when including 2 outliers: (r(89) = 0.45, P < 0.00001). The difference in slopes between the CD and CTL, as well as the CD and REC groups, was significant (*, z’s > 3.30, P’s < 0.001).
We used 2-sample t-tests on the component scores of each principal component (PC) to examine group effects and found no significant differences between CD and control groups: PC1 [“cognition”; t(178) = 0.30, P = 0.76] and PC2 [“emotion”; t(178) = −1.49, P = 0.14]. Then, to assess if PC1 and PC2 scores were differentially associated in the CD versus control groups, we conducted a regression of PC1 on PC2 scores, with a categorical moderator (CD vs. controls), and tested the interaction effect, which was significant [F(1) = 14.18; P = 2.3 × 10−4]. To further characterize these associations, we ran a correlation between the scores of PC1 and PC2 within each group, removing outliers > 3 SD from the mean (2 CD outliers; 2 REC outliers; 0 control outliers). Results showed that while these PCs representing cognition and emotion were not significantly correlated in control [r(89) = −0.05, P = 0.56) or REC (r(87) = −0.03, P = 0.53] groups, they were significantly associated in the CD group [r(87) = 0.40, P < 0.001], such that higher cognitive performance was correlated with higher positive emotionality and poorer performance with lower positive emotionality (Fig. 1d). The difference in slopes between CD and control (z = 3.51, P < 0.001) or between CD and REC groups (z = 3.36, P < 0.001) were significant.
Brain Imaging Analysis Linking Cognition and Emotion
We first tested main effects of group on brain activations via 2-sample t-tests of the cognition (WM: 2-back > 0-back) and emotion (angry/fearful faces > shapes) contrasts; results showed no significant differences between groups (Supplementary Fig. 1). Although the interaction effect between the first cognition fMRI component and the first emotion fMRI component did not reach significance [F(1) = 2.42; P = 0.12], follow-up analysis revealed that, similar to the behavioral results, the component scores of the first cognition fMRI component and the first emotion fMRI component were significantly correlated in the CD group [r(88) = 0.29, P = 0.007], but not in the REC group [r(67) = 0.058, P = 0.64], or in the matched control group [r(87) = 0.059, P = 0.59]. The regions with the strongest component loadings contributing to this result included the following: for the WM task, the dorsolateral/dorsomedial prefrontal, visual, anterior insular, and lateral parietal cortices, caudate, thalamus, and cerebellum; and for the emotion task similar regions were observed, with a lesser contribution from the lateral parietal regions (Fig. 2). The difference in slopes between CD and controls showed a trend for significance (z = 1.50, one-tailed P = 0.08).
Figure 2.
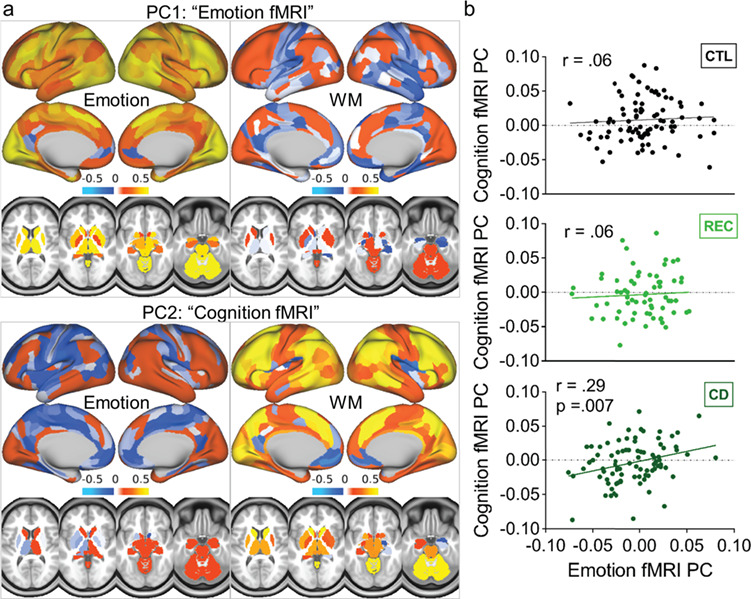
fMRI results. (a) Component loadings from a principle component analysis (PCA; n = 1005) that was used to reduce data dimensionality across parcels of brain activation for the emotion task (angry/fearful faces > shapes) and the WM task (2-back > 0-back). One component loaded mostly on activations for the emotion task (“emotion fMRI PC”) and one loaded mostly on activations for the WM task (“cognition fMRI PC”). (b) Scatter plots of the subject-weight eigenvectors for the 2 components. While a matched control group (CTL) and a group of recreational cannabis users (REC) did not show a significant correlation between the cognition and emotion fMRI PCs, the cannabis-dependent group (CD) showed a positive correlation such that activations to emotional stimuli were associated with activations to cognitive demand. This association was virtually identical when including one outlier: (r(89) = 0.29, P = 0.006).
Brain–Behavior Associations
To link results from behavioral and fMRI analysis and identify modes of population covariation in cognitive and emotional function, we performed CCA. In the CD group, one significant mode emerged [r(89) = 0.52, PFWE < 0.001] that was associated with both cognitive performance and self-reported emotionality; this mode loaded heavily onto the cognitive task activations but also substantially on the emotion task activations (Fig. 3). In particular, loadings on both imaging tasks were strong in lateral and medial frontoparietal regions, anterior insula, and caudate. To examine if this finding was similar among controls, we applied the CCA weights from the CD group to the control group. Using this approach, the first control mode was of comparable strength to the CD mode [r(89) = 0.52] but it was primarily associated with cognitive performance and cognitive task activations (Fig. 4). A highly similar pattern emerged when conducting a separate CCA analysis on the control group (i.e., conducting CCA only on control data without applying the CD weights first; Supplementary Fig. 2). For a complete list of behavioral loadings from these analyses, see Supplementary Table 3. Because the CD group showed significantly higher ratings of childhood conduct problems, externalizing symptoms, and thought problems than the CTL group, we conducted additional analyses using these ratings as additional covariates; these results were virtually identical (for the correlation between individual subject scores with and without these covariates, all r’s > 0.97), and we therefore only report the former analysis. Finally, we also conducted CCA analysis on the REC group. In line with the behavioral and brain imaging analyses, these results looked fairly similar to the control group results (i.e., one significant CCA mode emerged with top loadings primarily on the cognitive performance and cognitive task activations). However, this should be interpreted with caution, because CCA is highly sensitive to sample size and prone to overfitting (Egloff et al. 2005), and the REC group had 25% fewer individuals with imaging data. Indeed, reliability testing indicated that these results were less reliable than the primary CCA analysis, and therefore these results are reported in Supplementary Figure 4.
Figure 3.
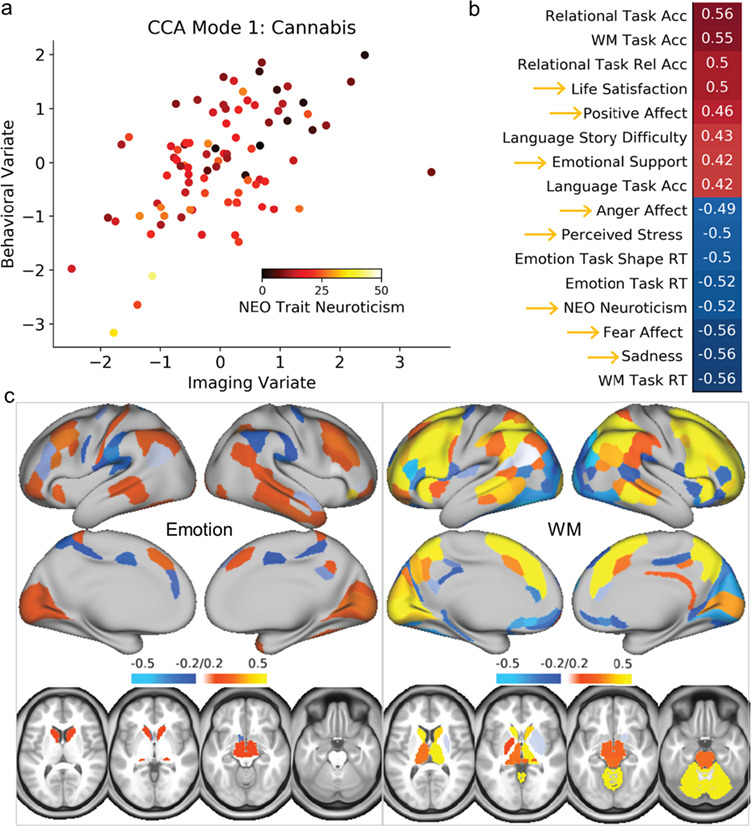
CCA results for the cannabis-dependent group. (a) Scatter plot of CCA mode 1, showing the correlation of the individual subject weights between the imaging and behavioral variates, color-coded by trait neuroticism, as an example measure. (b) Behavioral loadings for the CCA mode. Gold arrows indicate the measures of emotion. (c) Imaging loadings for the CCA mode. Note the mixture of cognitive task performance and self-reported emotionality among the top weights, as well as the overlapping weights for brain activation in both the emotion and WM cognitive fMRI task.
Figure 4.
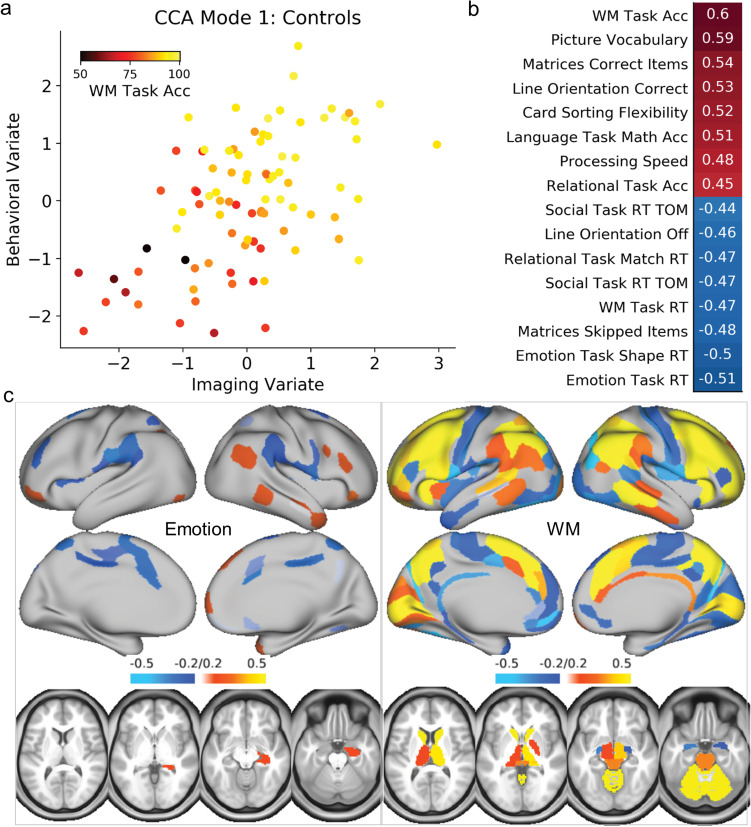
CCA results for the healthy control group. (a) Scatter plot of CCA mode 1, showing the correlation of the individual subject weights between the imaging and behavioral variates, color-coded by WM task accuracy, as an example measure. (b) Behavioral loadings for the CCA mode. (c) Imaging loadings for the CCA mode. Note the top measures are all related to cognitive task performance, and the top weights for brain activation are more restricted to the WM cognitive task activations than the cannabis group CCA analysis.
Reliability Testing
Split-half train-test analysis indicated that the CCA only mildly overfit the data (median training r = 0.56, median test r = 0.40, Supplementary Figure 3); these results are comparable to recent studies using CCA that performed extensive reliability testing (Moser et al. 2017, 2018). In addition, when testing the CCA with different numbers of brain and behavior component inputs (4, 6, 8, 10, and 12) the correlations for the behavioral variate subject weights (0.99, 0.97, 0.90, 0.87, and 0.81) and imaging variate subject weights (0.99, 0.94, 0.88, 0.84, and 0.75) with the original analysis were very high, indicating that different numbers of CCA inputs would yield very similar results.
Discussion
Here we investigated the relationship between cognition and emotion in a relatively large sample of young adults with CD. We found behavioral and neural evidence that these processes are less segregated in CD than in healthy young adults. These results may be more related to neuropsychological features of addiction rather than a general feature of individuals who are predisposed to use cannabis, for the heightened cognition–emotion link was not present among a sample of frequent cannabis users (>100 self-reported lifetime uses) without symptoms of dependence. These data have implications for the therapeutic management of cognitive and emotional dysfunction in CD.
We found that aggregate measures of strong cognitive performance and trait positive emotionality were more correlated among CD than controls, suggesting that these domains may be linked in CD. Though a large literature describes the strong interplay between emotion and cognition in healthy adults in an acute, trial-to-trial fashion (Verbruggen and De Houwer 2007; Inzlicht et al. 2015), little is known about how long-term measures of emotion and cognition are interconnected. Chepenik et al. (2007) induced a prolonged sad mood state by asking healthy young adults to vividly imagine the death of a loved one and found that, in line with the current results, standard measures of nonemotional cognition (e.g., response inhibition and WM) were not affected by the mood manipulation in healthy young adults. In CD, the link between emotion and cognition may be partially driven by anxiety. Anxiety is associated with negative emotionality (Kotov et al. 2010) and cognitive dysfunction (Eysenck et al. 2007; Bishop 2009; Basten et al. 2011), and individuals who are anxious develop CD faster than others, perhaps because they use cannabis to self-medicate (Buckner et al. 2012). Thus, the subgroup of individuals with CD who are high on trait negative emotionality may also have higher anxiety and impaired cognitive performance. In contrast, the subgroup with more positive emotionality may be less anxious and less vulnerable to the detrimental effects of anxiety on cognitive performance (for an exploratory analysis demonstrating partial support for this theory, see Supplementary Material). In line with these findings, we must consider that the reduced segregation between cognition and emotion might be predominantly driven by negative emotions, rather than general emotionality per se. This is supported by the brain imaging results to the emotion fMRI task, which specifically probed brain responses to faces expressing anger or fear. This interpretation is also consistent with the “dark side” theory of addiction, in which loss of cognitive control is specifically triggered by negative emotionality (Koob 2015).
Poor sleep quality may also contribute to the cognition–emotion association in CD. Daily cannabis users report poorer sleep quality than nondaily users and nonusers (Conroy et al. 2016), and chronic sleep problems are associated with a state of “hyperarousal” that is linked to emotional distress and cognitive dysfunction (Wassing et al. 2016). In regular cannabis users, both high doses of acute THC (Feinberg et al. 1975) and withdrawal from cannabis (Vandrey et al. 2011) are associated with poor sleep efficiency (percentage of time asleep while in bed) and shorter rapid eye movement sleep duration as assessed by polysomnography, even in carefully selected cohorts that have minimal comorbid alcohol and drug use (Bolla et al. 2008). These sleep disturbances mirror those observed in individuals with chronic anxiety (for a review, see Cox and Olatunji 2016). Critically, there are substantial individual differences in vulnerability to the negative effects of poor sleep (Van Dongen et al. 2011), in line with the wide variability in cognitive and emotional outcomes observed here.
We also observed that brain responses to negative emotional stimuli and cognitive demand were linked among the CD group. Substantial work has been devoted to understanding how different brain networks support the interplay of cognition and emotion (Pessoa 2018). One theory posits that there are “bottleneck” regions, such as medial prefrontal cortex and anterior insula, which are recruited by both emotional manipulations and cognitive load (Tombu et al. 2011; Pessoa 2015). If simultaneous emotional and cognitive load exceeds what these regions can process at a given time, then performance suffers. This theory was developed based on data using primarily acute manipulations, but it stands to reason that longer-term negative emotional states may also influence cognition, since for example trait anxiety is associated with reduced efficiency in these regions during cognitive task performance (Bishop 2009; Basten et al. 2011; Forster et al. 2015). It is possible that in CD the function of these regions is altered in a way that tightens the bottleneck. Indeed, resting-state functional connectivity of the insula and medial prefrontal cortex are altered in CD (Pujol et al. 2014), which is associated with blunted arousal to affective images (Blanco-Hinojo et al. 2017). Thus, the subset of CD individuals who have high negative emotionality may already be taxing a system with limited resources, leading to poor cognitive performance.
Finally, CCA identified one significant brain–behavior association in CD that ties the prior findings together: cognitive performance and emotionality were associated with brain activations to both cognitive demand and emotional stimuli. In contrast, in controls the strongest associations were mostly restricted to cognitive performance and cognitive fMRI task activations. These data expand on prior studies using CCA in the HCP dataset (see Supplementary Material for more discussion). Notably, the regions that shared strong loadings on both the emotion and the cognitive task were ones hypothesized to be bottleneck regions as previously discussed: anterior insula, dorsomedial, and lateral frontoparietal cortices (Tombu et al. 2011; Pessoa 2015). It remains unclear if segregation of resting-state brain networks, a characteristic that is thought to be important for healthy function (Chamberlain et al. 2009; Crossley et al. 2014), is an important marker underlying segregation of cognitive and emotional behaviors. Evidence is emerging of dedifferentiation between functional networks with aging and neuropathology (Grady et al. 2016; Chan et al. 2017) and that segregation of functional networks is associated with states of consciousness and cognitive performance (Naci et al. 2018; Wang et al. 2018). To the extent that CD is associated with loss of segregation between emotional and cognitive processing, future research is needed to assess if it accelerates the effects of aging on brain network typology.
These data highlight substantial individual differences in CD and provide new avenues for future research and treatment. Although attempts to improve cognition through “brain training” have been largely unsuccessful in healthy young adults (Kable et al. 2017), it is well established that psychotherapy can be very effective in reducing negative emotions (Leichsenring et al. 2004). If cognitive deficits in drug dependence stem largely from poor emotion regulation techniques (Verdejo-Garcia et al. 2012), then it is plausible that psychotherapy may be particularly effective in counteracting negative emotionality and improving cognitive function in CD, especially in the subset of individuals who are struggling in both domains. Extensive training may be necessary, for cannabis users have impaired emotion regulation (Zimmermann et al. 2017). Still, these strategies may be well worth the effort, since poor emotion regulation is strongly associated with withdrawal symptom severity (Buckner et al. 2017) and relapse (Bonn-Miller and Moos 2009). Emotion regulation training may also be useful for the prevention of cannabis use: higher use of emotion regulation strategies during the day was associated with lower likelihood of cannabis use later in the evening in a large sample of college students (Weiss et al. 2017). Future longitudinal studies should assess whether the benefits of emotion regulation training also extend to cognitive outcomes in CD.
The current study has several limitations. First, the HCP database has limited information describing detailed patterns of cannabis use. Thus, we cannot speak to how the current findings relate to more refined patterns of daily use, including last use of cannabis prior to imaging. Second, these data are cross-sectional and limited to one young adult population aged 22–35; as such, this work is inherently correlational and should therefore be considered exploratory. Future studies (using, e.g., confirmatory factor analysis) in more diverse samples are needed to do rigorous prospective testing of the hypotheses laid out here and to examine the trajectory of this effect over time. Third, we were unable to identify components that distinctly loaded on behavioral measures of motivation/reward-related behaviors, and therefore it will be critical in future experiments to have a rich characterization of this functional domain and how it relates to cognition and emotion in CD. A broader battery of phenotypic assessments will also lend insight into whether these findings truly represent a direct emotion–cognition association, or if instead there is a more generalized phenomenon in CD that underlies these findings.
In summary, convergent evidence from behavioral, neuroimaging, and brain–behavior association analyses consistently demonstrated that cognitive and emotional processes are linked in CD, in a way that was not present in healthy controls or recreational nonaddicted cannabis users. These findings suggest that, for a subgroup of individuals with CD, poor cognitive control may stem from an inability to segregate cognitive function from negative emotional states. Thus, in this subgroup, training strategies on emotion regulation may improve cognitive control and in executive function may improve emotion regulation to enhance patient outcomes in CD.
Funding
National Institute on Alcohol Abuse and Alcoholism (Y1AA-3009).
Notes
The authors would like to thank Carlos Blanco, Dardo Tomasi, Vijay Ramchandani, Corinde Wiers, Jonathan O’Rawe, and John Fedota for their feedback and helpful suggestions. Data were provided by the Human Connectome Project, WU-Minn Consortium (Principal Investigators: David Van Essen and Kamil Ugurbil; 1U54MH091657) funded by the 16 National Institutes of Health (NIH) institutes and centers that support the NIH Blueprint for Neuroscience Research, and by the McDonnell Center for Systems Neuroscience at Washington University. Conflict of Interest: None declared.
Supplementary Material
References
- Anderson AW, Heptulla RA, Driesen N, Flanagan D, Goldberg PA, Jones TW, Rife F, Sarofin H, Tamborlane W, Sherwin R et al. 2006. Effects of hypoglycemia on human brain activation measured with fMRI. Magn Reson Imaging. 24:693–697. [DOI] [PubMed] [Google Scholar]
- Anderson JC, Gerbing DW. 1984. The effect of sampling error on convergence, improper solutions, and goodness-of-fit indices for maximum likelihood confirmatory factor analysis. Psychometrika. 49:155–173. [Google Scholar]
- Barch DM, Burgess GC, Harms MP, Petersen SE, Schlaggar BL, Corbetta M, Glasser MF, Curtiss S, Dixit S, Feldt C et al. 2013. Function in the human connectome: task-fMRI and individual differences in behavior. Neuroimage. 80:169–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basten U, Stelzel C, Fiebach CJ. 2011. Trait anxiety modulates the neural efficiency of inhibitory control. J Cogn Neurosci. 23:3132–3145. [DOI] [PubMed] [Google Scholar]
- Bishop SJ. 2009. Trait anxiety and impoverished prefrontal control of attention. Nat Neurosci. 12:92–98. [DOI] [PubMed] [Google Scholar]
- Blanco-Hinojo L, Pujol J, Harrison BJ, Macià D, Batalla A, Nogué S, Torrens M, Farré M, Deus J, Martín-Santos R. 2017. Attenuated frontal and sensory inputs to the basal ganglia in cannabis users. Addict Biol. 22:1036–1047. [DOI] [PubMed] [Google Scholar]
- Blanco C, Hasin DS, Wall MM, Flórez-Salamanca L, Hoertel N, Wang S, Kerridge BT, Olfson M. 2016. Cannabis use and risk of psychiatric disorders prospective evidence from a US national longitudinal study. JAMA Psychiatry. 73:388–395. [DOI] [PubMed] [Google Scholar]
- Boehler CN, Schevernels H, Hopf JM, Stoppel CM, Krebs RM. 2014. Reward prospect rapidly speeds up response inhibition via reactive control. Cogn Affect Behav Neurosci. 14:593–609. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Lesage SR, Gamaldo CE, Neubauer DN, Funderburk FR, Cadet JL, David PM, Verdejo-Garcia A, Benbrook AR. 2008. Sleep disturbance in heavy marijuana users. Sleep. 31:901–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonn-Miller MO, Moos RH. 2009. Marijuana discontinuation, anxiety symptoms, and relapse to marijuana. Addict Behav. 34:782–785. [DOI] [PubMed] [Google Scholar]
- Brown TA. 2015. Confirmatory factor analysis for applied research. 2nd ed. New York, NY: Guilford Publications. [Google Scholar]
- Buckner JD, Heimberg RG, Schneier FR, Liu SM, Wang S, Blanco C. 2012. The relationship between cannabis use disorders and social anxiety disorder in the National Epidemiological Study of Alcohol and Related Conditions (NESARC). Drug Alcohol Depend. 124:128–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner JD, Walukevich KA, Zvolensky MJ, Gallagher MW. 2017. Emotion regulation and coping motives serially affect cannabis cessation problems among dually diagnosed outpatients. Psychol Addict Behav. 31:839–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaarani B, Spechler PA, Hudson KE, Foxe JJ, Potter AS, Garavan H. 2017. The neural basis of response inhibition and substance abuse In: The Wiley handbook of cognitive control, pp. 581–601. Hoboken, NJ: Wiley-Blackwell. [Google Scholar]
- Chamberlain SR, Hampshire A, Müller U, Rubia K, del Campo N, Craig K, Regenthal R, Suckling J, Roiser JP, Grant JE et al. 2009. Atomoxetine modulates right inferior frontal activation during inhibitory control: a pharmacological functional magnetic resonance imaging study. Biol Psychiatry. 65:550–555. [DOI] [PubMed] [Google Scholar]
- Chan MY, Alhazmi FH, Park DC, Savalia NK, Wig GS. 2017. Resting-state network topology differentiates task signals across the adult life span. J Neurosci. 37:2734–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chepenik LG, Cornew LA, Farah MJ. 2007. The influence of sad mood on cognition. Emotion. 7:802–811. [DOI] [PubMed] [Google Scholar]
- Chiew KS, Braver TS. 2016. Reward favors the prepared: incentive and task-informative cues interact to enhance attentional control. J Exp Psychol Hum Percept Perform. 42:52–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MX, Young J, Baek JM, Kessler C, Ranganath C. 2005. Individual differences in extraversion and dopamine genetics predict neural reward responses. Brain Res Cogn Brain Res. 25:851–861. [DOI] [PubMed] [Google Scholar]
- Conroy DA, Kurth ME, Strong DR, Brower KJ, Stein MD. 2016. Marijuana use patterns and sleep among community-based young adults. J Addict Dis. 35:135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa PT, McCrae RR. 1980. Influence of extraversion and neuroticism on subjective well-being: happy and unhappy people. J Pers Soc Psychol. 38:668–678. [DOI] [PubMed] [Google Scholar]
- Cox RC, Olatunji BO. 2016. A systematic review of sleep disturbance in anxiety and related disorders. J Anxiety Disord. 37:104–129. [DOI] [PubMed] [Google Scholar]
- Crossley NA, Mechelli A, Scott J, Carletti F, Fox PT, Mcguire P, Bullmore ET. 2014. The hubs of the human connectome are generally implicated in the anatomy of brain disorders. Brain. 137:2382–2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolcos F, McCarthy G. 2006. Brain systems mediating cognitive interference by emotional distraction. J Neurosci. 26:2072–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drobyshevsky A, Baumann SB, Schneider W. 2006. A rapid fMRI task battery for mapping of visual, motor, cognitive, and emotional function. Neuroimage. 31:732–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egloff B, Schwerdtfeger A, Schmukle SC, Mainz JG, Sherry A, Henson RK. 2005. Conducting and interpreting canonical correlation analysis in personality research: a user-friendly primer. J Pers Assess. 84:37–48. [DOI] [PubMed] [Google Scholar]
- Eysenck MW, Derakshan N, Santos R, Calvo MG. 2007. Anxiety and cognitive performance: attentional control theory. Emotion. 7:336–353. [DOI] [PubMed] [Google Scholar]
- Feinberg I, Jones R, Walker JM, Cavness C, March J. 1975. Effects of high dosage delta-9-tetrahydrocannabinol on sleep patterns in man. Clin Pharmacol Ther. 17:458–466. [DOI] [PubMed] [Google Scholar]
- Fillo J, Alfano CA, Paulus DJ, Smits JAJ, Davis ML, Rosenfield D, Marcus BH, Church TS, Powers MB, Otto MW et al. 2016. Emotion dysregulation explains relations between sleep disturbance and smoking quit-related cognition and behavior. Addict Behav. 57:6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster S, Nunez Elizalde AO, Castle E, Bishop SJ. 2015. Unraveling the anxious mind: anxiety, worry, and frontal engagement in sustained attention versus off-task processing. Cereb Cortex. 25:609–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser MF, Coalson TS, Robinson EC, Hacker CD, Harwell J, Yacoub E, Ugurbil K, Andersson J, Beckmann CF, Jenkinson M et al. 2016. A multi-modal parcellation of human cerebral cortex. Nature. 536:171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser MF, Sotiropoulos SN, Wilson JA, Coalson TS, Fischl B, Andersson JL, Xu J, Jbabdi S, Webster M, Polimeni JR et al. 2013. The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage. 80:105–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales MM, Tarumi T, Miles SC, Tanaka H, Shah F, Haley AP. 2010. Insulin sensitivity as a mediator of the relationship between BMI and working memory-related brain activation. Obesity. 18:2131–2137. [DOI] [PubMed] [Google Scholar]
- Gordon EM, Laumann TO, Adeyemo B, Huckins JF, Kelley WM, Petersen SE. 2016. Generation and evaluation of a cortical area parcellation from resting-state correlations. Cereb Cortex. 26:288–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, Sarraf S, Saverino C, Campbell K. 2016. Age differences in the functional interactions among the default, frontoparietal control and dorsal attention networks. Neurobiol Aging. 41:159–172. [DOI] [PubMed] [Google Scholar]
- Gray JR, Burgess GC, Schaefer A, Yarkoni T, Larsen RJ, Braver TS. 2005. Affective personality differences in neural processing efficiency confirmed using fMRI. Cogn Affect Behav Neurosci. 5:182–190. [DOI] [PubMed] [Google Scholar]
- Haas BW, Omura K, Amin Z, Constable RT, Canli T. 2006. Functional connectivity with the anterior cingulate is associated with extraversion during the emotional Stroop task.Soc Neurosci. 1:16–24. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Tessitore A, Mattay VS, Fera F, Weinberger DR. 2002. The amygdala response to emotional stimuli: a comparison of faces and scenes. Neuroimage. 17:317–323. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Sarvet AL, Cerdá M, Keyes KM, Stohl M, Galea S, Wall MM. 2017. US adult illicit cannabis use, cannabis use disorder, and medical marijuana laws: 1991–1992 to 2012–2013. JAMA Psychiatry. 74:579–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Akshoomoff N, Tulsky D, Mungas D, Weintraub S, Dikmen S, Beaumont J, Casaletto KB, Conway K, Slotkin J et al. 2014. Reliability and validity of composite scores from the NIH Toolbox Cognition Battery in adults. J Int Neuropsychol Soc. 20:588–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijbregts SCJ, Griffith-Lendering MFH, Vollebergh WAM, Swaab H. 2014. Neurocognitive moderation of associations between cannabis use and psychoneuroticism. J Clin Exp Neuropsychol. 36:794–805. [DOI] [PubMed] [Google Scholar]
- Inzlicht M, Bartholow BD, Hirsh JB. 2015. Emotional foundations of cognitive control. Trends Cogn Sci. 19:126–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable JW, Caulfield MK, Falcone M, McConnell M, Bernardo L, Parthasarathi T, Cooper N, Ashare R, Audrain-McGovern J, Hornik R et al. 2017. No effect of commercial cognitive training on brain activity, choice behavior, or cognitive performance. J Neurosci. 37:7390–7402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman L, Rousseeuw PJ. 1990. Finding groups in data: an introduction to cluster analysis. New York: Wiley. [Google Scholar]
- Kensinger EA, Corkin S. 2003. Effect of negative emotional content on working memory and long-term memory. Emotion. 3:378–393. [DOI] [PubMed] [Google Scholar]
- Koffarnus MN, Jarmolowicz DP, Mueller ET, Bickel WK. 2013. Changing delay discounting in the light of the competing neurobehavioral decision systems theory: a review. J Exp Anal Behav. 99:32–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. 2015. The dark side of emotion: the addiction perspective. Eur J Pharmacol. 753:73–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. 2010. Neurocircuitry of addiction. Neuropsychopharmacology. 35:217–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotov R, Gamez W, Schmidt F, Watson D. 2010. Linking “big” personality traits to anxiety, depressive, and substance use disorders: a meta-analysis. Psychological Bull. 136:768–821. [DOI] [PubMed] [Google Scholar]
- Krebs RM, Boehler CN, Egner T, Woldorff MG. 2011. The neural underpinnings of how reward associations can both guide and misguide attention. J Neurosci. 31:9752–9759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari V, Ffytche DH, Williams SCR, Gray JA. 2004. Personality predicts brain responses to cognitive demands. J Neurosci. 24:10636–10641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leichsenring F, Rabung S, Leibing E. 2004. The efficacy of short-term psychodynamic psychotherapy in specific psychiatric disorders. Arch Gen Psychiatry. 61:1208–1216. [DOI] [PubMed] [Google Scholar]
- Manza P, Tomasi D, Volkow ND. 2018. Subcortical local functional hyperconnectivity in cannabis dependence. Biol Psychiatry Cogn Neurosci Neuroimaging. 3:285–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martz ME, Trucco EM, Cope LM, Hardee JE, Jester JM, Zucker RA, Heitzeg MM. 2016. Association of marijuana use with blunted nucleus accumbens response to reward anticipation. JAMA Psychiatry. 370:2219–2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrae RR, Costa PT. 2004. A contemplated revision of the NEO Five-Factor Inventory. Pers Indiv Dif. 36:587–596. [Google Scholar]
- Moser DA, Doucet GE, Ing A, Dima D, Schumann G, Bilder RM, Frangou S. 2017. An integrated brain–behavior model for working memory. Mol Psychiatry. 23:1974–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser DA, Doucet GE, Lee WH, Rasgon A, Krinsky H, Leibu E, Ing A, Schumann G, Rasgon N, Frangou S. 2018. Multivariate associations among behavioral, clinical, and multimodal imaging phenotypes in patients with psychosis. JAMA Psychiatry. 75:386–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Bandettini PA. 2013. Resting-state fMRI confounds and cleanup. Neuroimage. 80:349–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naci L, Haugg A, MacDonald A, Anello M, Houldin E, Naqshbandi S, Gonzalez-Lara LE, Arango M, Harle C, Cusack R et al. 2018. Functional diversity of brain networks supports consciousness and verbal intelligence. Sci Rep. 8:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader DA, Sanchez ZM. 2018. Effects of regular cannabis use on neurocognition, brain structure, and function: a systematic review of findings in adults. Am J Drug Alcohol Abuse. 44:4–18. [DOI] [PubMed] [Google Scholar]
- Navas JF, Verdejo-García A, López-Gómez M, Maldonado A, Perales JC. 2016. Gambling with rose-tinted glasses on: use of emotion-regulation strategies correlates with dysfunctional cognitions in gambling disorder patients. J Behav Addict. 5:271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr JM, Paschall CJ, Banich MT. 2016. Recreational marijuana use impacts white matter integrity and subcortical (but not cortical) morphometry. Neuroimage Clin. 12:47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L. 2009. How do emotion and motivation direct executive control. Trends Cogn Sci. 13:160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L. 2015. Précis on the cognitive-emotional brain. Behav Brain Sci. 38:e71. [DOI] [PubMed] [Google Scholar]
- Pessoa L. 2018. Understanding emotion with brain networks. Curr Opin Behav Sci. 19:19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan KL, Angstadt M, Golden J, Onyewuenyi I, Popovska A, de Wit H. 2008. Cannabinoid modulation of amygdala reactivity to social signals of threat in humans. J Neurosci. 28:2313–2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA. 2006. Emotion and cognition: insights from studies of the human amygdala. Annu Rev Psychol. 57:27–53. [DOI] [PubMed] [Google Scholar]
- Pujol J, Blanco-Hinojo L, Batalla A, López-Solà M, Harrison BJ, Soriano-Mas C, Crippa JA, Fagundo AB, Deus J, De la Torre R et al. 2014. Functional connectivity alterations in brain networks relevant to self-awareness in chronic cannabis users. J Psychiatr Res. 51:68–78. [DOI] [PubMed] [Google Scholar]
- Reynolds B, Ortengren A, Richards JB, de Wit H. 2006. Dimensions of impulsive behavior: personality and behavioral measures. Pers Individ Dif. 40:305–315. [Google Scholar]
- Robinson EC, Jbabdi S, Glasser MF, Andersson J, Burgess GC, Harms MP, Smith SM, Van Essen DC, Jenkinson M. 2014. MSM: a new flexible framework for multimodal surface matching. Neuroimage. 100:414–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigo AH, Di Domenico SI, Graves B, Lam J, Ayaz H, Bagby RM, Ruocco AC. 2015. Linking trait-based phenotypes to prefrontal cortex activation during inhibitory control. Soc Cogn Affect Neurosci. 11:55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salsman JM, Butt Z, Pilkonis PA, Cyranowski JM, Zill N, Hendrie HC, Kupst MJ, Kelly MAR, Bode RK, Choi SW et al. 2013. Emotion assessment using the NIH Toolbox. Neurology. 80:S76–S86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schel MA, Scheres A, Crone EA. 2014. New perspectives on self-control development: highlighting the role of intentional inhibition. Neuropsychologia. 65:236–246. [DOI] [PubMed] [Google Scholar]
- Smith SM, Nichols TE, Vidaurre D, Winkler AM, J Behrens TE, Glasser MF, Ugurbil K, Barch DM, Van Essen DC, Miller KL. 2015. A positive-negative mode of population covariation links brain connectivity, demographics and behavior. Nat Neurosci. 18:1565–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JL, May AC, Poppa T, Davenport PW, Tapert SF, Paulus MP. 2014. You are the danger: attenuated insula response in methamphetamine users during aversive interoceptive decision-making. Drug Alcohol Depend. 142:110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombu MN, Asplund CL, Dux PE, Godwin D, Martin JW, Marois R. 2011. A unified attentional bottleneck in the human brain. Proc Natl Acad Sci U S A. 108:13426–13431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dongen HPA, Caldwell JA, Caldwell JL. 2011. Individual differences in cognitive vulnerability to fatigue in the laboratory and in the workplace. Prog Brain Res. 190:145–153. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Smith SM, Barch DM, Behrens TEJ, Yacoub E, Ugurbil K. 2013. The WU-Minn Human Connectome Project: an overview. Neuroimage. 80:62–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC, Ugurbil K, Auerbach E, Barch DM, Behrens TEJ, Bucholz R, Chang A, Chen L, Corbetta M, Curtiss SW et al. 2012. The Human Connectome Project: a data acquisition perspective. Neuroimage. 62:2222–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hell HH, Vink M, Ossewaarde L, Jager G, Kahn RS, Ramsey NF. 2010. Chronic effects of cannabis use on the human reward system: an fMRI study. Eur Neuropsychopharmacol. 20:153–163. [DOI] [PubMed] [Google Scholar]
- Vandrey R, Smith MT, McCann UD, Budney AJ, Curran EM. 2011. Sleep disturbance and the effects of extended-release zolpidem during cannabis withdrawal. Drug Alcohol Depend. 117:38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen F, De Houwer J. 2007. Do emotional stimuli interfere with response inhibition? Evidence from the stop signal paradigm. Cogn Emot. 21:391–403. [Google Scholar]
- Verdejo-Garcia A, Clark L, Dunn BD. 2012. The role of interoception in addiction: a critical review. Neurosci Biobehav Rev. 36:1857–1869. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Swanson JM, Evins AE, DeLisi LE, Meier MH, Gonzalez R, Bloomfield MAP, Curran HV, Baler R. 2016. Effects of cannabis use on human behavior, including cognition, motivation, and psychosis: a review. JAMA Psychiatry. 73:292–297. [DOI] [PubMed] [Google Scholar]
- Wang J, Khosrowabadi R, Ng KK, Hong Z, Chong JSX, Wang Y, Chen C-Y, Hilal S, Venketasubramanian N, Wong TY et al. 2018. Alterations in brain network topology and structural-functional connectome coupling relate to cognitive impairment. Front Aging Neurosci. 10:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassing R, Benjamins JS, Dekker K, Moens S, Spiegelhalder K, Feige B, Riemann D, van der Sluis S, Van Der Werf YD, Talamini LM et al. 2016. Slow dissolving of emotional distress contributes to hyperarousal. Proc Natl Acad Sci U S A. 113:2538–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiland BJ, Thayer RE, Depue BE, Sabbineni A, Bryan AD, Hutchison KE. 2015. Daily marijuana use is not associated with brain morphometric measures in adolescents or adults. J Neurosci. 35:1505–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss NH, Bold KW, Sullivan TP, Armeli S, Tennen H. 2017. Testing bidirectional associations among emotion regulation strategies and substance use: a daily diary study. Addiction. 112:695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesley MJ, Lile JA, Hanlon CA, Porrino LJ. 2016. Abnormal medial prefrontal cortex activity in heavy cannabis users during conscious emotional evaluation. Psychopharmacology (Berl). 233:1035–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE. 2014. Permutation inference for the general linear model. Neuroimage. 92:381–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler AM, Webster MA, Vidaurre D, Nichols TE, Smith SM. 2015. Multi-level block permutation. Neuroimage. 123:253–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yücel M, Harrison BJ, Wood SJ, Fornito A, Clarke K, Wellard RM, Cotton S, Pantelis C. 2007. State, trait and biochemical influences on human anterior cingulate function. Neuroimage. 34:1766–1773. [DOI] [PubMed] [Google Scholar]
- Zimmermann K, Walz C, Derckx RT, Kendrick KM, Weber B, Dore B, Ochsner KN, Hurlemann R, Becker B. 2017. Emotion regulation deficits in regular marijuana users. Hum Brain Mapp. 38:4270–4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


