Abstract
Aims
Alcohol use disorder is highly heterogeneous. One approach to understanding this heterogeneity is the identification of drinker subtypes. A candidate classification consists of reward and relief subtypes. The current study examines a novel self-report measure of reward, relief, and habit drinking for its clinical correlates and subjective response (SR) to alcohol administration.
Methods
Non-treatment-seeking heavy drinkers (n = 140) completed the brief reward, relief, habit drinking scale (RRHDS). A subset of this sample (n = 67) completed an intravenous alcohol administration. Individuals were classified into drinker subtypes. A crowdsourced sample of heavy drinkers (n = 187) completed the RRHDS and a validated reward relief drinking scale to compare drinking classification results.
Results
The majority of the sample was classified as reward drinkers (n = 100), with fewer classified as relief (n = 19) and habit (n = 21) drinkers. Relief and habit drinkers reported greater tonic alcohol craving compared to reward drinkers. Reward drinkers endorsed drinking for enhancement, while relief drinkers endorsed drinking for coping. Regarding the alcohol administration, the groups differed in negative mood, such that relief/habit drinkers reported a decrease in negative mood during alcohol administration, compared to reward drinkers. The follow-up crowdsourcing study found a 62% agreement in reward drinker classification between measures and replicated the tonic craving findings.
Conclusions
Our findings suggest that reward drinkers are dissociable from relief/habit drinkers using the brief measure. However, relief and habit drinkers were not successfully differentiated, which suggests that these constructs may overlap phenotypically. Notably, measures of dysphoric mood were better at detecting group differences than measures capturing alcohol’s rewarding effects.
This study classified non-treatment-seeking heavy drinkers into reward, relief and habit drinking subtypes. Clinically, reward and relief/habit drinkers differed on tonic alcohol craving and drinking motives. Regarding SR to alcohol, the relief/habit drinkers reported a decrease in negative mood during alcohol administration, compared to reward drinkers.
INTRODUCTION
Alcohol use disorder (AUD) is a heterogeneous disorder, and for many years, the field has attempted to identify subgroups within AUD. Early efforts used data primarily derived from treatment-seeking populations to cluster individuals with AUD into subgroups based on several criteria. Jellinek (1960) characterized five subtypes of problematic alcohol drinking based on etiological considerations, progression of alcohol use and resulting consequences of alcohol consumption. Cloninger et al. (1981) recommended the Type 1 versus Type 2 dichotomy based on age of onset of alcohol problems and personality traits. Babor et al. (1992) also proposed two types of drinkers, Type A and Type B, who can primarily be distinguished based on age onset of alcohol problems, number of childhood risk factors, sex, socioeconomic status, psychological dysfunction, polysubstance use, chronic treatment history, familial history of alcoholism and life stress. Lesch and Walter (1996) characterized four subtypes based on biological, psychological and sociological dysfunction. More recently, Moss et al. (2007) used national substance abuse survey data from the 2001 to 2002 National Epidemiological Survey on Alcohol and Related Conditions (NESARC-II) to distinguish between five clusters of alcohol dependency based on age of onset of alcohol dependency, familial alcoholism, antisocial personality traits, endorsement of DSM-IV alcohol abuse criteria and comorbid psychiatric and substance use disorders. Although these proposed typologies enhanced our understanding of the various facets of AUD, there is still a lack of consensus about which approach can best advance the field (Leggio et al., 2009).
Broadly speaking, the goal of classifying subgroups of patients with AUD is in the interest of providing more effective treatments that are tailored to common clinical features and putative pathophysiology. As such, attempts have been made to match behavioral treatments with specific alcohol drinking profiles. For example, using Babor’s classification method, patients classified as Type A were shown to have better outcomes after group psychotherapy but do worse with coping skills training. Conversely, patients classified as Type B had better outcomes with coping skills training yet worse ones with interactional group therapy (Litt et al., 1992). However, the lack of knowledge regarding other contributing factors to AUD may have led to the failure of large randomized clinical trials (i.e. Project MATCH) to target interventions to specific subgroups of alcohol-dependent patients (Project MATCH Research Group, 1997).
Recent efforts to identify discrete subgroups of patients with AUD have been informed by neurobiological models. Specifically, these neurobiological models of addiction have identified unique neural substrates underlying reward, relief and habit pathways. The allostatic model of addiction posits a heuristic framework that involves three stages of addiction: binge/intoxication, withdrawal/negative affect and preoccupation/intoxication stages (Koob and Le, 1997; Koob and Volkow, 2010; Koob, 2013). Initial alcohol use is characterized by positive reinforcement and impulsivity, such that alcohol’s rewarding properties increase the likelihood of continued alcohol seeking and consumption (Wise, 1987). These initial features are mediated by both the dopaminergic and opioidergic activity within the ventral striatum (Volkow et al., 2003; Gilman et al., 2008; Mitchell et al., 2012). In a subset of drinkers, repeated cycles of intoxication and withdrawal shift motivation to consume alcohol from positive reinforcement to negative reinforcement, wherein individuals drink alcohol to alleviate negative emotional states (Koob and Le Moal, 2005). The emergence of negative emotional states is mediated by neuroadaptations to stress systems in the extended amygdala (Koob and Kreek, 2007; Koob, 2008). Additionally, concurrent decreases in alcohol reward are associated with blunted dopaminergic activity in the ventral striatum (Koob and Bloom, 1988; Volkow et al., 2007). Although the rewarding effects of alcohol diminish, alcohol-associated stimuli develop incentive-salience via dopaminergic and glutamatergic signaling in the dorsal striatum, contributing to automaticity and habit learning (Robinson and Berridge, 1993; George and Koob, 2017). Over time, chronic alcohol consumption decreases executive functioning regulated by frontal lobe areas (Oscar-Berman and Marinković, 2007), leading to an overactive “Go” system that drives craving and habits, and an underactive “Stop” system that inhibits these behaviors (Bechara et al., 1999; Lobo and Nestler, 2011; Volkow and Morales, 2015; George and Koob, 2017). Taken together, compulsive alcohol drinking is the result of combined neuroadaptations in reward, stress, habit formation and executive function circuitry.
As with early efforts to classify AUD patients, neuroscience-informed clinical groupings are meant to inform treatment and personalize clinical care. Given that the initial stages of the addiction cycle are motivated by alcohol’s ability to indirectly increase dopamine levels in brain reward pathways, it is reasonable to assume that a pharmacological treatment that diminishes alcohol reward would be a viable therapeutic option for AUD. Naltrexone, a mu opioid antagonist, blunts alcohol-induced dopamine release but is only modestly effective in treating AUD (O'Malley et al., 1992; Volpicelli et al., 1992; Bouza et al., 2004). However, clinical responses to naltrexone are highly variable, which may be due, at least in part, to factors such as family history of alcoholism (King et al., 1997; Rubio et al., 2005; Krishnan-Sarin et al., 2007) and variation in the OPRM1 gene (Oslin et al., 2003; Ray and Hutchison, 2007; Anton et al., 2008). Two recent studies have found that classifying individuals into reward and relief drinking subtypes impacts the effectiveness of pharmacological treatments for AUD (Roos et al., 2017; Mann et al., 2018). In the first study, individuals in the COMBINE study who were classified as high relief drinkers, defined as those who drink alcohol mainly to relieve negative affect, had better drinking outcomes when treated with the glutamatergic modulator acamprosate compared to placebo. There was no significant effect found for reward drinkers and naltrexone in improving drinking outcomes (Roos et al., 2017). In contrast, Mann and colleagues utilized the PREDICT study sample and found that reward drinkers, defined as those who drink alcohol for its pleasurable/euphoric effects, benefited more from naltrexone in reducing heavy drinking compared to those treated with acamprosate (Mann et al., 2018). The discrepancy between these two findings may be due to the differing protocols of the COMBINE and PREDICT studies, particularly in the duration of pre-randomization abstinence. In the COMBINE study, the majority of participants were abstinent for only a short period prior to study randomization, whereas in the PREDICT study, participants underwent a full medication detoxification protocol. These differences likely impacted glutamatergic neurotransmission, which has been hypothesized as acamprosate’s mechanism of action (Holmes et al., 2013; Mann et al., 2018) and thereby limited the ability of Mann and colleagues to identify a relationship between relief drinking and acamprosate. Despite the contrasting medication findings, both studies reliably identified reward and relief AUD subtypes, which also replicates earlier work which classified reward and relief craving subtypes and identified specific clinical characteristics subserving them (Glockner-Rist et al., 2013). However, for these clinical groups to be reliably identified, clinical instruments must be developed and validated. In the current context of healthcare, brief measures that can be easily administered are most likely to be adopted and to impact practice. To that end, this study examines a novel self-report measure of reward, relief and habit drinking developed a priori by our group and administered in a human laboratory study of non-treatment seeking problem drinkers (Bujarski et al., 2018). In this report, we examine (a) the test-retest reliability of the new measure, (b) the clinical correlates of the three drinking subtypes and (c) patterns of subjective response (SR) to alcohol and self-administration of alcohol across the drinker subtypes. A follow-up study comprised of a crowdsourcing-based survey was undertaken to explore the agreement between our approach to identifying reward/relief drinkers compared to the approach undertaken by Mann et al. (2018).
METHOD
Participants
This study was approved by the Institutional Review Board at University of California, Los Angeles (UCLA). Non-treatment-seeking heavy drinkers were recruited between April 2015 and August 2016 from the Los Angeles community through fliers and online advertisements. Initial eligibility screening was conducted via online and telephone surveys and was followed by an in-person screening session. After providing written informed consent, participants were breathalyzed, provided urine for toxicology screening and completed a battery of self-report questionnaires and interviews. All participants were required to have a breath alcohol content (BrAC) of 0 mg% and to test negative on a urine drug screen for all drugs (except cannabis). Female participants were required to test negative on a urine pregnancy test. A physical examination was performed to ensure medical eligibility. Full inclusion and exclusion criteria for the experimental alcohol administration procedures have been published elsewhere (Bujarski et al., 2018). Participants were between the ages of 21 and 45 and were required to report drinking at (or above) heavy drinking guidelines (i.e. 14+ drinks/week for men or 7+ drinks/week for women).
Intravenous (IV) alcohol challenge and self-administration
Alcohol administration was conducted at the UCLA Clinical and Translational Research Center (CTRC). Detailed methodology can be found in (Bujarski et al., 2018). Briefly, at intake, vitals, height and weight were measured and IV lines were placed by a registered nurse. Participants then completed baseline assessments. The alcohol infusion paradigm lasted 180 minutes. Study staff remained in the room to monitor the infusion, breathalyze the participant, take vital signs, administer questionnaires and answer questions but did not significantly engage with participants otherwise. To enable precise control over BrAC and to dissociate biobehavioral responses to alcohol from responses to cues, alcohol was administered IV (6% ethanol v/v in saline) using a physiologically based pharmacokinetic model implemented in the Computerized Alcohol Infusion System (CAIS (Plawecki et al., 2008; Zimmermann et al., 2008, 2013). During the alcohol challenge, participants were administered alcohol designed to reach target BrACs of 20, 40 and 60 mg%, each over 15 minutes. BrACs were clamped at each target level while participants completed questionnaires (~5 minutes). Participants began the self-administration paradigm immediately after reaching the 60 mg% time point. Participants could exert effort (by pressing an electronic button) to obtain additional “drinks” through the CAIS system, according to a log-linear progressive ratio schedule. Ratio requirements ranged from 20 responses (1st completion) to 3139 responses (20th completion). Each “drink” increased BrAC by 7.5 mg% over 2.5 minutes, followed by a descent of −1 mg%/min (Zimmermann et al., 2008). A maximum BrAC safety limit was set at 120 mg%. If an infusion would exceed this limit, the response button was temporarily inactivated. Except for the first “drink”, participants were given no instruction with respect to their self-administration. After 180 minutes, the infusion ended and participants were instructed to wait until discharge. To ensure all participants were safe to discharge and to disincentivize low-levels of self-administration for early discharge, all participants were informed that they would remain at the CTRC for at least 4 additional hours following alcohol self-administration regardless of their self-administration profile. Discharge occurred when participant BrAC fell below 40 mg% or 0 mg% if they were driving. Participants were permitted to leave earlier than the 4 additional hour time requirement if they reached discharge BrAC levels early. However, participants were not informed of the discharge options until after the self-administration protocol was complete to further reduce the confound of low-levels of self-administration due to a desired earlier discharge time.
Measures
The UCLA reward, relief, habit drinking scale (UCLA RRHDS)
Reward, relief and habit drinking sub-types were assessed using a novel four-item self-report questionnaire (see Appendix 1). The first question asked participants to identify their primary reason for drinking alcohol (i.e. reward, relief or habit). The remaining questions asked participants to rate on a 1–7 scale how often they drank alcohol for its rewarding effects (i.e. to feel good), relief effects (i.e. reducing negative feelings) and out of habit (i.e. without thinking). Participants were classified into reward, relief and habit drinking sub-types based on their answers to questions 2–4, in which they rated how often they drank because of reward, relief or habit. Question 1 was used as a tie-breaker in cases where participants rated more than 1 dimension as the highest. The use of the tie-breaker question was required in 22% of the screening sample (n = 31) and 18% of the follow-up crowdsourcing sample (n = 33) to classify participants. Overall, there was a high level of consistency (93%) between participants’ self-identification of drinking type (i.e. question 1) and their highest frequency motive ratings (i.e. questions 2–4). This measure was assessed at the baseline visit and again at the eligibility physical visit. As the relief and habit drinking subtypes were largely indistinguishable in the larger screening sample (see Results) and previous research in this domain has only characterized reward and relief subtypes (Roos et al., 2017; Mann et al., 2018), the relief and habit subgroups were combined for analyses of the alcohol challenge experiment.
AUD severity measures
The Structured Clinical Interview for DSM-5 (SCID; adapted from (First et al., 2015)) assessed for lifetime and current AUD. The Clinical Institute Withdrawal Assessment for Alcohol (CIWA-Ar) assessed for the alcohol withdrawal severity (Sullivan et al., 1989). A 30-day timeline followback (TLFB) assessed drinking quantity and frequency (Sobell et al., 1988). Participants also completed the alcohol dependency scale (ADS (Skinner and Allen, 1982)), the Alcohol Use Disorders Identification Test (AUDIT (Allen et al., 1997)), the Drinkers Inventory of Consequences (DrINC-2r (Miller et al., 1995)), the penn alcohol craving scale (PACS (Flannery et al., 1999)), the obsessive compulsive drinking scale (OCDS (Anton, 2000)) and the Drinking Motives Questionnaire-Revised (Cooper, 1994).
Individual difference measures
Cigarette use and marijuana use were assessed using the TLFB (Sobell et al., 1988). The Fagerstrom Test for Nicotine Dependence (FTND) assessed for nicotine dependence (Heatherton et al., 1991). Depressive symptomatology was assessed via the beck depression inventory-II (BDI-II (Beck et al., 1996)). Anxiety symptomatology was assessed using the state-trait anxiety inventory (STAI (Spielberger, 2010)) and the beck anxiety inventory (BAI (Beck et al., 1988)).
SR measures
Based on previous factor analytic work, SR was assessed along four dimensions: stimulation/hedonia (stimulation), negative affect, sedation/motor intoxication (sedation) and craving (Bujarski et al., 2015). Participants completed SR assessments at baseline, 20, 40 and 60 mg% time points during the challenge. Stimulation included the biphasic alcohol effects scale stimulation subscale (BAES (Martin et al., 1993)) and the profile of mood states positive mood and vigor subscales (POMS (McNair et al., 1992)). Sedation included the BAES sedation subscale and select items from the subjective high assessment scale (SHAS (Schuckit, 1984)); specifically items assessing feelings of floating, clumsiness and drunk were assessed (questions #11, 3, and 6 from the SHAS). Negative Affect included the POMS Negative Mood and Tension subscales. Craving was measured by the alcohol urge questionnaire (AUQ (Bohn et al., 1995)). To incorporate multiple scales per SR domain, and equally weight scales with discrepant ranges, combined scores were computed within each SR domain by first Z-score transforming each measure across the entire challenge and then summing these scaled scores. These methods are consistent with our previous report from this study (Bujarski et al., 2018).
Data analysis
All analyses were conducted in SPSS v. 24. Test-retest analyses were conducted on the reward, relief and habit measure using the sample that completed both the screening and eligibility physical visits (n = 73). The analysis of clinical characteristics of drinker subtypes used the entire screening sample (n = 140). Univariate analyses of variance (ANOVAs) were conducted to evaluate group differences. Post-hoc Tukey t-tests were conducted to evaluate the direction of differences between the reward, relief and habit groups. The analyses of SR and self-administration behavior only used the sample of participants who completed the alcohol infusion experimental session (n = 67). Repeated measures ANOVAs were conducted to identify group differences in SR to alcohol across four domains: stimulation, sedation, negative affect and craving. For each test, we were interested in the main effect of group (reward drinkers vs. relief/habit drinkers), the main effect of trial (i.e. baseline, 20, 40, and 60% time-points) and the group × trial interaction. A series of analyses were conducted to evaluate the effect of drinking group (reward drinkers vs. relief/habit drinkers) on self-administration behavior. Regarding the self-administration outcomes, four domains were evaluated: total number of drinks self-administered, total number of button presses made, progressive ratio breakpoint, defined as the number of button presses in the last completed progressive ratio set, and maximum BrAC reached during the self-administration period. The number of button presses and progressive ratio breakpoint were log-transformed, as button-pressing requirements were generated from an exponential function and were not normally distributed. The self-administration domains were analyzed using t-tests. Given that this is an initial study designed to evaluate the newly developed measure, no P-value correction was implemented to account for multiple comparisons.
Follow-up study
In order to compare the UCLA RRHDS approach to identifying reward and relief drinkers to the approach taken by Mann et al. (2018), we conducted an online survey using a crowdsourcing platform Amazon Mechanical Turk (MTurk). In doing so, we followed methodological recommendations by Strickland and Stoops (2019) and approached this survey as a complement to the human laboratory methods reported in this manuscript (Strickland and Stoops, 2018, 2019). The first step consisted of screening a large unselected sample of MTurk users (n = 1000) for their alcohol use based on the AUDIT. We then invited back individuals who scored ≥8 on the AUDIT, indicating a hazardous drinking pattern (Reinert and Allen, 2007) to complete a second series of questionnaires. The follow-up survey was comprised of the alcohol dependence scale (ADS), the UCLA RRHDS and the inventory of drinking situations ((IDS); (Davis et al., 1987)), which was the primary instrument used by Mann et al. (2018) to classify reward/relief drinkers. As with the main study, the PACS and OCDS measures were also administered. Cross-tabulation analyses examined the agreement between the two methods in identifying reward drinkers. T-tests also compared reward/relief drinkers on the PACS, OCDS and ADS to replicate findings from the in-person assessment study.
RESULTS
Test-retest reliability of the UCLA RRHDS
For these analyses, a subset of individuals (n = 73) who completed the initial assessment were again given the UCLA RRHDS at the time of the physical exam required for medical clearance to the alcohol administration component of the study. On average, there were 10.83 ± 9.02 days between the completion of the reward, relief, habit measure conducted at the screening and physical exam (range = 2–48 days). First, we analyzed the test-retest reliability of the overall group identification. There was modest reliability of overall group identification; φc = 0.53, P < 0.001. We then assessed the test-retest reliability of each drinker sub-type. The reward drinker sub-type had strong reliability, 93% of individuals (n = 50/54) were classified as a reward drinker at baseline and at the physical. The relief and habit drinker sub-types were less reliable, 60% (n = 6/10) were classified as relief drinkers at both time-points, while only 33% (n = 3/9) were classified as habit drinkers at both time-points. Overall, 14 individuals were classified into different drinker sub-types at both time-points. Of this group, 12% (n = 9) were classified as relief or habit at the baseline visit and reward at the physical visit. The remaining individuals were classified as the following: reward at baseline, relief at physical (n = 1); reward at baseline, habit at physical (n = 3) and relief at baseline, habit at physical (n = 1). These results suggest that the reward-drinking subtype showed initial evidence of strong reliability, whereas the relief and habit drinking subtypes were not nearly as reliable, albeit with limited sample sizes.
Clinical correlates of drinker type
For these analyses, the full screening sample of 140 participants was included, because clinical characteristics were available on the full sample, whereas only a subset (n = 67) completed the alcohol administration procedures. The majority of the screening sample was classified as reward drinkers (n = 100; 71%), while 19 participants (14%) were classified as relief drinkers and 21 participants (15%) were classified as habit drinkers. The three groups did not differ on demographic characteristics (age, sex, cigarette smoking or cannabis use). Relief and habit drinkers had higher depressive symptomology and trait anxiety than reward drinkers. The groups did not differ on AUD severity, recent drinking behavior or dependence severity. Relief and habit drinkers had greater scores on alcohol craving measures (PACS and OCDS) than reward drinkers. Habit drinkers had significantly greater alcohol withdrawal symptoms than reward drinkers although all groups reported subclinical levels of withdrawal. The groups also differed on drinking motives. Reward drinkers were more likely to endorse drinking for enhancement motives, while relief drinkers were more likely to endorse drinking for coping motives. See Table 1 for complete results.
Table 1.
Clinical characteristics of reward, relief and habit drinkers
| Reward drinkers (n = 100) | Relief drinkers (n = 19) | Habit drinkers (n = 21) | Statistic | P | Effect size | |
|---|---|---|---|---|---|---|
| Age | 28.77 ± 6.50 | 30.42 ± 5.65 | 28.14 ± 5.20 | F = 0.75 | 0.50 |
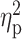 = 0.01 = 0.01 |
| Sex (M/F) | 51/49 (51/49%) | 10/9 (53/47%) | 9/12 (43/57%) | χ2 = 0.52 | 0.77 | φ = 0.06 |
| Cigarette smoker | 43 (43%) | 12 (63%) | 9 (43%) | χ2 = 2.70 | 0.26 | φ = 0.14 |
| THC+ | 20 (20%) | 2 (11%) | 0 (0%) | χ2 = 5.69 | 0.06 | φ = 0.20 |
| BDI-II a , b | 7.09 ± 7.32 | 11.34 ± 7.90 | 11.29 ± 11.89 | F = 3.77 | 0.03 |
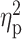 = 0.05 = 0.05
|
| STAI Trait a , b | 37.12 ± 9.28 | 47.42 ± 7.96 | 41.76 ± 10.01 | F = 10.53 | <0.001 |
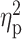 = 0.13 = 0.13
|
| STAI state | 53.32 ± 2.96 | 52.53 ± 4.18 | 52.67 ± 3.75 | F = 0.70 | 0.50 |
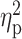 = 0.01 = 0.01 |
| BAI a | 4.83 ± 5.00 | 10.05 ± 9.74 | 7.52 ± 7/47 | F = 6.42 | 0.002 |
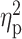 = 0.09 = 0.09
|
| AUD severity (none/mild/moderate/severe) | 51/21/19/9 | 8/3/6/2 | 7/6/4/4 | χ2 = 4.95 | 0.55 | φ = 0.19 |
| Total drinking days (TLFB) | 16.25 ± 6.47 | 16.84 ± 6.44 | 19.05 ± 7.49 | F = 1.55 | 0.22 |
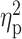 = 0.02 = 0.02 |
| Drinks per day (TLFB) | 2.94 ± 2.13 | 2.83 ± 1.53 | 3.39 ± 2.70 | F = 0.44 | 0.65 |
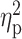 = 0.01 = 0.01 |
| Drinks per week (TLFB) | 20.56 ± 14.92 | 19.79 ± 10.72 | 23.70 ± 18.91 | F = 0.44 | 0.65 |
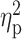 = 0.01 = 0.01 |
| Drinks per drinking day (TLFB) | 5.41 ± 2.94 | 5.35 ± 2.94 | 5.01 ± 2.45 | F = 0.17 | 0.85 |
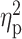 = 0.002 = 0.002 |
| Binge days (TLFB) | 8.16 ± 6.66 | 8.79 ± 7.39 | 8.10 ± 7.30 | F = 0.07 | 0.93 |
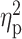 = 0.001 = 0.001 |
| CIWA-Ar b | 0.71 ± 1.36 | 1.16 ± 1.74 | 2.14 ± 3.09 | F = 5.81 | 0.004 |
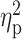 = 0.08 = 0.08
|
| ADS | 10.33 ± 5.56 | 12.37 ± 5.05 | 11.81 ± 5.87 | F = 1.46 | 0.24 |
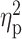 = 0.02 = 0.02 |
| AUDIT | 12.66 ± 6.13 | 14.95 ± 6.88 | 13.52 ± 7.06 | F = 1.08 | 0.34 |
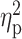 = 0.02 = 0.02 |
| OCDS a , b | 6.97 ± 4.22 | 9.95 ± 5.39 | 9.33 ± 5.48 | F = 4.87 | 0.009 |
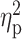 = 0.07 = 0.07
|
| PACS a | 7.59 ± 5.43 | 10.89 ± 5.59 | 9.95 ± 7.08 | F = 3.58 | 0.03 |
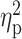 = 0.05 = 0.05
|
| Drinking motives—enhancement a , b | 16.00 ± 4.49 | 13.58 ± 4.52 | 13.71 ± 4.65 | F = 3.87 | 0.02 |
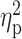 = 0.05 = 0.05
|
| Drinking motives—social | 16.69 ± 4.71 | 15.74 ± 5.24 | 15.67 ± 5.40 | F = 0.59 | 0.56 |
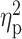 = 0.01 = 0.01 |
| Drinking motives—conform | 7.31 ± 2.85 | 6.84 ± 2.46 | 8.48 ± 4.54 | F = 1.61 | 0.20 |
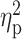 = 0.02 = 0.02 |
| Drinking motives—coping a , c | 10.11 ± 4.08 | 15.37 ± 3.39 | 11.57 ± 5.21 | F = 12.80 | <0.001 |
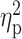 = 0.16 = 0.16
|
aReward and relief groups differ, P < 0.05.
bReward and habit groups differ, P < 0.05.
cRelief and habit groups differ, P < 0.05.
Characteristics that significantly differ between groups are displayed in bold type.
SR to alcohol by drinker type
For these analyses, only the subset of individuals who completed the experimental session (Bujarski et al., 2018) were included (n = 67) and the relief and habit subgroups were combined. Forty-eight individuals (72%) were classified as reward drinkers, and 19 individuals (28%) were classified as relief/habit drinkers.
As previously reported (Bujarski et al., 2018), across the sample, alcohol administration significantly increased ratings of stimulation (F(3,63) = 4.99, P = 0.002, 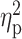 = 0.16), sedation (F(3,63) = 14.21, P < 0.001,
= 0.16), sedation (F(3,63) = 14.21, P < 0.001, 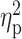 = 0.40) and craving for alcohol (F(3,63) = 10.84, P < 0.001,
= 0.40) and craving for alcohol (F(3,63) = 10.84, P < 0.001, 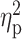 = 0.34), while it decreased ratings of negative mood (F(3,63) = 8.42, P < 0.001,
= 0.34), while it decreased ratings of negative mood (F(3,63) = 8.42, P < 0.001, 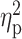 = 0.29). Across the rising BrAC levels, there was a trend towards a main effect of group on sedation (F(1,65) = 3.65, P = 0.06,
= 0.29). Across the rising BrAC levels, there was a trend towards a main effect of group on sedation (F(1,65) = 3.65, P = 0.06, 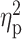 = 0.05), such that relief/habit drinkers reported greater levels of sedation than reward drinkers (Fig. 1b). There was also a significant interaction between group (reward drinker vs. relief/habit drinker) and rising BrAC (baseline, 20, 40, and 60% target BrAC) for ratings of negative affect (F(3,63) = 2.70, P = 0.05,
= 0.05), such that relief/habit drinkers reported greater levels of sedation than reward drinkers (Fig. 1b). There was also a significant interaction between group (reward drinker vs. relief/habit drinker) and rising BrAC (baseline, 20, 40, and 60% target BrAC) for ratings of negative affect (F(3,63) = 2.70, P = 0.05, 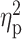 = 0.11), such that relief/habit drinkers reported high levels of negative affect at baseline and these sharply decreased upon alcohol administration (i.e. decrease from baseline to BrAC at 0.20 in the relief/habit group) (Fig. 1c). There was no significant main effect of group, nor a group × trial interaction, for ratings of stimulation or alcohol craving (P’s > 0.10; see Fig. 1).
= 0.11), such that relief/habit drinkers reported high levels of negative affect at baseline and these sharply decreased upon alcohol administration (i.e. decrease from baseline to BrAC at 0.20 in the relief/habit group) (Fig. 1c). There was no significant main effect of group, nor a group × trial interaction, for ratings of stimulation or alcohol craving (P’s > 0.10; see Fig. 1).
Fig. 1.
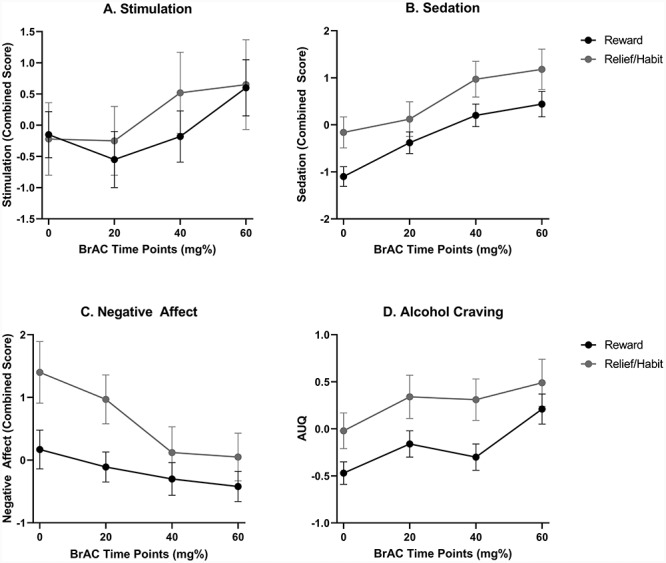
SR to alcohol during the alcohol challenge, by group. (A) The stimulation outcome combined measures from the BAES stimulation, POMS Vigor and POMS positive mood subscales. There were no group differences in stimulation over the challenge. (B) The sedation outcome combined measures from the BAES Sedation and SHAS scales. There was a trend towards increased sedation in the relief/habit group compared to the reward group across the challenge. (C) The negative affect outcome combined measures from the POMS tension and negative mood subscales. There was an interaction between group and BrAC on negative affect, such that the reward/relief drinkers reported a large decrease in negative affect following alcohol administration, compared to reward drinkers. (D) Alcohol craving was measured using the AUQ. There were no group differences on alcohol craving over the challenge.
Self-administration of alcohol by drinker type
For these analyses, only the subset of individuals included in the experimental session (Bujarski et al., 2018) were included (n = 67). Four measures of self-administration were evaluated: number of drinks self-administered, total number of button presses, breakpoint and maximum BrAC reached. The reward and relief/habit drinking groups did not differ on any measure of self-administration (all P’s > 0.30; see Table 2).
Table 2.
Alcohol self-administration by drinking type
| Reward (n = 48) | Relief + habit (n = 19) | Statistic | P | Effect size (Cohen’s d) | |
|---|---|---|---|---|---|
| Number of drinks | 11.25 ± 4.84 | 9.84 ± 5.21 | t = 1.05 | 0.30 | 0.28 |
| Maximum BrAC | 97.70 ± 21.09 | 91.79 ± 22.22 | t = 1.02 | 0.31 | 0.27 |
| Number of button presses (log transformed) | 3.10 ± 0.82 | 2.92 ± 0.86 | t = 0.79 | 0.63 | 0.21 |
| Breakpoint (log transformed) | 2.58 ± 0.56 | 2.42 ± 0.60 | t = 1.00 | 0.66 | 0.28 |
Comparing approaches to identifying reward/relief drinkers
From the initial crowdsourcing sample (n = 1,000) screened for drinking levels, a total of 187 (n = 125 male, 67%) heavy drinkers (i.e. AUDIT ≥8) of an average age of 30.9 ± 7.98 completed the entire follow-up survey in MTurk. The sample reported drinking an average of 19 ± 11.18 standard drinks in the past week and had an average ADS score of 17.70 ± 9.27. Based on the IDS criteria for identifying reward drinkers, 131 (64.53%) of the sample was classified as reward drinker and the remaining 72 (35.47%) were relief drinkers. Based on the UCLA RRHDS, 142 (69.95%) individuals were classified as reward drinkers and 61 (30.05%) were relief drinkers. Habit drinkers identified on the UCLA RRHDS were excluded from these analyses given that habit was not assessed in the IDS. In order to examine the agreement between UCLA RRHDS and IDS, we conducted a cross-tabulation procedure and found that 126 (62.1%) drinkers were identified as reward drinkers on both methods, whereas for the remaining 77 (38.9%) of drinkers, the UCLA RRHDS and IDS methods resulted in non-concordant classification.
Comparison between reward (n = 119) and relief (n = 68) drinkers identified by the UCLA RRHDS on the PACS, OCDS and ADS was undertaken to replicate the in-person assessment study. Results revealed group differences, such that relief drinkers reported higher craving than reward drinkers on the OCDS [relief mean = 24.04 ± 7.54; reward mean = 16.54 ± 9.06], t(185) = 5.78, P < 0.0001. The same pattern emerged for the PACS [relief mean = 13.19 ± 4.88; reward mean = 9.93 ± 4.59], t(185) = 4.56, P < 0.0001. Unlike the main study, ADS scores were higher among relief drinkers compared to reward drinkers [relief mean = 20.07 ± 8.55; reward mean = 15.61 ± 9.42], t(184) = 3.22, P < 0.01.
DISCUSSION
This study sought to develop a brief measure designed to characterize drinking motivations into one of three primary categories, namely reward, relief and habit. To do so, clinical variables and alcohol administration variables were considered in groups comprised of reward, relief and habit drinkers. Regarding clinical variables, our findings suggest that, in this sample, relief and habit were not dissociable groups, yet they both differed from the reward drinking group. Specifically, relief and habit drinkers reported higher levels of anxiety and depressive symptoms, higher levels of tonic craving and higher endorsement of drinking for coping motives than reward drinkers. Conversely, reward drinkers reported higher levels of drinking for enhancement motives, compared to relief/habit drinkers. Additionally, habit drinkers had greater withdrawal symptoms than reward drinkers. Our mood-related findings replicate earlier work characterizing reward and relief drinkers (Mann et al., 2018), such that relief/habit drinkers report higher depressive symptomology and higher trait anxiety. In further support of the current drinking classification, the groups differed on their self-reported drinking motives, such that reward drinkers endorsed drinking for enhancement (increase positive affect) while the relief group endorsed drinking to cope (decrease negative affect). In individuals with high coping motivation, negative affect induction increases automatic alcohol-approach associations, compared to individuals with low coping motivation (Ostafin and Brooks, 2011), which highlights the relief categorization of individuals with high coping motivation. Notably, however, the groups did not differ significantly on measures of alcohol use and AUD severity, suggesting that the distinction between reward and relief/habit drinkers represents a construct that is dissociable from AUD severity and drinking patterns. Importantly, the initial assessment of test-retest reliability suggested that only the reward drinker subtype benefited from strong reliability, whereas the relief and habit subtypes were clearly less reliable.
Regarding the experimental portion of the study, there were noteworthy group differences with regard to SRs to alcohol. Specifically, relief/habit drinkers reported a great decrease in negative mood during alcohol administration, as compared to reward drinkers. Moreover, there was a trend toward a main effect of drinker type on sedation, such that relief/habit drinkers reported higher sedation than reward drinkers. These effects were largely driven by baseline differences, suggesting that tonic levels of sedation may differentiate the two groups. Notably, the two groups did not differ on measures of self-administration during the self-administration phase of the task. This is somewhat consistent with our finding that the groups did not differ on clinical measures of AUD severity and alcohol use. Importantly, the self-administration phase of the task directly followed the alcohol challenge procedure, i.e. study participants had already received alcohol infusions up to a BrAC of 60 mg%. This methodology may have limited our ability to identify group differences in self-administration, as changes in negative mood and sedation had already occurred during the initial alcohol exposure. Further, this initial alcohol exposure may have masked other group differences in self-administration that would be present if the paradigm was tested on individuals without a priming dose of alcohol. Overall, these results provide some support for the allostatic model (Koob and Le, 1997), as relief/habit drinkers appeared to be in the “withdrawal/negative affect” state prior to the administration of alcohol. However, we did not find experimental evidence to support the categorization of reward drinkers into the “binge/intoxication” state, i.e. there were no significant group differences on ratings of alcohol-induced stimulation throughout the challenge.
The clinical and experimental correlates of reward and relief/habit drinking may be used to facilitate a personalized medicine approach to AUD pharmacotherapy. In the current study, reward drinkers reported drinking to increase positive affect had lower levels of trait anxiety, depression symptomology, withdrawal symptoms and less alcohol-induced sedation than relief drinkers. Thus, individuals classified as reward drinkers may respond better to pharmacotherapies targeting the positive rewarding effects of alcohol, such as naltrexone and nalmefene, which are both opioid antagonists. Indeed, in the PREDICT sample, reward drinkers who received naltrexone had a large reduction in the return to heavy drinking compared to those receiving placebo (Mann et al., 2018). This approach has yet to be examined with nalmefene; however, given that nalmefene is structurally similar to naltrexone (Mason et al., 1999), we would hypothesize that reward drinkers would show a positive response to nalmefene treatment on reductions in heavy drinking outcomes. In the current study, relief/habit drinkers reported drinking to reduce negative affect had significantly higher withdrawal symptoms, depressive symptoms and trait anxiety. Furthermore, the alcohol infusion resulted in a larger reduction in reports of negative affect in relief drinkers than reward drinkers. Therefore, relief/habit drinkers may respond better to medications designed to relieve the withdrawal and negative affect symptoms associated with chronic alcohol use, such as acamprosate and gabapentin. In the COMBINE study sample, high relief/moderate reward drinkers had better drinking outcomes when treated with acamprosate compared to placebo (Roos et al., 2017). While the drinker subtype classification precision-medicine approach has not yet been applied to gabapentin, available evidence supports the potential use of this medication to treat relief/habit drinkers. Gabapentin, a GABA analog that acts on voltage-gated calcium channels (Hendrich et al., 2008), has been shown to reduce alcohol-withdrawal symptoms (Myrick et al., 2009). Furthermore, in individuals with high levels of alcohol withdrawal, such as was seen in relief/habit drinkers in the current study, treatment with gabapentin had higher percent days abstinent compared to placebo, whereas in individuals with low levels of alcohol withdrawal treatment with placebo produced better outcomes than treatment with gabapentin (Anton et al., 2009). Finally, this drinking classification framework may be useful as novel medications are developed. For example, ibudilast, a neuroimmune modulator, has been shown to improve mood during alcohol and stress exposure, particularly in individuals who have higher depressive symptomatology (Ray et al., 2017). These promising findings indicate that ibudilast may be more effective in relief/habit drinkers.
Notably, we were unable to differentiate relief and habit drinkers using the UCLA RRHDS. The role of habit in addiction has largely been studied with animal models (Vandaele and Janak, 2018). There has been significant interest in translating these preclinical findings into clinical substance use disorder populations. To that end, behavioral tasks have been developed to interrogate the construct of habit in substance using populations (Voon et al., 2015; Ersche et al., 2016; Luijten et al., 2019) as well as its neural substrates (Sjoerds et al., 2013; Sebold et al., 2017; Grodin et al., 2018). In a similar vein as the UCLA RRDHS instrument, a self-report questionnaire has recently been developed to capture habit-, reward- and fear-related motivations to drink (Piquet-Pessoa et al., 2019). Habitual alcohol use was associated with a higher AUD severity, which corresponds to predictions from the addiction cycle model, where individuals with more severe AUD would have greater alcohol-induced neuroadaptations resulting in decreased executive control of goal-directed behavior. As we were unable to differentiate the relief and habit drinkers in the current study, it may be that a longer self-report measure or a behavioral task may be needed to fully capture habit drinkers and differentiate this group from relief drinkers.
Our measure of reward and relief/habit drinking patterns differs from previous work in several ways. The 30-item version of the Inventory of Drinking Situation (Annis et al., 1987) used by Mann et al. (2018) categorizes reward and relief drinking profiles based on the frequency of heavy drinking in various contexts over the past year. In contrast, our four-item measure asks participants to identify their primary reason for drinking alcohol and how often they drink alcohol for reward, relief or out of habit. In order to directly examine how our measure relates to previous work, particularly that of Mann et al. (2018), we used a crowdsourcing approach to complement the present study and to identify heavy drinkers who could complete both assessments of reward/relief drinking as well as measures of craving and alcohol problems. Results indicated that the agreement (or reliability) between the two methods was 62%, which lies in the questionable range. This finding suggests only moderate overlap between the two approaches. Further, the crowdsourcing-based follow-up survey replicated the original finding that relief drinkers report greater tonic craving for alcohol while also suggesting that relief drinkers have higher ADS scores.
Taken together, our findings suggest that reward drinkers are dissociable from relief/habit drinkers using the brief measure developed by our group. However, relief and habit drinkers were not differentiated in our study, which suggests that these two constructs overlapped phenotypically, at least in this sample of non-treatment seeking heavy drinkers and individuals with AUD. In terms of group differences, clinical measures suggested that relief/habit drinkers experience more mood dysphoria, tonic craving and report drinking for coping while reward drinkers report drinking for enhancement. This supports the face-validity of the constructs, especially when no differences in AUD severity and alcohol use were observed. The experimental component of the study added to the dysphoria findings by suggesting that relief/habit drinkers were more likely to report sedation across the alcohol administration and to endorse greater decreases in negative mood as BrAC levels increased. It is notable that measures of dysphoric mood were better at detecting group differences in this study, than were measures capturing the rewarding and stimulant effects of alcohol.
These results should be considered in light of the study’s strengths and limitations. Strengths include the combination of clinical and experimental variables, as well as an initial evaluation of test-retest reliability. Limitations include the relatively modest sample size, particularly in light of the disproportionate number of reward drinkers in this sample. The lack of strong reliability for the relief and habit subtypes may have been impacted by the relatively low numbers of drinkers identified in these categories, and large-scale studies of more diverse drinker groups are needed to fully evaluate this novel assessment tool. Further, about 20% of the sample in the initial and follow-up studies required the use of a tie-breaker question (Question 1) to classify their drinker subtype, indicating that a sizeable minority of individuals endorsed drinking for reward, relief and/or habit equally. We recommend that future studies using the UCLA RRHDS expand the numerical range of the scale from 1–7 to 1–10 to allow for greater differentiation between drinker subtypes. Moreover, due to ethical considerations, this study only enrolled non-treatment-seeking individuals who were able to produce a 0 mg% BrAC reading at each study visit. These methodological limitations reduced our ability to recruit participants with moderate and severe AUD. Based on the addiction cycle model, we would hypothesize that individuals with moderate and severe AUD would be more likely to be classified as relief and habit drinkers; therefore, the lower enrollment of individuals with severe AUD may have limited our ability to identify relief and habit drinkers. Additionally, as this was a preliminary study of the reward relief measure, we did not include a correction for multiple comparisons. Therefore, the results in this study may include false positives and should be replicated in a larger sample. In conclusion, this study reports on a novel scale developed by our team to capture drinking subtype with an eye towards a brief and readily disseminable instrument that can inform treatment matching.
Appendix 1
UCLA RRHDS
-
1) Do you drink alcohol primarily because (please circle 1):
It gives you a pleasant feeling (e.g. makes you feel good, excited, or confident)
It reduces negative feelings (e.g. makes you feel less bad, sad, or nervous)
It is a habit (e.g. without thinking about the effects of alcohol)
2) How often do you drink alcohol for the pleasant effects it has (e.g. makes you feel good, excited, or confident)?
Never Half of the time Always
1 -‐-‐-‐-‐-‐ 2 -‐-‐-‐-‐-‐ 3 -‐-‐-‐-‐-‐ 4 -‐-‐-‐-‐-‐ 5 -‐-‐-‐-‐-‐ 6 -‐-‐-‐-‐-‐ 7
3) How often do you drink alcohol because it reduces negative feelings (e.g. makes you feel bad, sad, or nervous)?
Never Half of the time Always
1 -‐-‐-‐-‐-‐ 2 -‐-‐-‐-‐-‐ 3 -‐-‐-‐-‐-‐ 4 -‐-‐-‐-‐-‐ 5 -‐-‐-‐-‐-‐ 6 -‐-‐-‐-‐-‐ 7
4) How often do you drink alcohol out of habit (e.g. without thinking about the effects of drinking)?
Never Half of the time Always
1 -‐-‐-‐-‐-‐ 2 -‐-‐-‐-‐-‐ 3 -‐-‐-‐-‐-‐ 4 -‐-‐-‐-‐-‐ 5 -‐-‐-‐-‐-‐ 6 -‐-‐-‐-‐-‐ 7
ACKNOWLEDGEMENT
This work was supported by a grant from the National Institute on Alcohol Abuse and Alcoholism (NIAAA) to LAR (K24AA025704 and R21AA022752). LAR has received study medication from Pfizer Medicinova and consulted for GSK and Mitsubishi Tanabe. Support for the development of the Computer-Assisted Infusion System was provided by Sean O’Connor, Martin Plawecki and Victor Vitvitskiy, Indiana Alcohol Research Center (P60 AA007611), Indiana University School of Medicine. None of the authors have conflicts of interest to disclose.
References
- Allen JP, Litten RZ, Fertig JB, Babor T (1997) A review of research on the alcohol use disorders identification test (AUDIT). Alcohol Clin Exp Res 21:613–9. [PubMed] [Google Scholar]
- Annis H, Graham JM, Davis CS, Addiction Research Foundation of O (1987) Inventory of Drinking Situations (IDS): User's Guide. Toronto: Addiction Research Foundation. [Google Scholar]
- Anton RF. (2000) Obsessive-compulsive aspects of craving: development of the obsessive compulsive drinking scale. Addiction 95:211–7. [DOI] [PubMed] [Google Scholar]
- Anton RF, Myrick H, Baros AM, et al. (2009) Efficacy of a combination of flumazenil and gabapentin in the treatment of alcohol dependence: relationship to alcohol withdrawal symptoms. J Clin Psychopharmacol 29:334–42. [DOI] [PubMed] [Google Scholar]
- Anton RF, Oroszi G, O'Malley S, et al. (2008) An evaluation of mu-opioid receptor (OPRM1) as a predictor of naltrexone response in the treatment of alcohol dependence: results from the combined pharmacotherapies and Behavioral interventions for alcohol dependence (COMBINE) study. Arch Gen Psychiatry 65:135–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babor TF, Hofmann M, DelBoca FK, et al. (1992) Types of alcoholics, I. evidence for an empirically derived typology based on indicators of vulnerability and severity. Arch Gen Psychiatry 49:599–608. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR, Lee GP (1999) Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. J Neurosci Off J Soc Neurosci 19:5473–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown G, Steer RA (1988) An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol 56:893. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK (1996) Beck depression inventory-II, Vol. 78 San Antonio: Psychological Corporation; 490–8. [Google Scholar]
- Bohn MJ, Krahn DD, Staehler BA (1995) Development and initial validation of a measure of drinking urges in abstinent alcoholics. Alcohol Clin Exp Res 19:600–6. [DOI] [PubMed] [Google Scholar]
- Bouza C, Angeles M, Munoz A, Amate JM (2004) Efficacy and safety of naltrexone and acamprosate in the treatment of alcohol dependence: a systematic review. Addiction 99:811–28. [DOI] [PubMed] [Google Scholar]
- Bujarski S, Hutchison KE, Roche DJ, Ray LA (2015) Factor structure of subjective responses to alcohol in light and heavy drinkers. Alcohol Clin Exp Res 39:1193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujarski S, Jentsch JD, Roche DJO, et al. (2018) Differences in the subjective and motivational properties of alcohol across alcohol use severity: application of a novel translational human laboratory paradigm. Neuropsychopharmacology 43:1891–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloninger CR, Bohman M, Sigvardsson S (1981) Inheritance of alcohol abuse. Cross-fostering analysis of adopted men. Arch Gen Psychiatry 38:861–8. [DOI] [PubMed] [Google Scholar]
- Cooper ML. (1994) Motivations for alcohol use among adolescents: development and validation of a four-factor model. Psychol Assess 6:117. [Google Scholar]
- Davis CS, Graham JM, Foundation OAR (1987) Inventory of Drinking Situations (IDS): User's Guide. Toronto, Ontario: Addiction Research Foundation. [Google Scholar]
- Ersche KD, Gillan CM, Jones PS, et al. (2016) Carrots and sticks fail to change behavior in cocaine addiction. Science 352:1468–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Williams J, Karg R, Rl S (2015) Structured Clinical Interview for the DSM-5. Arlington, VA: American Psychiatric Association. [Google Scholar]
- Flannery B, Volpicelli J, Pettinati H (1999) Psychometric properties of the Penn alcohol craving scale. Alcohol Clin Exp Res 23:1289–95. [PubMed] [Google Scholar]
- George O, Koob GF (2017) Individual differences in the neuropsychopathology of addiction. Dialogues Clin Neurosci 19:217–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman JM, Ramchandani VA, Davis MB, et al. (2008) Why we like to drink: a functional magnetic resonance imaging study of the rewarding and anxiolytic effects of alcohol. J Neurosci Off J Soc Neurosci 28:4583–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glockner-Rist A, Lemenager T, Mann K, Group PSR (2013) Reward and relief craving tendencies in patients with alcohol use disorders: results from the PREDICT study. Addict Behav 38:1532–40. [DOI] [PubMed] [Google Scholar]
- Grodin EN, Sussman L, Sundby K, et al. (2018) Neural correlates of compulsive alcohol seeking in heavy drinkers. Biol Psychiatry Cogn Neurosci Neuroimaging 3:1022–31. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO (1991) The Fagerström test for nicotine dependence: a revision of the Fagerstrom tolerance questionnaire. Br J Addict 86:1119–27. [DOI] [PubMed] [Google Scholar]
- Hendrich J, Van AT, Heblich F, et al. (2008) Pharmacological disruption of calcium channel trafficking by the alpha2delta ligand gabapentin. Proc Natl Acad Sci U S A 105:3628–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Spanagel R, Krystal JH (2013) Glutamatergic targets for new alcohol medications. Psychopharmacology 229:539–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellinek EM. (1960) Alcoholism, a genus and some of its species. Can Med Assoc J 83:1341–5. [PMC free article] [PubMed] [Google Scholar]
- King AC, Volpicelli JR, Frazer A, O'Brien CP (1997) Effect of naltrexone on subjective alcohol response in subjects at high and low risk for future alcohol dependence. Psychopharmacology 129:15–22. [DOI] [PubMed] [Google Scholar]
- Koob G, Kreek MJ (2007) Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry 164:1149–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. (2008) A role for brain stress systems in addiction. Neuron 59:11–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. (2013) Theoretical frameworks and mechanistic aspects of alcohol addiction: alcohol addiction as a reward deficit disorder. Curr Top Behav Neurosci 13:3–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Bloom FE (1988) Cellular and molecular mechanisms of drug dependence. Science 242:715–23. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le M (1997) Drug abuse: hedonic homeostatic dysregulation. Science 278:52–8. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M (2005) Plasticity of reward neurocircuitry and the 'dark side' of drug addiction. Nat Neurosci 8:1442–4. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND (2010) Neurocircuitry of addiction. Neuropsychopharmacology 35:217–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan-Sarin S, Krystal JH, Shi J, et al. (2007) Family history of alcoholism influences naltrexone-induced reduction in alcohol drinking. Biol Psychiatry 62:694–7. [DOI] [PubMed] [Google Scholar]
- Leggio L, Kenna GA, Fenton M, et al. (2009) Typologies of alcohol dependence. From Jellinek to genetics and beyond. Neuropsychol Rev 19:115–29. [DOI] [PubMed] [Google Scholar]
- Lesch OM, Walter H (1996) Subtypes of alcoholism and their role in therapy. Alcohol and Alcoholism 31:63–7. [PubMed] [Google Scholar]
- Litt MD, Babor TF, DelBoca FK, et al. (1992) Types of alcoholics. II Application of an empirically derived typology to treatment matching. Arch Gen Psychiatry 49:609–14. [DOI] [PubMed] [Google Scholar]
- Lobo MK, Nestler EJ (2011) The striatal balancing act in drug addiction: distinct roles of direct and indirect pathway medium spiny neurons. Front Neuroanat 5:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luijten M, Gillan CM, De Wit S, et al. (2019) Goal-directed and habitual control in smokers. Nicotine Tob Res. http://10.1093/ntr/ntz001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann K, Roos CR, Hoffmann S, et al. (2018) Precision medicine in alcohol dependence: a controlled trial testing pharmacotherapy response among reward and relief drinking phenotypes. Neuropsychopharmacology 43:891–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CS, Earleywine M, Musty RE, et al. (1993) Development and validation of the biphasic alcohol effects scale. Alcohol Clin Exp Res 17:140–6. [DOI] [PubMed] [Google Scholar]
- Mason BJ, Salvato FR, Williams LD, et al. (1999) A double-blind, placebo-controlled study of oral nalmefene for alcohol dependence. Arch Gen Psychiatry 56:719–24. [DOI] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppleman LF (1992) Profile of Mood States: Manual. EdITS. [Google Scholar]
- Miller W, Tonigan J, Longabaugh R (1995) The Drinker Inventory of Consequences (DrInC): An Instrument for Assessing Adverse Consequences of Alcohol Abuse, Vol. 4 Rockville, MD: National Institute on Alcohol Abuse and Alcoholism. [Google Scholar]
- Mitchell JM, O'Neil JP, Janabi M, et al. (2012) Alcohol consumption induces endogenous opioid release in the human orbitofrontal cortex and nucleus accumbens. Sci Transl Med 4:116ra116. [DOI] [PubMed] [Google Scholar]
- Moss HB, Chen CM, Yi HY (2007) Subtypes of alcohol dependence in a nationally representative sample. Drug Alcohol Depend 91:149–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myrick H, Malcolm R, Randall PK, et al. (2009) A double-blind trial of gabapentin versus lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res 33:1582–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Malley SS, Jaffe AJ, Chang G, et al. (1992) Naltrexone and coping skills therapy for alcohol dependence. A controlled study. Arch Gen Psychiatry 49:881–7. [DOI] [PubMed] [Google Scholar]
- Oscar-Berman M, Marinković K (2007) Alcohol: effects on neurobehavioral functions and the brain. Neuropsychol Rev 17:239–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oslin DW, Berrettini W, Kranzler HR, et al. (2003) A functional polymorphism of the mu-opioid receptor gene is associated with naltrexone response in alcohol-dependent patients. Neuropsychopharmacology 28:1546–52. [DOI] [PubMed] [Google Scholar]
- Ostafin BD, Brooks JJ (2011) Drinking for relief: negative affect increases automatic alcohol motivation in coping-motivated drinkers. Motiv Emot 35:285–95. [Google Scholar]
- Piquet-Pessoa M, Chamberlain SR, Lee RSC, et al. (2019) A study on the correlates of habit-, reward-, and fear-related motivations in alcohol use disorder. CNS Spectr 1–8. http://10.1017/S1092852918001554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plawecki MH, Han JJ, Doerschuk PC, et al. (2008) Physiologically based pharmacokinetic (PBPK) models for ethanol. IEEE Trans Biomed Eng 55:2691–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Project MATCH Research Group (1997) Matching alcoholism treatments to client heterogeneity: project MATCH posttreatment drinking outcomes. J Stud Alcohol 58:7–29. [PubMed] [Google Scholar]
- Ray LA, Bujarski S, Shoptaw S, et al. (2017) Development of the neuroimmune modulator ibudilast for the treatment of alcoholism: a randomized, placebo-controlled, human laboratory trial. Neuropsychopharmacology 42:1776–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Hutchison KE (2007) Effects of naltrexone on alcohol sensitivity and genetic moderators of medication response: a double-blind placebo-controlled study. Arch Gen Psychiatry 64:1069–77. [DOI] [PubMed] [Google Scholar]
- Reinert DF, Allen JP (2007) The alcohol use disorders identification test: an update of research findings. Alcohol Clin Exp Res 31:185–99. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC (1993) The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev 18:247–91. [DOI] [PubMed] [Google Scholar]
- Roos CR, Mann K, Witkiewitz K (2017) Reward and relief dimensions of temptation to drink: construct validity and role in predicting differential benefit from acamprosate and naltrexone. Addict Biol 22:1528–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio G, Ponce G, Rodriguez-Jimenez R, et al. (2005) Clinical predictors of response to naltrexone in alcoholic patients: who benefits most from treatment with naltrexone? Alcohol and Alcoholism 40:227–33. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. (1984) Subjective responses to alcohol in sons of alcoholics and control subjects. Arch Gen Psychiatry 41:879–84. [DOI] [PubMed] [Google Scholar]
- Sebold M, Nebe S, Garbusow M, et al. (2017) When habits are dangerous: alcohol expectancies and habitual decision making predict relapse in alcohol dependence. Biol Psychiatry 82:847–56. [DOI] [PubMed] [Google Scholar]
- Sjoerds Z, de Wit S, van den Brink W, et al. (2013) Behavioral and neuroimaging evidence for overreliance on habit learning in alcohol-dependent patients. Transl Psychiatry 3:e337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner HA, Allen BA (1982) Alcohol dependence syndrome: measurement and validation. J Abnorm Psychol 91:199. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB, Leo GI, Cancilla A (1988) Reliability of a timeline method: assessing normal drinkers’ reports of recent drinking and a comparative evaluation across several populations. Br J Addict 83:393–402. [DOI] [PubMed] [Google Scholar]
- Spielberger C. D. (1983) Manual for the State-Trait Anxiety Inventory: STAI (Form Y). Palo Alto, CA: Consulting Psychologists Press
- Strickland JC, Stoops WW (2018) Feasibility, acceptability, and validity of crowdsourcing for collecting longitudinal alcohol use data. J Exp Anal Behav 110:136–53. [DOI] [PubMed] [Google Scholar]
- Strickland JC, Stoops WW (2019) The use of crowdsourcing in addiction science research: amazon mechanical Turk. Exp Clin Psychopharmacol 27:1–18. [DOI] [PubMed] [Google Scholar]
- Sullivan JT, Sykora K, Schneiderman J, et al. (1989) Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). Addiction 84:1353–7. [DOI] [PubMed] [Google Scholar]
- Vandaele Y, Janak PH (2018) Defining the place of habit in substance use disorders. Prog Neuro-Psychopharmacol Biol Psychiatry 87:22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ (2003) The addicted human brain: insights from imaging studies. J Clin Invest 111:1444–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Morales M (2015) The brain on drugs: from reward to addiction. Cell 162:712–25. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, et al. (2007) Profound decreases in dopamine release in striatum in detoxified alcoholics: possible orbitofrontal involvement. J Neurosci Off J Soc Neurosci 27:12700–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpicelli JR, Alterman AI, Hayashida M, O'Brien CP (1992) Naltrexone in the treatment of alcohol dependence. Arch Gen Psychiatry 49:876–80. [DOI] [PubMed] [Google Scholar]
- Voon V, Derbyshire K, Ruck C, et al. (2015) Disorders of compulsivity: a common bias towards learning habits. Mol Psychiatry 20:345–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. (1987) The role of reward pathways in the development of drug dependence. Pharmacol Ther 35:227–63. [DOI] [PubMed] [Google Scholar]
- Zimmermann US, Mick I, Vitvitskyi V, et al. (2008) Development and pilot validation of computer-assisted self-infusion of ethanol (CASE): a new method to study alcohol self-administration in humans. Alcohol Clin Exp Res 32:1321–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann US, O'Connor S, Ramchandani VA (2013) Modeling alcohol self-administration in the human laboratory. Curr Top Behav Neurosci 13:315–53. [DOI] [PubMed] [Google Scholar]


