To the Editor
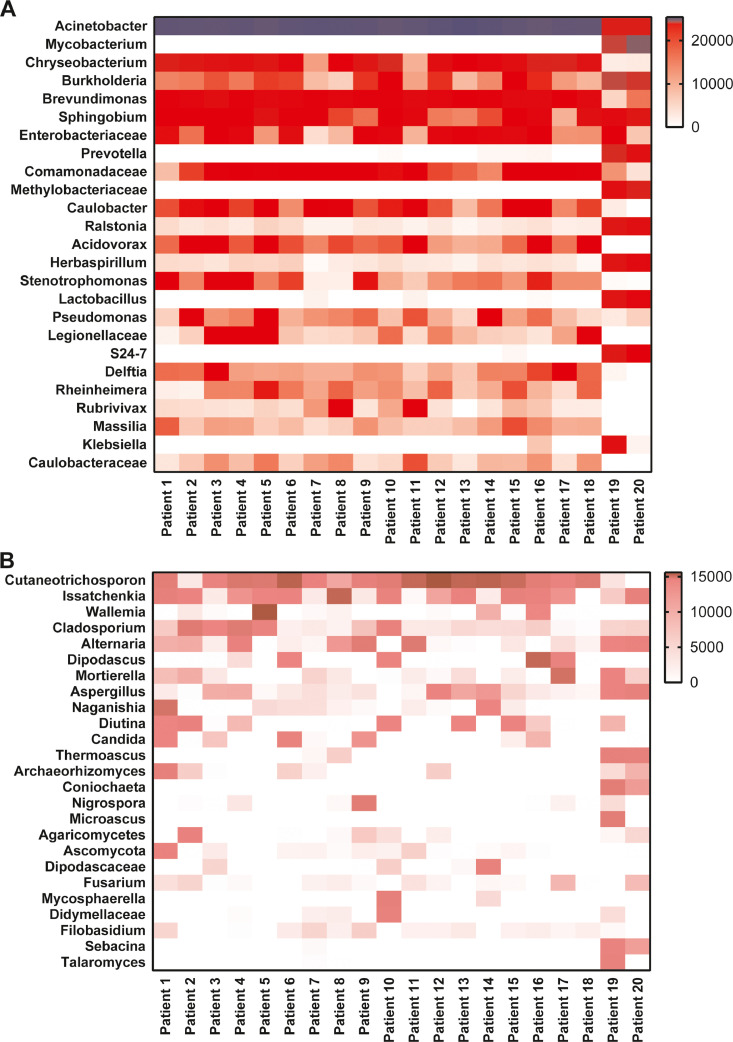
We read with great interest the article by Yanan Chu and colleagues, accepted for publication in the Journal of Infection.1 Secondary infection and sepsis are common complications in critically ill patients with COVID-19,2 , 3 but the underlying pathogen is not clear. We investigated the microbiota characteristics of lung tissue from 20 deceased COVID-19 patients. All cases met the COVID-19 clinical diagnostic criteria provided by the National Health Commission of China, and died at Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, between February 1, 2020, and March 31, 2020. The median age was 66 years (interquartile range, IQR, 60.75–77.0 years), 16 (80%) were older than 60 years and 14 (70.0%) were male. One patient had a history of exposure to the Huanan seafood market. On admission, most patients had fever (15 [75.0%]) and cough (15[75.0%]). Half of the patients had dyspnoea (10 [50.0%]) and fatigue or myalgia (10 [50.0%]). One-fifth of the patients had diarrhoea (4 [20%]). Other symptoms included chest pain, vomiting and headache. All 20 (100.0%) patients had findings of bilateral infiltrates on radiographic imaging. Fifteen (75.0%) patients had comorbidities, including cardiovascular disease (10 [50%]), hypertension (9 [45%]), malignancy (7 [35.0%]), diabetes (2 [10.0%]), chronic kidney disease (2 [10.0%]), and chronic lung disease (1 [5%]). The median time from symptom onset to hospital admission was 10 days (IQR, 6.75–14 days). The median time from hospital admission to death was 23 days (IQR, 20–30 days). Measures of vital signs were recorded on the day of hospital admission for all patients. The patients often developed tachypnoea, and the median respiratory rate was 25 (IQR, 20–30 breaths per minute). The patients had a median percutaneous oxygen saturation of 92% (IQR, 86.8%−97.3%)) on admission. The most common complications were respiratory failure (20 [100%]) and sepsis (18 [90%]), followed by liver dysfunction (17 [85%]), acute respiratory distress syndrome (14 [70%]), acute kidney injury (14 [70%]) and acute cardiac injury (14 [60%]). All patients received antibacterial therapy (20 [100%]) and antiviral therapy (20 [100%]). Most patients received glucocorticoid therapy (18 [90%]), and 12 patients received antifungal therapy (60%). In terms of ventilation modes, 4 patients (20%) received noninvasive ventilation, and invasive mechanical ventilation was required in 16 patients (80%). A total of 5 patients (25%) received continuous kidney replacement therapy (Table 1 ). In agreement with previous studies, our study confirmed that older men (>65 years) and those with comorbidities (especially malignancy) were more vulnerable to SARS-CoV-2 infection. Notably, sepsis was the most frequently observed complication. Immediately after death, postmortem needle core biopsies were performed on bilateral lungs in the negative-pressure isolation ward. The procedures were guided with ultrasound, and specimens were collected aseptically. Tissue cores were fixed in 10% neutral formalin immediately after being removed from the body, fixed for over 24 h, dehydrated in a graded series of ethanol, cleared in xylene, and then embedded in paraffin. A QIAamp DNA Mini Kit (QIAGEN, Hilden, Germany) was used to extract the total DNA from every FFPE tissue. The V3-V4 regions of the 16S rRNA samples were amplified by PCR, and the specific primers were 338F: 5-ACTCCTACGGGAGGCAGCA-3 and 806R: 5-GGACTACHVGGGT WTCTAAT-3. The ITS1 genes were amplified using barcoded ITS 5F: 5-GGAAGTAAAAGTCGTAACAAGG-3 and ITS1R: 5-GCTGCGTTCTTCATCGATGC-3. The quantified amplicons were pooled in equal amounts, and pair-end 2 × 300 bp sequencing was performed using the Illumina MiSeq platform with a MiSeq Reagent Kit v3. The sequencing data were analysed using QIIME V1.8.0 package. The bacterial distribution was characterized by the relative abundance of operational taxonomy units (OTUs). The most prevalent genera were Acinetobacter (80.70% of the total sequences), Chryseobacterium (2.68%), Burkholderia (2.00%), Brevundimonas (1.18%), Sphingobium (0.93%), and Enterobacteriaceae (0.68%), together comprising 92.32% of the total sequences and regularly detected in all subjects. Mycobacterium (3.59%) and Prevotella (0.56%) were detected mainly in patients 19 and 20(Fig. 1 A). The fungal community in the lung microbiome of each patient was analysed by ITS gene sequencing. The most common genus was Cutaneotrichosporon (Cryptococcus, 28.14%), followed by Issatchenkia (8.22%), Wallemia (4.77%), Cladosporium (4.67%), Alternaria (4.46%), Dipodascus (4.01%), Mortierella (3.22%), Aspergillus (2.72%), Naganishia (2.53%), Diutina (2.15%), and Candida (1.42%). Each patient's fungal infection status is shown in a heat map (Fig. 1B). More remarkably, the vast majority of patients had mixed bacterial and fungal infections.
Table 1.
Demographic Characteristics of fatal Patients With COVID-19.
| Characteristics | n = 20, n (%) |
|---|---|
| Median (IQR) age, years | 66 (60.75–77) |
| <40 years | 1 (5) |
| 40–60 years | 3 (15) |
| ≥60 years | 16 (80) |
| Sex | |
| Female | 6 (30) |
| Male | 14 (70) |
| Huanan Seafood Market exposure | 1 (5) |
| Initial common symptoms | |
| Fever | 15 (75) |
| Cough | 15 (75) |
| dyspnea | 10 (50) |
| Chest pain | 1 (5) |
| Headache | 1 (5) |
| Vomiting | 1 (5) |
| Diarrhoea | 4 (20) |
| Fatigue or myalgia | 10 (50) |
| Chest imaging, infiltratea | |
| Unilateral | 0 (0) |
| Bilateral | 20 (100) |
| Comorbidities | |
| Hypertension | 9 (45) |
| Diabetes | 2 (10) |
| Cardiovascular disease | 10 (50) |
| Chronic lung diseases | 1 (5) |
| Chronic kidney disease | 2 (10) |
| Malignancy | 7 (35) |
| Median (IQR) time from onset of symptom to hospital admission, days | 10 (6.75–14) |
| Median (IQR) time from hospital admission to death, days | 23 (20–30) |
| Vital signs on admission | |
| Disorders of consciousness | 0 (0) |
| Median (IQR) arterial pressure, mm Hg | 137 (120–150) |
| Median (IQR) heart rate, beat per minute | 91 (79–103) |
| Median (IQR) respiratory rate, breaths per minute | 25 (20–30) |
| Median (IQR) percutaneous oxygen saturation, | 92% (86.8%−97.3%) |
| Complications | |
| Sepsis | 18 (90) |
| Respiratory failure | 20 (100) |
| Acute respiratory distress syndrome | 14 (70) |
| Liver dysfunction | 17 (85) |
| Acute kidney injury | 14 (70) |
| Acute cardiac injury | 12 (60) |
| Treatment | |
| Antibacterial therapy | 20 (100) |
| Antiviral therapy | 20 (100) |
| Antifungal therapy | 12 (60) |
| Glucocorticoids therapy | 18 (90) |
| Intravenous immunoglobulin therapy | 11 (55) |
| Interferon inhalation | 3 (15) |
| Oxygen treatment | |
| High flow nasal cannula | 1 (5) |
| Noninvasive ventilation | 3 (15) |
| Invasive mechanical ventilation | 16 (80) |
| Continuous renal replacement therapy | 5 (25) |
| Extracorporeal membrane oxygenation | 0 (0) |
Fig. 1.
(A) Distribution of bacterial genera (OTUs) found in the lung samples of fatal COVID-19 patients defined by sequencing of the 16S rRNA gene. (B) Distribution of fungal genera (OTUs) found in the lung samples of fatal COVID-19 patients defined by sequencing of the ITS gene. Increasing depth of colour indicates the relative abundance of the OTU in an individual sample.
Although originally believed to be sterile, the lung exhibits a microbiota that varies in both physiological and pathological conditions.4 Many authors agree that healthy lung tissue has a low density of microbial populations, represented mainly by genera such as Prevotella, Veillonella, Streptococcus and Tropheryma.5 In our study, disorder of the lung microbiome was characterized by enrichment with the OTU of the Acinetobacter spp., which is usually comprised of Acinetobacter calcoaceticus, Acinetobacter baumannii, Acinetobacter nosocomialis, and Acinetobacter pittii, with Acinetobacter baumannii (AB) being the most clinically important species responsible for the highest incidence of multidrug resistance and mortality.6 It should be noted that Enterobacteriaceae spp., a kind of abundant taxonomic group in the human gut microbiome, was common in the lung tissues of fatal COVID-19 patients. The Enterobacteriaceae family of bacteria (a kind of taxon comprising many potentially pathogenic bacteria including Klebsiella, Escherichia coli, Proteus, Enterobacter, etc.) might release a large amount of endotoxin in the intestinal lumen, which would inhibit protein synthesis in intestinal epithelial cells.7 Significantly, we observed that the fungal communities in lung specimens were usually dominated by Cryptococcus spp. Cryptococcus infections have high morbidity and mortality rates worldwide, particularly in the context of immune suppression and central nervous system involvement.8 Moreover, we successfully detected Issatchenkia spp., Cladosporium spp., Cladosporium spp., Alternaria spp., Aspergillus spp., and Candida spp., all of which are important species for opportunistic invasive mycosis in immunocompromised patients. In general, persistent lymphocytic depletion, mechanical ventilation, corticosteroid therapy and prolonged hospital stays may lead to the development of opportunistic infections in fatal COVID-19. What is more important is that fatal COVID-19 is associated with complex mixed bacteria and fungal infections in the lungs. Therefore, it is urgent to serially monitor the microbiota in the lower respiratory tract for timely personalized treatment.
Declaration of Competing Interest
All authors declare no competing interests.
Acknowledgments
Ethics approval
The study was approved by the health commission of Hubei Province and the ethics committee of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology (approval number: 2020–0043–1).
Acknowledgments
We thank all patients and their families involved in the study.This study was financially supported by grant from the Key Special Project of Ministry of Science and Technology, China (No.2020YFC 0845700); the National Natural Science Foundation of China (No. 81773022); the Fundamental Research Funds for the Central Universities (No. 2020 kfyXG YJ101).
References
- 1.Chu Y., Li T., Fang Q., Wang X. Clinical features of critically ill patients with confirmed COVID-19. J. Infect. Jul 2020;81(1):147–178. doi: 10.1016/j.jinf.2020.03.023. PubMed PMID: 32360498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan. China: Retrosp Cohort Study. Lancet. 2020 Mar 11 doi: 10.1016/S0140-6736(20)30566-3. PubMed PMID: 32171076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen T., Wu D., Chen H., Yan W., Yang D., Chen G. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. Bmj. 2020 Mar 26;368:m1091. doi: 10.1136/bmj.m1091. PubMed PMID: 32217556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beck J.M., Young V.B., Huffnagle G.B. The microbiome of the lung. Transl Res: J Lab Clin Med. Oct 2012;160(4):258–266. doi: 10.1016/j.trsl.2012.02.005. PubMed PMID: 22683412. Pubmed Central PMCID: 3440512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Man W.H., de Steenhuijsen Piters W.A., Bogaert D. The microbiota of the respiratory tract: gatekeeper to respiratory health. Nat Rev Microbiol. May 2017;15(5):259–270. doi: 10.1038/nrmicro.2017.14. PubMed PMID: 28316330. Pubmed Central PMCID: 7097736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doi Y., Murray G.L., Peleg A.Y. Acinetobacter baumannii: evolution of antimicrobial resistance-treatment options. Semin Respir Crit Care Med. Feb 2015;36(1):85–98. doi: 10.1055/s-0034-1398388. PubMed PMID: 25643273. Pubmed Central PMCID: 4465586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zeng Y., Chen S., Fu Y., Wu W., Chen T., Chen J. Gut microbiota dysbiosis in patients with hepatitis B virus-induced chronic liver disease covering chronic hepatitis, liver cirrhosis and hepatocellular carcinoma. J. Viral Hepat. Feb 2020;27(2):143–155. doi: 10.1111/jvh.13216. PubMed PMID: 31600845. [DOI] [PubMed] [Google Scholar]
- 8.Levitz S.M., Dupont M.P., Smail E.H. Direct activity of human T lymphocytes and natural killer cells against Cryptococcus neoformans. Infect. Immun. Jan 1994;62(1):194–202. doi: 10.1128/iai.62.1.194-202.1994. PubMed PMID: 8262627. Pubmed Central PMCID: 186086. [DOI] [PMC free article] [PubMed] [Google Scholar]