Abstract
A subgroup of COVID-19 patients develop very severe disease with requirement for ICU treatment, ventilation, and ECMO therapy. Laboratory tests indicate that the immune and clotting system show marked alterations with hyper-activation, hyper-inflammation, cytokine storm development. Furthermore, organ-specific biomarkers demonstrate the involvement of cardiac muscle, kidney, and liver dysfunction in many patients. In this article the use of laboratory biomarkers is discussed with regard to their use for diagnosis, disease progression, and risk assessment.
Keywords: Biomarker, COVID-19, Cytokine storm, Laboratory diagnosis, SARS-CoV2
Introduction
Although only a minority of COVID-19 patients show critical disease progression from moderate to severe stages of the disease including requirement for ventilation and ECMO therapy, this subgroup of COVID-19 patients requires particular attention. Data collection from several regions of the world including China, Europe, and the United States clearly demonstrate that COVID-19 is not only a disease of the lung and the airways. Many other organ systems are involved and contribute to disease variety and progression. With regard to the immune system, hyper-inflammation together with the development of exorbitant increased cytokine production represents a hallmark of severe patients requiring ventilation. Some of these patients develop bacterial superinfections with increased levels of sepsis markers. Another important systems which recently caused increased attention is the clotting system. This is particularly highlighted by the detection of increased levels of D-dimers. Organ dysfunction has been reported in many patients including the heart (myocardial muscle damage), the kidney, and the liver. Laboratory diagnostics play not only an important role in disease diagnosis, but also in assessing progression and severity in these patients. Furthermore, laboratory diagnostics allow early detection of organ dysfunction in many cases. Moreover, biotests are used to assess an increased mortality risk in severe lethal patients. In this article we summarize the most prominent findings in COVID-19 patients and discuss the use of these markers for diagnosis, disease progression, and risk assessment. (Fig. 1 and Table 1, Table 2)
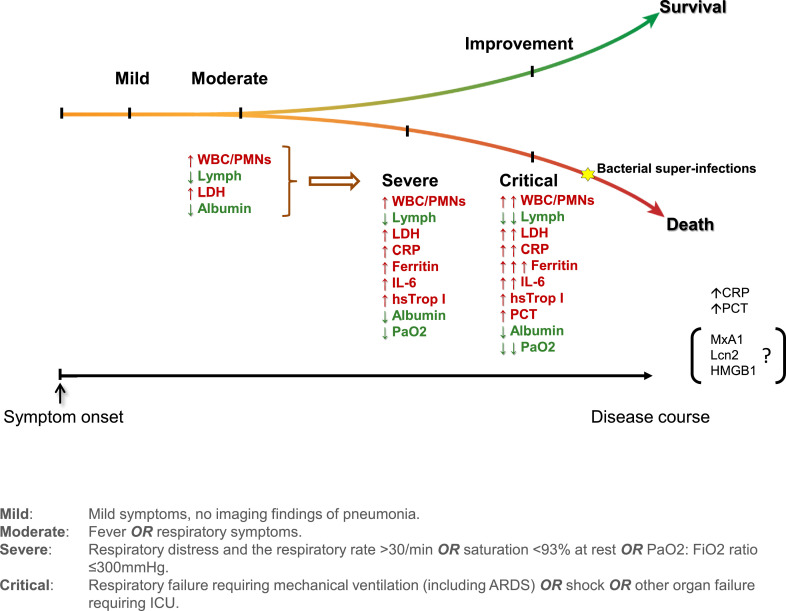
Fig. 1.
Schematic overview of key laboratory characteristics during SARS-CoV-2 infections. The latter induce an increase (depicted in red) or a reduction (depicted in green) in the concentration and/or counts of a wide range of laboratory biomarkers. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Table 1.
Laboratory parameters and associated pathophysiology in adult COVID-19 patients.
| BIOMARKER | PATHOPHYSIOLOGY | CLINICAL UTILITY IN ADULT COVID-19 | REFERENCES |
|---|---|---|---|
| Hematological indices | |||
| Hemoglobin | Reduced erythropoiesis due to inflammatory cytokines |
Lower levels associated with: Lack of improvement No clear association with disease severity and outcomes |
(16, 5, 11) |
| Lymphocytes | Absolute count reduction, functional exhaustion of all populations (especially cytotoxic T-cells) Unknown exact mechanisms |
Lower levels associated with: ↑Severity, ↑Mortality PMN/CD8+ ratio and PMN/Lymphocyte ratio may be used as prognostic markers |
(52, 5, 1–4, 6, 10–13, 7) |
| Monocytes/ Basophils/ Eosinophils |
Absolute count reduction, Unknown exact mechanisms |
No clear association with disease severity and outcomes | (14, 3, 10) |
| Total white blood cells/Neutrophils | Increased due to inflammation | Higher levels associated with: ↑Severity, ↑Mortality, Bacterial superinfections | (1–4) |
| Acute phase reactants | |||
| Albumin | Reduced production due to inflammatory cytokines |
Lower levels associated with: ↑Severity, ↑Mortality, Lack of improvement Low levels on admission may be used as prognostic marker for severity |
(1, 2, 11, 6, 16, 19) |
| C-reactive protein (CRP) | Increased production due to inflammatory cytokines | Higher levels associated with: ↑Severity, Lack of improvement, Bacterial superinfections | (1, 3, 16) |
| Erythrocyte sedimentation rate | Increased in inflammation | Tendency for higher levels associated with: ↑Mortality | (1) |
| Ferritin | Increased production due to inflammatory cytokines, released by activated macrophages |
Higher levels associated with: ↑Severity, ↑Mortality High levels are indicators ofCSS/sHLH development |
(1, 2, 6, 5) |
| Procalcitonin | Increased production due to inflammatory cytokines | Higher levels associated with: ↑Severity, ↑Mortality, Bacterial superinfections | (4, 3, 18, 2, 6, 5, 19) |
| Serum amyloid A | Increased production due to inflammatory cytokines | High levels seen among: all COVID-19 patients | (3) |
| Biochemistry indices | |||
| Cholinesterase | Unknown exact mechanism |
Lower levels associated with: ↑ Severity |
(53) |
| Electrolytes (Na, K, Cl) | Multiple mechanisms (e.g. SIADH, acidosis etc.) | No clear association with disease severity and outcomes | (1, 11, 18, 51) |
| Lactate dehydrogenase (LDH) | Released by cell injury |
Higher levels associated with: ↑Severity, ↑Mortality, Lack of improvement High levels on admission may be used as prognostic marker for severity |
(11, 2, 1, 6, 5, 19, 16) |
| Triglycerides | Reduced lipoprotein lipase activity due to high TNF-α levels | Higher levels have been reported in fatal cases but not enough data Component of HScore for CSS/sHLH diagnosis |
(1) |
| TSH/FT3 | Possible euthyroid sick syndrome of critical illness | Higher levels have been reported in fatal cases, not enough data | (1) |
| Cardiac biomarkers | |||
| Hs-troponin I | Released by myocardial injury |
Higher levels associated with:↑igher levels associa, lack of improvement High levels on admission or gradual increase may be used as prognostic marker for severity and mortality |
(54, 31, 33, 4) |
| Troponin T | Released by myocardial injury |
Higher levels associated with: ↑igher levels associa |
(32) |
| CK-MB | Released by myocardial injury | Higher levels associated with:↑igher le | (33, 4) |
| NT-proBNP | Increased production due to heart failure | Higher levels associated with:↑igher le | (31, 33) |
| Renal function indices | |||
| Creatinine | Decreased discharge due to renal injury |
Higher levels associated with: ↑igher levels associa |
(4, 2, 55, 34, 31) |
| BUN | Decreased discharge due to renal injury |
Higher levels associated with: ↑igher levels associa |
(31, 34, 55, 4) |
| Urinary protein | Possiblypositive due to renal dysfunction | Proteinuria may associated with:↑roteinuri (limited data) | (34) |
| Urinary erythrocyte | Possibly positive due to renal dysfunction | Hematuria may associated with:↑ematuria (limited data) | (34) |
| Liver function indices | |||
| ALT | Possibly liver injury, unknown exact mechanism |
Higher levels associated with: ↑igherity, (↑Mortality, indeterminate data) |
(2, 31, 4, 18, 36) |
| AST | Possibly myocardial or liver injury, unknown exact mechanism |
Higher levels associated with: ↑igher leve(↑Mortality, indeterminate data) |
(31, 4, 18, 36) |
| TBIL | Unknown exact mechanism |
Higher levels associated with: ↑igher le |
(36, 31) |
| GGT | Unknown exact mechanism |
Higher levels associated with: ↑igher le (limited data) |
(36) |
| ALP | Increased levels in some patients, unknown exact mechanism | No clear association with disease severity and outcomes | (36, 56) |
| Coagulation profile | |||
| D-dimer | Elevated levels possibly due to hypercoagulability and secondary fibrinolysis |
Higher levels associated with: ↑igher levels associa D-dimer>1 ng/mlon admission or gradual increase may be used as prognostic marker for severity and mortality |
(4, 3, 2, 55, 31) |
| PT | Prolonged PT possibly duehypercoagulability and secondary fibrinolysis |
Higher levels associated with: ↑igher lev |
(2, 4, 55, 44, 57) |
| INR | Elevated levels possibly duehypercoagulability and secondary fibrinolysis | Higher levels may associated with:↑Severity (limited data) | (36) |
| APTT | Unknown exact mechanism | Indeterminate association with disease severity and outcomes | (44, 5, 55) |
| Fibrinogen | Elevated as an acute phase protein and may decreasedue to hypercoagulability | Higher levels may associated with:↑igher levels may ass (limited data) | (44, 43) |
| Cytokines and chemokines | |||
| IL-1β | Increased production/Associated with CSS/sHLH |
Higher levels may be associated with: ↑Mortality Indeterminate data for severity |
(58, 1, 6, 11, 10) |
| IL-2/ soluble IL-2R | Increased production/Associated with CSS/sHLH | Higher levels associated with: ↑Severity, ↑Mortality | (58, 1, 11, 6, 10) |
| IL-6 | Increased production/Associated with CSS/sHLH |
Higher levels associated with: ↑Severity, ↑Mortality IL-6 levels may monitor disease progression Higher of IL-6 to IFN-γ ratio may distinguish severe from moderate cases |
(5, 24, 58, 1, 11, 6, 2, 10) |
| IL-7 | Increased production/Associated with CSS/sHLH | Higher levels associated with: ↑Severity | (58, 11) |
| IL-8 | Increased production/Associated with CSS/sHLH | Higher levels may be associated with:↑Severity(Indeterminate data) | (6, 10) |
| IL-10 | Increased production by macrophages | Higher levels associated with: ↑Severity (also ↑Mortality, but not enough data) | (58, 1, 11, 6) |
| IL-17 | Increased production/Associated with CSS/sHLH | Higher levels may be associated with:↑Severity (Not enough data) | (10) |
| IP10 (CXCL10) | Increased production/Associated with CSS/sHLH | Higher levels associated with: ↑Severity | (58, 11) |
| G-CSF/GM-CSF | Increased production/Associated with CSS/sHLH | Higher levels associated with: ↑Severity | (58, 11, 10) |
| TNF-α | Increased production/Associated with CSS/sHLH | Higher levels associated with: ↑Severity(also ↑Mortality, but not enough data) | (58, 1, 11, 6, 10) |
| MCP1 (CCL2) | Increased production/Associated with CSS/sHLH | Higher levels associated with: ↑Severity | (58, 11, 10) |
| MIP-1α (CCL3) | Increased production/Associated with CSS/sHLH | Higher levels associated with: ↑Severity | (58, 11, 6, 10) |
| INF-γ | Reduced production by CD4+ T cells |
Lower levels may be associated with: ↑Severity Higher of IL-6 to IFN-γratio may distinguish severe from moderate cases |
(24, 11, 58, 6) |
| Complement | Possible activation of the alternative and lectin-based complement pathways from viral proteins | Deposits of C5b-9, C4d and MASP 2 in the microvasculature of lungs (from autopsy specimens) No differences in C3/C4 levels among survivors- non survivors |
(1, 27, 59) |
| Immunoglobulins (IgA, IgG, IgM) | In theory, increased production induced by activated B-cells | No differences in IgA/IgG/IgM levels among survivors- non survivors | (1) |
| Soluble urokinase plasminogen activator receptor (suPAR) | Increased due to endothelial activation | High levels may be associated with: prediction of respiratory failure | (52) |
| Arterial blood gases parameters | |||
| pH | Respiratory alkalosis driven by hypoxemia, metabolic acidosis due organ hypoperfusion | Conflicting data on pH and associated mortality. One study found statistically higher frequency of acidosis among fatal cases | (1, 2) |
| Bicarbonates | Decreased due to respiratory alkalosis and metabolic acidosis | Not enough data – possibly lower among non-survivors | (1) |
| PaO2 | Decreased due to alveolar and microvasculature injury (direct and indirect) | Frequency of type I respiratory failure is significantly higher among non survivors Markedly low PaO2 (<60 mmHg) levels are seen in fatal cases |
(1, 2) |
| PaCO2 | Decreased due to high respiratory rate driven by hopoxia/shunt | Not enough data – possibly lower among non-survivors | (1) |
| PaO2:FiO2 ratio | Decreased due to alveolar and microvasculature injury (direct and indirect) | PaO2:FiO2 ratio of ≤300 associated with ↑Mortality | (1) |
Cl: Chloride, CSS/sHLH: Cytokine storm syndrome/secondary Hemophagocytic lymphohistiocytosis, FiO2: Fraction of inspired oxygen, FT3: Free triiodothyronine, G-CSF: Granulocyte-colony stimulating factor, GM-CSF: Granulocyte-macrophage colony-stimulating factor, IL: Interleukin, IP10:Interferon gamma-induced protein 10, K: Potassium, MASP 2: mannose binding lectin associated serine protease 2. MCP1 (CCL2): Monocyte chemoattractant protein 1, MIP-1α (CCL3): Macrophage inflammatory protein 1-alpha), Na: Sodium,PaCO2:Arterial carbon dioxide partial pressure, PaO2: Arterial oxygen partial pressure, SIADH: Syndrome of inappropriate antidiuretic hormone secretion, soluble IL-2R: soluble Interleukin 2 receptor, TSH: Thyroid stimulating hormone, BUN, blood urea nitrogen; ALT, alanine transaminase; AST, aspartate transaminase; TBIL, total bilirubin; GGT,gamma-glutamyl transpeptidase; ALP, alkaline phosphatase; CK-MB, creatine kinase MB; PT, prothrombin time; APTT, activated partial thromboplastin time; INR, international normalized ratio; Hs-troponin I, high sensitivity troponin I.
Table 2.
Laboratory parameters in pediatric COVID-19 patients.
| BIOMARKER | PEDIATRIC COVID-19 DATA | REFERENCES |
|---|---|---|
| Hematological indices | ||
| Hemoglobin | Potentially similar to adults | (52, 53) |
| Lymphocytes | Higher lymphocyte counts compared to adults Normal lymphocyte counts common Lymphopenia in 0–35% of children Lymphocytosis is rare |
(52, 54–57) |
| Total white blood cells/Neutrophils |
Higher levels associated with: Symptomatic disease, Younger age (<2 y.o.) Lower neutrophil counts compared to adults Leukocytosis is more frequent Leukopenia is rare |
(52, 55–57) |
| Acute phase reactants | ||
| Albumin | Less frequently decreased compared to adults | (57, 53) |
| C-reactive protein (CRP) | Lower CRP levels compared to adults High CRP in 10%−83% of children |
(52, 55, 56) |
| Erythrocyte sedimentation rate | Less frequently elevated compared to adults | (57) |
| Procalcitonin | Can be high in hospitalized children More frequently elevated compared to adults |
(56, 57) |
| Biochemistry indices | ||
| Lactate dehydrogenase (LDH) | Normal LDH levels commonly Higher LDH levels compared to adults in one report |
(52, 55, 57) |
| Cytokines and chemokines | ||
| IL-6 | Lower IL-6 levels compared to adults | (52) |
Hemoglobin and white blood cells
Retrospective analyses from China demonstrated that leukocyte counts were higher among non-survivors compared to recovered patients1 , 2; in particular, Zhou et al. reported that COVID-19 patients who did not survive had a median of 9.8 × 109/L WBC count compared to 5.2 × 109/L among those who survived (p<0.0001), although the exact time point of measurement was not defined in their methods. Furthermore, another study of 140 hospitalized patients in Wuhan, demonstrated significantly higher leukocyte counts among those with severe COVID-19 disease, compared to patients with milder infection (p = 0.003).3 Finally, a series from the same center, and possibly overlapped populations with the previous study, reported significantly higher WBC counts upon hospital admission among patients requiring critical care, although median values were within normal range (WBC count median 6.6 × 109/L for ICU vs 4.3 × 109/L for non-ICU admission, p = 0.003).4
The observed leukocytosis is attributed to an elevation of neutrophils, as the other WBC populations seem to drop in severely ill and eventually fatal COVID-19 cases.5 Absolute lymphopenia is commonly observed in patients with COVID-19, but pronounced lymphocyte depletion is a cardinal marker of enhanced disease severity and an indicator of imminent death, that has been consistently depicted by almost all currently published reports, coming mainly from China.1, 2, 3, 4, 5, 6, 7 Importantly, not only the degree of lymphocyte drop, but also the persistence of low lymphocyte counts throughout the disease course have been associated with critical illness and death.1 , 2 , 4 In contrast to previous reports for SARS-CoV, peripheral blood smears reveal the presence of reactive lymphocytes, including some lymphoplasmacytoids, in the majority of COVID-19 patients7, 8, 9. Severe SARS-CoV-2 infection depletes all lymphocyte subsets, including CD4+ T cells, CD8+ T cells, B cells and natural killer (NK) cells, but CD4+/ CD8+ ratio is not inverted as seen in other viral infections.10, 11, 12, 13 Not only the absolute numbers of T-cells are reduced, but also receptors suppressing their cytotoxic effects, like the CD94/NKG2A receptor, are up-regulated leading to diminished defense mechanisms against the virus.10
Monocyte, eosinophil and basophil counts are also decreased in COVID-19, but the magnitude of this reduction has not been associated with disease severity, in currently published data from Chinese centers.3 , 10 , 14 Moreover, pro-inflammatory cytokines are known to blunt erythropoiesis.15 However, aside from one study that found significantly higher frequencies of decreased hemoglobin concentrations among severe (43.6%) and critical cases (37.2%) compared to mild/moderate ones (23.1%) (p<0.001), solid evidence of significant hemoglobin reduction in severe COVID-19 has not been consistently reported as yet.5 , 11 , 16 In one particular study, lower hemoglobin concentration was associated with increased odds for lack of disease improvement but not death (odds ratio 1.731, p = 0.008).16
Preliminary reports imply that high neutrophil counts and persistently deep lymphocyte nadir counts during hospitalization as well as high neutrophil to lymphocyte ratios (NLR) are indicators of adverse outcomes such as ICU admission and death10. A retrospective Chinese study reported that NLR, along with the SARS-CoV-2 IgG levels, could be used as a simple discriminative tool for severity between COVID-19 patients, and further predict the clinical outcome of these patients14. However, whether these indices can actually risk stratify patients and predict poor outcomes, most importantly at an early stage of the disease, remains to be addressed and validated in large prospective trials.
Common inflammatory markers – Acute phase reactants
The regulation of ferritin synthesis is cytokine-controlled17; hence, the extreme immune activation in the context of the cytokine storm observed in critical, and usually fatal, cases of COVID-19, leads to an up-regulation of serum ferritin levels. Indeed, preliminary patient data demonstrate that excessive ferritin levels are observed among COVID-19 patients, ranging from 400 μg/L to as high as >2000 μg/L, with the highest trends being observed in severe cases and in non-survivors.1 , 2 , 6 Direct correlation between serum ferritin concentration and poor survival, as reported by the meta-analysis conducted by Henry et al. (weighted mean difference: 408.28 μg/L, 95%CI: 311.12–505.44 μg/L, Cochran's Q p-value=0.01), suggests its use as a surrogate marker of immune dysregulation and a prognostic marker of disease severity and imminent death.5
Only scarce data have contextualized the erythrocyte sedimentation rate (ESR) kinetic in patients with COVID-19. One study reported that fatal cases had a tendency for higher ESR compared to those who recovered (median ESR 38.5 vs 28 mm/h) without reporting the statistical significance of the observed difference among the two groups.1 A similar trend was also depicted for C-reactive protein (CRP) concentration by the same study, with median levels being 4-fold higher among non-survivors (median concentration 113 vs 26.2 mg/L).1 Between severe and non-severe cases, reported CRP differences are not that striking (median (IQR): 47.6 mg/L (20.6–87.1) vs 28.7 mg/L (9.5–52.1), p<0.001), but significantly increased frequency of higher concentrations among severe and critical cases compared to mild/moderate ones are nevertheless evident (mild/moderate cases: 50.5%, severe cases: 79.2% and critical cases: 92%, p<0.001).3 , 16 Finally, one Chinese study with 663 COVID-19 patients reported that higher CRP levels are inversely associated with disease improvement (odds ratio 4.697, p<0.0001).16
Individual studies demonstrate that greater procalcitonin (PCT) concentrations (usually ≥0.05 ng/ml) can significantly distinguish between non-severely from severely ill and fatal cases, thus possibly acting as a prognostic marker.2, 3, 4 , 6 , 18 , 19 However, a meta-analysis found that severe from non-severe COVID-19 could be differentiated by a marginally higher PCT (by 0.2 ng/ml).5 Increments of both CRP and PCT may be associated, not only with the immense inflammatory response, but also with the higher frequency of bacterial superinfections among critically ill COVID-19 patients (up to 50% rate among non-survivors).5 The differentiation between severe SARS-CoV-2 infection and a bacterial superinfection is often difficult in clinical practice. Though markedly elevated PCT and CRP are consistent with bacterial co-infection, there is not a clear cut-off. Other markers that have been proposed as differentiators between bacterial and viral infections (such as Myxoma resistance protein (MxA1), Lipocalin 2 (Lcn2), High mobility group box one protein (HMGB1)) have not been studied in COVID-19 disease.20
Albumin is a negative acute phase reactant whose synthesis is down-regulated by inflammatory cytokines.21 Therefore, it is not surprising that hypoalbuminemia (usually <30 g/L) has been persistently noticed among patients with severe or fatal COVID-191,2,6,11. Moreover, one study demonstrated that low albumin concentration was associated with lack of disease improvement (odds ratio 2.377, p<0.0001), while hypoalbuminemia was also introduced as a risk factor, among other parameters, in a proposed risk prediction nomogram for severe COVID-19.16,19
Serum amyloid A (SAA) is another acute phase reactant inhibiting monocyte mobilization, platelet activation and various chemotactic pathways.21 High concentrations of SAA among all COVID-19 patients have only been reported by Zang et al., without a significant difference between severe and non-severe cases.3
Cytokines, chemokines, pathology findings and other markers
Exuberant release of pro-inflammatory cytokines is associated with multi-organ injury and acute respiratory distress syndrome (ARDS), which is inevitably fatal if left untreated.22 Fulminant hypercytokinemia has been increasingly recognized among critically ill COVID-19 patients.
Distinct pro-inflammatory cytokines (such as interleukin (IL)−1β, IL-2 and its soluble receptor, IL-6, IL-8, IL-17, Granulocyte colony-stimulating factor (G-CSF), Granulocyte-macrophage colony-stimulating factor (GM-CSF), tumor necrosis factor alpha (TNF-α)), inflammatory chemokines (such as the monocyte chemoattractant protein 1 (MCP1or CCL2) and the macrophage inflammatory protein 1-alpha (MIP-1α or CCL3)), as well as the anti-inflammatory cytokine IL-10, have been consistently found significantly elevated in patients with severe COVID-19, those admitted to ICU or patients who died compared to milder forms of SARS-CoV-2 infection.1 , 2 , 6 , 10 , 11 , 23 Notably, monitoring of IL-6 levels has been proposed as a candidate index for disease progression.5 Moreover, higher of IL-6 to Interferon gamma (IFN-γ) ratios may distinguish severe from moderate COVID-19 cases (standardized mean difference of 0.739, 95% CI = 0.131–1.383). 24 All these data converge into the conclusion that major immune dysregulation occurs in severe COVID-19, leading to many clinical manifestations of the fatal form. Although measurement of these indices is not widely available, following up such markers may be an integral part of relevant prognostic and diagnostic tools.
On the other hand, complement components C3 and C4 and immunoglobulin (IgG, IgM and IgA) levels are not specific markers of the cytokine storm syndrome and the few data that are available showed no clinically significant differences between deceased and recovered patients1. However, exuberant SARS-CoV-2-specific IgG responses were associated with increased disease severity, in a retrospective Chinese study with 222 COVID-19 patients.14
Cytokine storm can certainly, but only partially, explain the observed clinical features of COVID-19 disease. Angiotensin-converting enzyme 2 (ACE2) receptors, the mediators of SARS-CoV-2 invasion into host-cells, are expressed by numerous cells, including endothelium; therefore, direct viral endothelial injury cannot be excluded.25 Indeed, preliminary histopathological data from fatal cases demonstrated lesions consistent with endotheliitis (endothelialitis) in many organs including lungs, small lung vessels’ congestion, mononuclear cell infiltrates within the intima of organs’ vasculature, viral inclusion bodies in peritubular spaces and viral particles in endothelial cells of the glomerular capillary loops.26
Another series reported the identification of C5b-9, C4d and mannose binding lectin-associated serine protease (MASP) 2 terminal complement component deposits in pulmonary microvasculature; furthermore, co-localization of spike glycoproteins with C4d and C5b-9 in inter-alveolar septa and on skin microvasculature were evident is some cases.27 This observation is consistent with a systemic complement activation leading to a catastrophic pauci-inflammatory septal capillary injury and a pro-coagulant state.27 Importantly, hallmarks of classic ARDS such as typical diffuse alveolar damage (DAD) were not prominent.27
Moreover, the connection of viral spike protein to ACE2 receptor, down-regulates ACE2 levels in lungs; this in turn, increases the angiotensin II (AngII) levels, reduces angiotensin 1–7 (Ang-(1–7)), and imbalances the renin-angiotensin system in the lung, leading to vasoconstriction.28 These data are in concordance with a notably distinct type of ARDS with highly compliant lungs, which is seen in a major subset of COVID-19 patients; this manifestation is quite possibly consistent with an underlying vasoconstriction and microvasculature injury leading to loss of lung perfusion regulation29. Though neither histopathology specimens nor lung ACE2 or AngII levels are easily obtainable in daily clinical practice, they would definitely be useful in research settings in order to elucidate the disease's pathophysiology and may assist diagnosis in the future.
Cardiac biomarkers
Cardiac troponin I and T are highly sensitive and specific biomarkers of myocardial injury which can be caused by myocardial ischemia, inflammation, immune response, and toxin. 30 Elevated troponin at admission was observed in more than half of dead patients and associated with increased severity and mortality in COVID-19 patients.1 , 2 , 31 Regardless of underlying cardiovascular disease, patients with dynamic increases of troponin during the hospitalization were more likely to have fatal outcomes. 2 , 31, 32, 33 Although some COVID-19 patients were reported with comorbidity of chronic heart disease, the underlying mechanism for troponin elevation in patients with COVID-19 is not clear. The myocardial injury in COVID-19 patients might associate with a systemic hyperinflammation13 , 31 rather than a virus attack directly. Increased levels of CK-MB and NT-proBNP can also be found in severe COVID-19 patients compared to non-severe patients.4 , 31 , 33
Renal function tests
According to a cohort study 34 of 701 patients with COVID-19, the proportion of proteinuria, hematuria, abnormal serum creatinine and urea nitrogen at admission and were 43.9, 26.7, 14.4 and 13.1%, respectively. In addition, there was a high prevalence (5.1%) of acute kidney injury (AKI) during the study period. The result showed proteinuria, hematuria, and elevated serum creatinine/urea nitrogen at admission and acute kidney injury (AKI) during hospitalization over stage 2 were associated with in-hospital death. However, the other largest retrospective study to date found that the prevalence of serum creatinine abnormalities and AKI was only 1.6% and 0.5%.18 This may be due to the different proportions of severe patients between the two studies and the different definitions of the normal reference range for serum creatinine. From the result of autopsy of 26 COVID-19 patients,35 the histopathology of the kidney revealed significant acute tubular injury and found that the tubular epithelial cells were directly infected by SARS-CoV2. Therefore, SARS-CoV2 may cause kidney injury or exacerbate existing kidney disease. Attention should be paid to monitoring renal function and the occurrence of AKI.
Liver function tests
Abnormal liver function tests, such as increased levels of ALT, AST, TBIL, GGT and decreased level of albumin were relatively common in patients with COVID-19, and 10–33% of these patients had abnormal ALT or AST.2 , 4 , 18 , 31 , 36 Although patients with severe COVID-19 seem to have higher rates of liver dysfunction, it is reassuring that the levels of ALT, AST, TBIL, GGT in COVID-19 patients were not significantly different in compared with hospitalized community-acquired pneumonia patients and even the median or average transaminase level in severe COVID-19 patients was lower than twice upper reference limit.4 , 36 , 37 Therefore, the clinical effect of these elevated indicators may not be evident in COVID-19 patients. Liver dysfunction may be related to severe infection, inflammation induced liver injury, medication associated hepatotoxicity and hypoxia.38
Coagulation profile
D-dimer is a degradation product of fibrin. Elevated D-dimer levels were consistently reported in COVID-19 patients with prevalence ranging from 43 to 68%.2 , 3 , 31 D-dimer>1 ng/ml at admission were associated with increased severity and odds of death with COVID-19, and the gradual increasing of D-dimer during disease course was particularly associated with disease worsening and mortality.2 , 4 Serum D-dimer can reflect fibrinolytic activities and is also an inflammatory biomarker. Furtherly, recent studies found that severe cases of COVID-19 were commonly complicated with thrombosis,39 , 40 markedly elevated D-dimer was related to thrombosis and poor prognosis of severe COVID-19 patients. Prothrombin time (PT) reflects the activity of exogenous coagulation factors. COVID-19 associated lung tissue damage may induce the release of tissue factors to circulation and promotes secondary fibrinolysis through exogenous coagulation pathways. This may explain the elevated D-dimer and prolonged PT in COVID-19 as well as CAP patients.2 , 4 , 18 , 31 , 36 The fibrinogen is a kind of coagulation factor, but also an acute phase protein 41. It can be induced by infection or other stress factors.42 Several literatures reported fibrinogen levels was elevated in severe patients or non-survivors with COVID-19.43 , 44 However, Du et al. found fibrinogen increased in 47.1% of fatal cases and decreased in 22.4% of fatal cases.45 In fact, the fibrinogen would decrease when excessive consumption happened due to hypercoagulability or the worst disseminated intravascular coagulation occurred. Hence, the abnormality of the coagulation profile should be interpreted individually.
Biochemistry markers and arterial blood gases
Lactate dehydrogenase (LDH) is a cytoplasmic enzyme that is present in every tissue, and high serum concentrations indicate underlying organ damage. Thus, LDH is expected to rise in severe COVID-19 cases, where multi-organ damage occurs.23 Current data support that critically ill patients as well as fatal cases of COVID-19 have significantly higher LDH levels (usually >320 U/L) compared to moderate infections.1 , 2 , 5 , 6 , 11 , 16 Moreover, higher LDH quadruples the odds for lack of disease improvement (odds ratio: 4.381. p<0.0001).16 Lastly, greater LDH concentrations upon admission correlate with a higher risk for serious COVID-19, and therefore it has been added in a proposed early predictive tool for severe infection.19 These data favor the utilization of LDH as a candidate prognostic marker for disease severity.
Hypertriglyceridemia is commonly encountered in hyperinflammatory states, like the CSS and the secondary HLH, due to the reduced lipoprotein lipase activity driven by the high TNF-α levels.46 Therefore, triglyceride concentration is a key component of the HScore [http://saintantoine.aphp.fr/score/] that is currently being proposed by the European Society of Intensive Care Medicine as a predictive tool for SARS-CoV-2-driven sHLH in COVID-1922,47 However, only scarce data on triglycerides levels in COVID-19 disease are currently available; one study reported higher concentrations in fatal cases as compared to patients who survived the disease (median 1.8 vs 1.2 mmol/L), without stating the statistical significance of this finding1. The same study demonstrated lower thyroid stimulating hormone and free triiodothyronine concentrations in deceased patients, possibly due to critical illness-associated eythyroid sick syndrome1.
Hyponatremia is a known sequela of lower respiratory tract infections, that is possibly induced by the inappropriate secretion of anti-diuretic hormone.48, 49, 50 However, not many studies currently report measurement of electrolytes, including sodium. Among these studies, none has depicted statistically or clinically significant differences of sodium or potassium concentrations between severe/fatal and less severe COVID-19 patient groups.1 , 11 , 18 , 51
Although acid-base balance disturbances are expected among COVID-19 patients with multi-organ injury, few data are available; Zhou et al. disclosed significantly higher frequency of acidosis in non-survivors compared to survivors (30% vs 1% respectively, p<0.0001), while Chen et al. reported lower bicarbonate concentration in patients who died, without reporting the statistical significance of this finding.1 , 2 Importantly, but not surprisingly, in the latter study more than 50% of the deceased patients had arterial partial pressure of oxygen (PaO2) of <60 mmHg (compared to 0% in the survivor group), while none in the same group had a partial pressure of oxygen to fraction of inspired oxygen ratio (PaO2:FiO2) of >3001. Hence, arterial blood gases constitute important prognostic tools for disease severity and poor outcomes, as they are directly associated with the degree of functional lung damage.
Conclusion
Recent clinical research among COVID-19 patients indicates that SARS-CoV-2 infection causes systemic disease, involving multiple organs and systems, including hyperactivation of the immune system, the nervous system and the clotting system. These in turn leading to pathologies in several organs, including the heart, liver and kidneys. In order to stratify patients at risk and to monitor high risk patients at intensive care units, tight laboratory diagnostics provide instrumental information. Laboratory tests can be used as prognostic markers for increased risk and mortality. The spectrum of currently available biomarkers is sufficient to fullfill this purpose. A major limitation of available studies is that the time point of sampling/biomarker assessment since onset of symptoms and/or presentation at health care facilities is not clearly mentioned. Furthermore, currently, there are no internationally acceptable criteria regarding disease severity, which renders evaluation of data quite subjective, depending on individual study investigations. Over the next months and years, with the use of further knowledge on the pathogenesis of SARS-CoV-2 infections, an even more comprehensive list of suitable biomarkers will be developed (Fig. 1 and Tables 1, 2).
Declaration of Competing Interest
CS and HR received consultancy fees and research funding from Hycor Biomedical and Thermo Fisher Scientific, research funding from Mead Johnson Nutrition (MJN), and consultancy fees from Bencard Allergie.
Footnotes
Harald Renz is funded by the Universities Giessen Marburg Lung Center and the German Center for Lung Disease (DZL German Lung Center, no. 82DZL00502) for UGMLC.
Chrysanthi Skevaki is funded by Universities Giessen and Marburg Lung Center and the German Center for Lung Research, University Hospital Gießen and Marburg (UKGM) research funding according to article 2, section 3 cooperation agreement, the Deutsche Forschungsgemeinschaft (DFG)–funded- SFB 1021 (C04), -KFO 309 (P10) and SK 317/1–1 (Project number 428518790).
Paraskevi C Fragkou is funded by a doctorate scholarship by the State Scholarships Foundation (IKY), Partnership Agreement (PA) 2014–2020, co-financed by Greece and the European Union (European Social Fund - ESF) through the Operational Program «Human Resources Development, Education and Lifelong Learning 2014–2020
References
- 1.Chen T., Wu Di, Chen H. Clinical characteristics of 113 deceased patients with coronavirus disease 2019, retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China, a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang J.-.J., Dong X., Cao Y.-.Y. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;00:1–12. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 4.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henry B.M., Oliveira MHS de, Benoit S., Plebani M., Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19), A meta-analysis. Clin Chem Lab Med. 2020;58:1021–1028. doi: 10.1515/cclm-2020-0369. [DOI] [PubMed] [Google Scholar]
- 6.Chen G., Wu Di, Guo W. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130:2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fan B.E., Chong V.C.L., Chan S.S.W. Hematologic parameters in patients with COVID-19 infection. Am J Hematol. 2020;95:E131–E134. doi: 10.1002/ajh.25774. [DOI] [PubMed] [Google Scholar]
- 8.Chng W.J., Lai H.C., Earnest A., Kuperan P. Haematological parameters in severe acute respiratory syndrome. Clin Lab Haematol. 2005;27:15–20. doi: 10.1111/j.1365-2257.2004.00652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee N., Hui D., Wu A. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348:1986–1994. doi: 10.1056/NEJMoa030685. [DOI] [PubMed] [Google Scholar]
- 10.Cao X. COVID-19, Immunopathology and its implications for therapy. Nat Rev Immunol. 2020;20:269–270. doi: 10.1038/s41577-020-0308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu Z., Shi L., Wang Y. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qin C., Zhou L., Hu Z. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang B., Zhou X., Zhu C., et al. Immune phenotyping based on neutrophil-to-lymphocyte ratio and IgG predicts disease severity and outcome for patients with COVID-19, 2020. [DOI] [PMC free article] [PubMed]
- 15.Nemeth E., Ganz T. Anemia of inflammation. Hematol Oncol Clin North Am. 2014;28:671–681. doi: 10.1016/j.hoc.2014.04.005. vi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang J., Wang X., Jia X. Risk factors for disease severity, unimprovement, and mortality of COVID-19 patients in Wuhan, China. Clin Microbiol Infect. 2020;26:767–772. doi: 10.1016/j.cmi.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kernan K.F., Carcillo J.A. Hyperferritinemia and inflammation. Int Immunol. 2017;29:401–409. doi: 10.1093/intimm/dxx031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guan W.-.J., Ni Z.-.Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gong J., Ou J., Qiu X. A tool to early predict severe corona virus disease 2019 (COVID-19), a multicenter study using the risk nomogram in Wuhan and Guangdong, China. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomas J., Pociute A., Kevalas R., Malinauskas M., Jankauskaite L. Blood biomarkers differentiating viral versus bacterial pneumonia aetiology, A literature review. Ital J Pediatr. 2020;46:4. doi: 10.1186/s13052-020-0770-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gulhar R., Ashraf M.A., StatPearls I.Jialal. StatPearls Publishing. Treasure Island (FL); 2020. Physiology, Acute Phase Reactants. [PubMed] [Google Scholar]
- 22.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19, consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu D., Yang X.O. TH17 responses in cytokine storm of COVID-19, An emerging target of JAK2 inhibitor Fedratinib. J Microbiol Immunol Infect. 2020;53:368–370. doi: 10.1016/j.jmii.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lagunas-Rangel F.A., Chávez-Valencia V. High IL-6/IFN-γ ratio could be associated with severe disease in COVID-19 patients. J Med Virol. 2020 doi: 10.1002/jmv.25900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferrario C.M., Jessup J., Chappell M.C. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111:2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- 26.Varga Z., Flammer A.J., Steiger P. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Magro C., Mulvey J.J., Berlin D. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection, A report of five cases. Transl Res. 2020;220:1–13. doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li S.-.R., Tang Z.-.J., Li Z.-.H., Liu X. Searching therapeutic strategy of new coronavirus pneumonia from angiotensin-converting enzyme 2, The target of COVID-19 and SARS-CoV. Eur J Clin Microbiol Infect Dis. 2020;39:1021–1026. doi: 10.1007/s10096-020-03883-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gattinoni L., Coppola S., Cressoni M., Busana M., Rossi S., Chiumello D. Covid-19 does not lead to a "Typical" acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020;201:1299–1300. doi: 10.1164/rccm.202003-0817LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mair J., Lindahl B., Hammarsten O. How is cardiac troponin released from injured myocardium? Eur Heart J Acute Cardiovasc Care. 2018;7:553–560. doi: 10.1177/2048872617748553. [DOI] [PubMed] [Google Scholar]
- 31.Li X., Xu S., Yu M. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020 doi: 10.1016/j.jaci.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo T., Fan Y., Chen M. Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deng Q., Hu B., Zhang Y. Suspected myocardial injury in patients with COVID-19, Evidence from front-line clinical observation in Wuhan, China. Int J Cardiol. 2020;311:116–121. doi: 10.1016/j.ijcard.2020.03.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheng Y., Luo R., Wang K. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97:829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Su H., Yang M., Wan C. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98:219–227. doi: 10.1016/j.kint.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Y., Zheng L., Liu L., Zhao M., Xiao J., Zhao Q. Liver impairment in COVID-19 patients, A retrospective analysis of 115 cases from a single center in Wuhan city, China. Liver Int. 2020;00:1–9. doi: 10.1111/liv.14455. [DOI] [PubMed] [Google Scholar]
- 37.Bangash M.N., Patel J., Parekh D. COVID-19 and thE liver, Little cause for concern. Lancet Gastroenterol Hepatol. 2020;5:529–530. doi: 10.1016/S2468-1253(20)30084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang C., Shi L., Wang F.-.S. Liver injury in COVID-19, Management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428–430. doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tavazzi G., Civardi L., Caneva L., Mongodi S., Mojoli F. Thrombotic events in SARS-CoV-2 patients, An urgent call for ultrasound screening. Intensive Care Med. 2020;46:1121–1123. doi: 10.1007/s00134-020-06040-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fish R.J., Neerman-Arbez M. Fibrinogen gene regulation. Thromb Haemost. 2012;108:419–426. doi: 10.1160/TH12-04-0273. [DOI] [PubMed] [Google Scholar]
- 42.Amaral A., Opal S.M., Vincent J.-.L. Coagulation in sepsis. Intensive Care Med. 2004;30:1032–1040. doi: 10.1007/s00134-004-2291-8. [DOI] [PubMed] [Google Scholar]
- 43.Feng Y., Ling Y., Bai T. COVID-19 with different severity, a multi-center study of clinical features. Am J Respir Crit Care Med. 2020;201:1380–1388. doi: 10.1164/rccm.202002-0445OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Du Y., Tu L., Zhu P. Clinical features of 85 fatal cases of COVID-19 from Wuhan, a retrospective observational study. Am J Respir Crit Care Med. 2020;201:1372–1379. doi: 10.1164/rccm.202003-0543OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.George M.R. Hemophagocytic lymphohistiocytosis, Review of etiologies and management. J Blood Med. 2014;5:69–86. doi: 10.2147/JBM.S46255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.ESICM. Important Announcement of new SSC Guidelines – COVID-19. (Accessed April 27, 2020, at https://www.esicm.org/ssc-covid19-guidelines/).
- 48.Dixon B.S., Anderson R.J. Pneumonia and the syndrome of inappropriate antidiuretic hormone secretion, Don't pour water on the fire. Am Rev Respir Dis. 1988;138:512–513. doi: 10.1164/ajrccm/138.3.512. [DOI] [PubMed] [Google Scholar]
- 49.Hausman-Kedem M., Reif S., Danino D. Mechanism of hyponatremia in community-acquired pneumonia, does B-type natriuretic peptide play a causative role? Pediatr Emerg Care. 2018;34:641–646. doi: 10.1097/PEC.0000000000000814. [DOI] [PubMed] [Google Scholar]
- 50.Shepshelovich D., Leibovitch C., Klein A. The syndrome of inappropriate antidiuretic hormone secretion, distribution and characterization according to etiologies. Eur J Intern Med. 2015;26:819–824. doi: 10.1016/j.ejim.2015.10.020. [DOI] [PubMed] [Google Scholar]
- 51.Xu X.-.W., Wu X.-.X., Jiang X.-.G. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China, Retrospective case series. BMJ. 2020;368:m606. doi: 10.1136/bmj.m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rovina N., Akinosoglou K., Eugen-Olsen J. Soluble urokinase plasminogen activator receptor (suPAR) as an early predictor of severe respiratory failure in patients with COVID-19 pneumonia. Crit Care. 2020;24(1):187. doi: 10.1186/s13054-020-02897-4. [DOI] [PMC free article] [PubMed] [Google Scholar]