Dear editor:
Since the first case of novel coronavirus disease 2019 (COVID-19) was identified in December 2019 in Wuhan (Hubei, China), the virus has continued to spread around the world. Patients infected with the novel coronavirus, designated as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), develop the disease COVID-19, which can cause severe pneumonia and damage the liver, heart and kidneys. The inflammatory cytokine storm has been recognized as the primary cause of death [1], which is defined by the excessive and uncontrolled release of pro-inflammatory cytokines, as has been reported in other infections caused by pathogenic coronaviruses [2]. For instance, inflammatory cytokines released by macrophages (IL-6, IL-10, and TNF-α) increase in patients with severe COVID-19 disease, resulting in damage to the lungs and other organs [1]. Consequently, measurements of plasma inflammatory markers could be useful for predicting the disease progress.
Studies on COVID-19 patients have reported the levels of some inflammatory markers such as procalcitonin, C-reactive protein, erythrocyte sedimentation rate and serum amyloid A. However, little attention has been paid to ferritin, even though hyperferritinemia has been shown to be associated with complications in other viral diseases such as dengue fever [3]. In order to determine if the circulating ferritin concentration could be used to predict COVID-19 progression, and to associate hyperferritinemia with the development of the cytokine storm, we reviewed all published studies that documented serum ferritin levels in patients with severe and non-severe COVID-19 disease, along with other inflammatory factors, which are summarized in Table 1 [1], [2], [3], [4], [5], [6], [7], [8]. Table 1 also includes studies reporting ferritin and cytokine levels in COVID-19 survivors and non-survivors.
Table 1.
Ferritin levels on hospital admission among patients with non-severe and severe COVID-19 disease (and survivors and non-survivors).
| Non-severe disease |
Severe disease |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hospital | Timeline | Sample size | COVID-19 diagnosis | Severity classification | N | Avg. age | Comor-bidity | Avg. ferritin (μg/L) | N | Avg. age | Comor-bidity | Avg. ferritin (μg/L) | P value | Comments | Ref. |
| Tongji Wuhan China |
Late Dec to Jan 27, 2020 | 21 | RT-PCR assay for respiratory specimens | NHCCguidelines | 10 | 52 | 20% | 337.4 | 11 (4 died) |
61 | 46% | 1598.2 | 0.049 | Ferritin higher than 800 μg/L in 100% of patients with severe and 30% of patients with non-severe disease. Cytokines (IL-2R, IL-6, IL-10, and TNF-α) and CRP were high. | [1] |
| Tongji Wuhan China | Jan 10 to Feb 12, 2020 | 452 | RT-PCR assay for respiratory specimens | NHCC guidelines | 286 | 53 | 33% | 523.7 | 166 | 61 | 51% | 800.4 | <0.001 | Cytokines (IL-2R, IL-6, which was really high, IL-8 and IL-10) higher in patients with severe disease. EDR and CRP were high. | [2] |
| PLA General Beijing China | Jan 20 to Feb 16, 2020 | 49 | RT-PCR assay for respiratory specimens | NHCC guidelines | 34 | 38 | 9% | 318.1 | 15 (1 died) |
57 | 80% | 907.4 | <0.001 | Ferritin > 400 μg/L (Hazard Ratio: 7.1) as risk factor for progression to severe disease | [3] |
| Union Wuhan China | Jan 5 to Jan 24, 2020 | 40 | RT-PCR assay for respiratory specimens | NHCC guidelines | 27 | 43 | 26% | 367.8 | 13 (2 died) |
60 | 54% | 835.5 | 0.015 | Cytokines (IL-2, IL-6, IL-10, IFN-γ) and CRP were higher for patients with severe disease. | [4] |
| Union Wuhan China | Jan 21 to Feb 16, 2020 | 80 | RT-PCR assay for respiratory specimens | NHCC guidelines | 11 | 31 | 28% | 155.7 | 69 | 56 | 36% | 827.2 | <0.001 | CRP, ESR higher for patients with severe disease. IL-6 correlated to lung damage, body temperature, CRP and ferritin. With the remission of disease, ferritin, CRP and IL-6 decreased. | [5] |
| Tongji Wuhan China | Jan 13 to Feb 12, 2020 | 274 | RT-PCR assay for respiratory specimens | Survivors vs. non-survivors | 161 | 51 | 39% | 481.2 | 113 | 68 | 63% | 1418.3 | – | CRP, ESR and cytokines (IL-6, IL-10 and TNF-α) were higher among non-survivors | [6] |
| Jinyintan Wuhan China | Dec 26, to Jan 31, 2020 | 127 | RT-PCR assay for respiratory specimens | Survivors vs. non-survivors | 91 | 50 | 23% HT, 11% D | ≈500 | 36 | 67 | 42% HT, 14% D | ≈1900 | <0.001 | Non-survivors had higher CRP and IL-6 on-admission, but not ESR (it was high for both groups) | [7] |
| Jinyintan and Wuhan Pulmo-nary Wuhan China | Dec 29, 2019 to Jan 31, 2020 | 191 | SARS-CoV-2 detection in respiratory specimens according to WHO | Survivors vs. non-survivors | 137 | 52 | 40% | 503.2 | 54 | 69 | 67% | 1435.3 | <0.001 | Ferritin on N = 128. Ferritin > 300 μg/L in 71% of survivors and 96% of non-survivors. Ferritin and IL-6 showed higher levels in non-survivors throughout the clinical course, and increased with disease deterioration. | [8] |
CRP: C-reactive protein; D: diabetes; EDR: erythrocyte sedimentation rate; HT: hypertension; NHCC: National Health Commission of China; RT-PCR: real-time reverse transcription polymerase chain reaction; WHO: World Health Organization. P value refers to the difference in ferritin between the 2 groups. Ferritin normal range: 30–400 μg/L [3].
It should be noticed that most of the studies presented in Table 1 were retrospective in design and were performed at single centers in Wuhan. These studies report ferritin concentrations of COVID-19 patients only at the time of hospital admission. It can be observed that the concentrations of ferritin are generally within the normal range (30–400 μg/L [3]) in patients with non-severe disease (according to the National Health Commission of China guidelines for COVID-19 severity classification). However, hyperferritinemia (ferritin level > 400 μg/L), was observed in patients with severe disease on admission. In fact, the average ferritin concentration was >800 μg/L for patients with severe disease. Moreover, ferritin levels on admission were between 1.5 and 5.3 times higher in patients classified with severe disease in comparison to patients with less-severe COVID-19 disease. Table 1 also presents studies comparing ferritin levels on admission between COVID-19 patients that did not survive and died at the hospital and patients that were discharged after being successfully treated. These studies reported that non-survivors showed ferritin levels on admission around 1400 μg/L, which is between 3 and 4 times higher than that observed in survivors. These studies also reported the levels of serum cytokines such as IL-6, which are especially high on admission in those patients developing severe disease. One study reported that both ferritin and IL-6 concentrations showed higher values in non-survivors in comparison to discharged patients throughout the clinical course, and increased as the patient deteriorates [8]. Liu et al. reported that, when patients began to recover, the ferritin and IL-6 concentrations decreased [5]. This may confirm that hyperferritinemia is associated with inflammatory states in SARS-CoV-2 infection, and therefore, ferritin can be a useful parameter to predict disease severity and the extent of the cytokine storm.
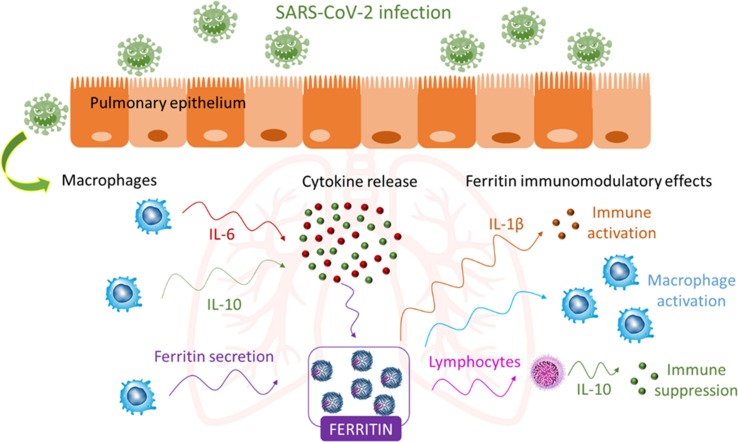
However, we should ask what the source of the increased plasma ferritin concentration is and the potential role of this protein during inflammation following COVID-19 disease development. Active ferritin production during the course of inflammatory diseases can occur (Fig. 1 ). Macrophages, which produce cytokines and account for the majority of the immune cells in the lung parenchyma, might be responsible of the secretion of serum ferritin [9]. Moreover, ferritin synthesis can be induced by several inflammatory stimuli including cytokines, such as IL-6 [9]. Interestingly, high IL-6 concentrations in COVID-19 patients have been correlated to disease severity [5]. Thus, since ferritin might be actively secreted at the site of infection, it is possible that ferritin can assume other functions apart from its classic role as an iron storage protein. Accumulated data have implicated a role for ferritin as a signaling molecule and direct mediator of the immune system [9]. Complex feedback mechanisms between ferritin and cytokines in the control of pro-inflammatory and anti-inflammatory mediators might exist as cytokines can induce ferritin expression, but ferritin can induce the expression of pro- and anti-inflammatory cytokines as well, as presented in Fig. 1 [9]. A debate between different schools of thought exists regarding the pathogenic role of ferritin during inflammation [10]. An interesting area for future research would be the analysis of the structure of plasma ferritin in COVID-19 patients. Ferritin is composed of 2 different subunits, H and L. Different studies have suggested that H subunit expression is driven by inflammatory stimuli and H-ferritin may work as an immunomodulatory molecule, displaying both pro-inflammatory and immunosuppressive functions [9], [10].
Fig. 1.
Potential role of ferritin during inflammation following COVID-19 infection. Active ferritin production by macrophages and cytokines may lead to hyperferritinemia, which in turn, might promote the production of several pro-inflammatory (IL-1β) and anti-inflammatory cytokines (IL-10) [9], [10].
Finally, if ferritin is involved as a pathogenic mediator in COVID-19, techniques such as therapeutic plasma exchange might be beneficial for SARS-CoV-2 infected patients as this will decrease the levels of ferritin and cytokines. Plasma exchange is an automated process whereby the patient’s plasma is removed and replaced by donor plasma from the blood bank, and has been shown to be very beneficial in certain diseases. Lastly, it is worth mentioning that most of the studies presented in Table 1 were performed in Wuhan during the early phase of the outbreak, where hospitals with inadequate medical facilities and insufficient staff were overwhelmed with patients. These patients were on the vanguard of the pandemic, and SARS-CoV-2 itself may have had changes in virulence during human-to-human dissemination. Therefore, hyperferritinemia in affected patients should be further verified in multi-center studies with larger sample sizes and performed in other countries. Nevertheless, we believe that longitudinal monitoring of ferritin during hospitalization may help to identify severe patients and predict the progression of COVID-19 towards a worse clinical prognosis.
Acknowledgements
Financial support from the National Heart, Lung, and Blood Institute from the United States National Institutes of Health (1R01HL131720-01A1) is acknowledged. The authors are also grateful to all the healthcare operators as well as all the essential workers on the frontlines during the COVID-19 pandemic.
References
- 1.Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H., Wang T., Zhang X., Chen H., Yu H., Zhang X., Zhang M., Wu S., Song J., Chen T., Han M., Li S., Luo X., Zhao J., Ning Q. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Invest. 2020 doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y., Xie C., Ma K., Shang K., Wang W., Tian D.S. Dysregulation of Immune Response in Patients With Coronavirus 2019 (COVID-19) in Wuhan, China. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ji D., Zhang D., Chen Z., Xu Z., Zhao P., Zhang M., Zhang L., Cheng G., Wang Y., Yang G., Liu H., Li B., Ji J., Lau G., Qin E. Clinical characteristics predicting progression of COVID-19. Lancet. 2020 doi: 10.2139/ssrn.3539674. [DOI] [Google Scholar]
- 4.Liu J., Li S., Liu J., Liang B., Wang X., Wang H., Li W., Tong Q., Yi J., Zhao L., Xiong L., Guo C., Tian J., Luo J., Yao J., Pang R., Shen H., Peng C., Liu T., Zhang Q., Wu J., Xu L., Lu S., Wang B., Weng Z., Han C., Zhu H., Zhou R., Zhou H., Chen X., Ye P., Zhu B., He S., He Y., Jie S., Wei P., Zhang J., Lu Y., Wang W., Zhang L., Li L., Zhou F., Wang J., Dittmer U., Lu M., Hu Y., Yang D., Zheng X. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;55 doi: 10.1016/j.ebiom.2020.102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.T. Liu, J. Zhang, Y. Yang, H. Ma, Z. Li, J. Zhang, J. Cheng, X. Zhang, Y. Zhao, Z. Xia, L. Zhang, G. Wu, J. Yi, The potential role of IL-6 in monitoring severe case of coronavirus disease 2019. Available online: https://www.medrxiv.org/content/10.1101/2020.03.01.20029769v2 (accessed on May 24, 2020). [DOI] [PMC free article] [PubMed]
- 6.Chen T., Wu D., Chen H., Yan W., Yang D., Chen G., Ma K., Xu D., Yu H., Wang H., Wang T., Guo W., Chen J., Ding C., Zhang X., Huang J., Han M., Li S., Luo X., Zhao J., Ning Q. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368 doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bai T., Tu S., Wei Y., Xiao L., Jin Y., Zhang L., Song J., Liu W., Zhu Q., Yang L., Chen H., Hou X. Clinical and laboratory factors predicting the prognosis of patients with COVID-19: an analysis of 127 patients in Wuhan, China. Lancet. 2020 doi: 10.2139/ssrn.3546118. [DOI] [Google Scholar]
- 8.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosário C., Zandman-Goddard G., Meyron-Holtz E.G., D’Cruz D.P., Shoenfeld Y. The Hyperferritinemic Syndrome: macrophage activation syndrome, Still’s disease, septic shock and catastrophic antiphospholipid syndrome. BMC Med. 2013;11:185. doi: 10.1186/1741-7015-11-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kernan K.F., Carcillo J.A. Hyperferritinemia and inflammation. Int. Immunol. 2017;29:401–409. doi: 10.1093/intimm/dxx031. [DOI] [PMC free article] [PubMed] [Google Scholar]