Highlights
-
•
Proportion positive tests were positively associated with marginalized statuses.
-
•
Low testing and high positivity were associated with public transportation use.
-
•
We recommend testing and health care resources be directed to eastern Brooklyn.
Keywords: Infectious disease, Health inequalities, Urban health, Cluster analysis
Abstract
Identifying areas with low access to testing and high case burden is necessary to understand risk and allocate resources in the COVID-19 pandemic. Using zip code level data for New York City, we analyzed testing rates, positivity rates, and proportion positive. A spatial scan statistic identified clusters of high and low testing rates, high positivity rates, and high proportion positive. Boxplots and Pearson correlations determined associations between outcomes, clusters, and contextual factors. Clusters with less testing and low proportion positive tests had higher income, education, and white population, whereas clusters with high testing rates and high proportion positive tests were disproportionately black and without health insurance. Correlations showed inverse associations of white race, education, and income with proportion positive tests, and positive associations with black race, Hispanic ethnicity, and poverty. We recommend testing and health care resources be directed to eastern Brooklyn, which has low testing and high proportion positives.
1. Introduction
At the close of March 2020, New York City became the new epicenter in the global COVID-19 pandemic (McKinley, 2020). According to the Coronavirus COVID-19 Global Cases by the Center for Systems Science and Engineering at Johns Hopkins University, New York City had 104,410 cases as of April 12, 2020 (COVID-19 Map, 2020). New York City has a highly diverse population of 8.3 million people spread across five boroughs interconnected by a subway system that also extends into neighboring areas (U.S. Census Bureau, 2020). While disease transmission is widespread, the case burden is known to be heterogeneous within the city (Buchanan et al., 2020). Understanding this spatial pattern is critical for identifying who is most at risk and for allocating resources and proper responses to hotspots. Spatial analysis is required to identify clusters of the hardest-hit areas and to understand associations with contextual factors of vulnerability, such as minority race or low-income areas. Recently released COVID-19 testing data by the New York City Department of Health at the zip code level allows for fine resolution spatial analysis to guide resource distribution (NYC Health, 2020). Zip codes are United States Postal Service-defined mail delivery routes that are understood by the public to roughly delineate neighborhoods. Since zip codes are not explicit geographic units, the United States Census Bureau provides zip code tabulation area (ZCTA) files (U.S. Census Bureau, 2020), which convert mail route-defined zip codes into areal units. ZCTAs, herein referred to as zip codes, are the primary unit of analysis for this study.
Infectious disease distribution and diffusion is an explicitly spatial process. Not only do cases and deaths exhibit heterogeneous distributions, but the process of contagion itself moves through areas as the virus is seeded and expands in particular locations (Chinazzi et al., 2020). Places that are near one another may be more likely to experience similar infection rates due to close proximity and increased social and cultural ties (Arthur et al., 2017). Furthermore, diffusion effects from especially high infection rates in one area may increase infection rates in neighboring areas (Charu et al., 2017). This interdependence of infection rates across areas suggests that the case burden may exhibit clustering in space.
Using ecosocial theory as a guide for hypothesis generation (Krieger, 1994), particular contextual factors are theorized to increase the risk of COVID-19 infection, which may intersect at particular spatial and temporal scales. Given the primary transmission of COVID-19 by respiratory droplets (CDC, 2020), individuals that rely on public transportation, subways and buses, to commute to work may be at increased risk due to contact with fellow passengers. Racial and ethnic minorities, non-citizens, and those without health insurance may also be at increased risk due to discouragement of obtaining appropriate medical care due to their marginalized status (Brondolo et al., 2008; Derose et al., 2007). Despite the New York shelter-in-place order in effect since March 22, 2020 (Cuomo, 2020), low-income individuals and households with high rent burden may not be able to suspend work and may be forced to leave their homes to maintain their income putting them at greater risk of infection. These demographic and socioeconomic characteristics may put particular individuals and neighborhoods both at greater health risk and greater economic risk at this particular moment when cases and deaths are rising exponentially, which will reverberate throughout the course of the pandemic.
The first objective of this study is to identify clusters of testing rates, positivity rates, and proportions of tests that were positive to understand test access and case burden. The second objective is to evaluate contextual factors associated with these clusters as well as across all of New York City. Combined, they advance current knowledge on how existing inequities in the city are exposed by the current COVID-19 pandemic.
2. Methods
2.1. Data
The total number of COVID-19 tests and the total number of positive COVID-19 tests aggregated by zip code were provided by New York City Department of Health as of April 12, 2020 (NYC Health, 2020). Zip code total population, race, Hispanic ethnicity, citizenship status, health insurance status, mode of transportation to work, educational attainment, median household income, receipt of public assistance payments, rent as a proportion of income, and poverty data were obtained from the 2014–2018 5-year American Community Survey via the IPUMS National Historical Geographic Information System (Manson et al., 2019).
Three outcome variables were created. First, the testing rate, calculated as the number of tests performed divided by the total population of a given zip code multiplied by 1000. Second, the positivity rate, obtained as the number of positive tests divided by the total population of a given zip code multiplied by 1000. Third, the proportion of positive tests, calculated as the number of positive tests divided by the total number of tests performed for a given zip code. All covariates except median household income were converted to proportions by zip code.
Of note, positivity rate as an outcome is difficult to interpret because the numerator, positive tests, is conditional on having been tested, whereas the denominator, total population, is unconditional; therefore, while included in the analysis, it is de-emphasized as an outcome of interest.
2.2. Cluster analysis
Choropleth maps for the three testing outcomes and each covariate were created using a four quantile categorization, which ensures an equal number of zip codes in each category and is recommended for use in public health studies (Brewer and Pickle, 2002). Therefore, the highest category captures zip codes in the 75th to 100th percentile of the distribution of a covariate, while the lowest category captures those zip codes falling below the 25th percentile. All maps except the bivariate maps described below were produced in ArcGIS 10.6.1 (ESRI, 2018).
We used a global Moran's I test, using simple adjacency as the neighborhood definition, to investigate the presence of spatial autocorrelation (clustering) in each outcome (Moran, 1950). Moran's I is a test that evaluates the covariance of a value X at an index location i with the average of the values X of its neighbors j. Moran's I values range from −1, indicating dissimilar values cluster together, to +1, indicating similar values cluster together. A Moran's I value of 0 indicates complete spatial randomness. To assess the occurrence of local clusters in each outcome we used a discrete Poisson spatial scan statistic (Kulldorff, 1997). This procedure scans a circular window of varying radii across zip code centroids and calculates the likelihood of the observed number of COVID-19 tests or positive tests for zip codes within the circular window to that expected by a Poisson distribution. The statistic produces a relative risk measure that compares the risk of having a test or a positive test for zip codes inside the window to those outside the window. A P value adjusted for multiple and dependent testing estimates the significance of each detected cluster by Monte Carlo hypothesis testing of the likelihood ratio test of no difference in risk inside versus outside the window (Kulldorff, 1997).
In our analysis, we limited the maximum cluster size to no more than two percent of the population at risk to ensure clusters of manageable size. The average percentage of the population contained in each zip code was 0.56 percent, meaning that this maximum cluster size would create clusters of approximately one to six zip codes. The procedure was also limited to identifying the top ten most likely clusters for each outcome to further focus the analysis. We defined a prioi four constructs of greatest interest to understand inequality of test access and case burden: clusters of high test rates, clusters of low test rates, clusters of high positivity rates, and clusters of high positive test proportions. Cluster analyses were performed in SaTScan v9.6 (Kulldorff, 1997) and Moran's I analyses were calculated using GeoDa 1.14.0 (Anselin et al., 2010).
2.3. Contextual factor analysis
Associations between the testing outcomes and contextual factors at the zip code level were assessed using descriptive statistics and correlations. Covariates included proportion white, proportion black, proportion Asian, proportion Hispanic, proportion non-citizens, proportion without health insurance, proportion of the working population using public transportation, proportion of those aged 25 years or older with at least a bachelor's degree, median household income, proportion of households receiving public assistance, proportion of households with rent greater than 50 percent of their income, and proportion of households living in poverty. We created maps showing the joint spatial distribution of key covariates, proportion black and education, with the positive test proportion outcome using a three quantile categorization. The distribution of each covariate stratified by and limited to the zip codes in each type of cluster identified in the scan statistics analysis was visualized using boxplots. Formal statistical tests of differences in these distributions were not performed due to relatively low sample sizes and interdependence among tests with zip codes appearing in multiple different cluster definitions. Using all zip codes, we performed Pearson correlations between each testing outcome and each covariate to understand directional associations. We used R 3.6.2 for the correlation analyses and bivariate maps (R Core Team 2019).
3. Results
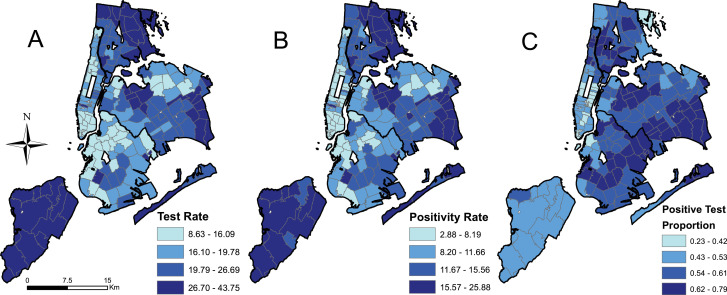
There were 177 zip codes for which testing data were available. The mean COVID-19 testing rate across zip codes was 21.6 tests per 1000 people, ranging from a minimum (MIN) of 8.6 and a maximum (MAX) of 43.8 (standard deviation [SD] of 7.3). The mean positivity rate was 12.1 per 1000 people (MIN=2.9, MAX=25.9, SD=4.7). The mean proportion of COVID-19 positive tests was 0.55 (MIN=0.23, MAX=0.79, SD=0.097). Fig. 1 shows the spatial distribution of each outcome categorized into quantiles.
Fig. 1.
Test rate (A), positivity rate (B), and the positive test proportion (C) categorized into quantiles.
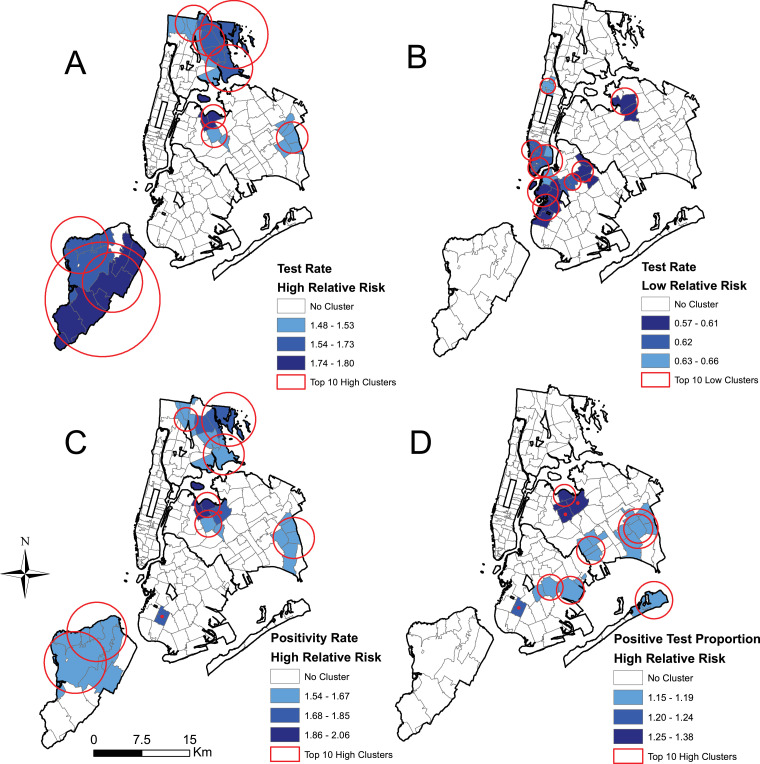
Global Moran's I analyses showed strong positive spatial autocorrelation for all three outcomes (0.698, 0.695, and 0.707 for testing rate, positivity rate, and proportion of positive tests, respectively) demonstrating clustering is present in these data. The results of the spatial scan statistic cluster analysis are presented in Fig. 2 . Clusters of high testing rates were located in Staten Island and the East Bronx as well as isolated areas in Queens including Jackson Heights (Fig. 2A). The relative risk of testing in these clusters ranged from 1.48 to 1.80. Clusters of low testing rates were observed in lower Manhattan, western Brooklyn, and Flushing, Queens, with relative risks of 0.57 to 0.66 (Fig. 2B). Clusters of high positivity rates largely mirrored those for high testing rates (Fig. 2C). Clusters of high proportion of positive tests occurred in parts of Brooklyn and Queens (Fig. 2D), with some overlap with areas in Queens that were also significant for high testing rates. Relative risks for clusters of high proportion of positive tests ranged from 1.15 to 1.38.
Fig. 2.
Top ten statistically significant spatial scan statistic clusters for (A) high test rates, (B) low test rates, (C) high positivity rates, and (D) high positive test proportions.
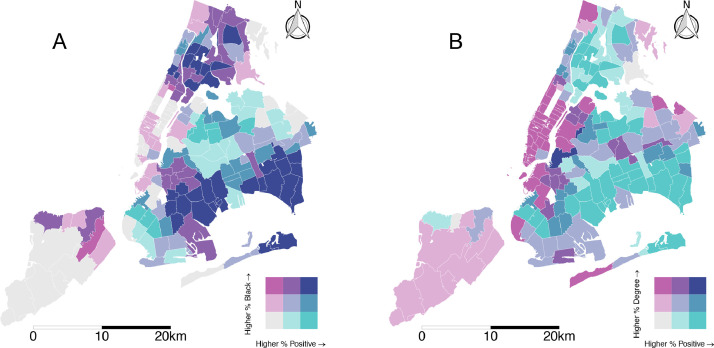
The spatial distribution of the twelve covariates categorized by quantiles is included in the supporting information (S1 Figure). Fig. 3 shows bivariate maps of the joint spatial distributions of the proportion of black population and the proportion of the population with at least a bachelor's degree each with proportion of positive tests. Areas with simultaneously high proportions of positive tests and black population are concentrated in eastern Brooklyn, southeast Queens, and parts of the Bronx. Areas with joint distribution of low education and high proportion of positive tests encompass a wide swath of the South Bronx, eastern Brooklyn, and southern Queens.
Fig. 3.
Bivariate maps of proportion positive tests and (A) proportion black and (B) proportion bachelor's degree or higher. Categorization is by 3 quantiles.
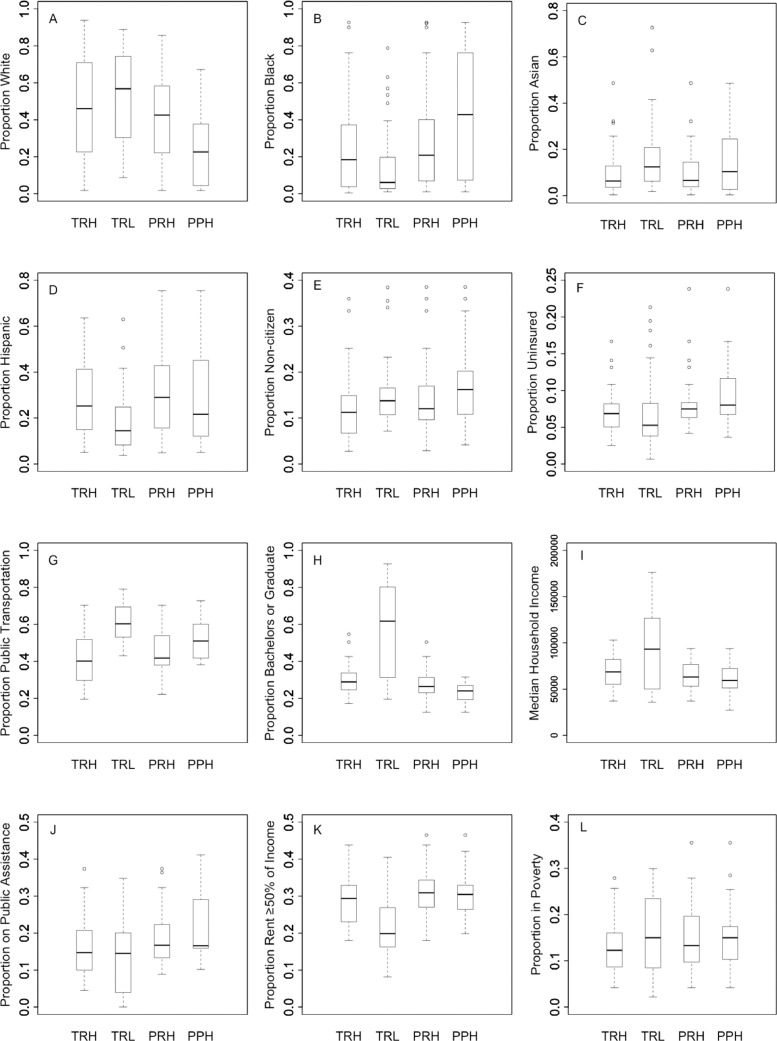
Fig. 4 shows boxplots of the distribution of covariates across zip codes identified in each cluster type. Zip codes identified as clusters of low testing rates had lower proportions of black population, Hispanic population, uninsured, and those with rent ≥50 percent of their income compared with clusters of high testing rates. In contrast, these clusters had higher proportions of white population, Asian population, use of public transportation, non-citizens, at least a bachelor's degree, and higher median household income compared with clusters of high testing rates. Zip codes with clusters of high proportion of positive tests had lower white population, higher black population, lower educational attainment, and higher proportion receiving public assistance compared to clusters of low testing rates.
Fig. 4.
Covariate distribution by zip codes in clusters of high testing rates (TRH), clusters of low testing rates (TRL), clusters of high positivity rates (PRH), and clusters of high proportion of positive test (PPH).
Pearson correlation results between the outcomes and the covariates are shown in Table 1 . Significant negative correlations of test rate were found with Asian race, use of public transportation, education, non-citizens, and median income, while a positive correlation was found with rent ≥50 percent of income. Notably, many of these associations reversed signs and became much stronger when correlated with the proportion of positive tests, particularly use of public transportation, non-citizens, and to a lesser extent poverty and lack of health insurance. Also striking is that the proportions of black and white populations show no correlations with testing rates, but strong positive and negative correlations, respectively, with proportion of positive tests.
Table 1.
Correlation results for covariates and COVID-19 test rate, positivity rate, and proportion of positive tests, New York City, United States, April 12, 2020. Bolded values are significant at P <0.05.
| Variables | Test rate |
Positivity rate |
Proportion of positive tests |
|||
|---|---|---|---|---|---|---|
| Correlation (95% CI) | P value | Correlation (95% CI) | P value | Correlation (95% CI) | P value | |
| White | 0.0026 | −0.3 | −0.69 | |||
| (−0.14, 0.15) | 0.97 | (−0.43, −0.16) | < 0.001 | (−0.76, −0.6) | < 0.001 | |
| Black | 0.083 | 0.27 | 0.45 | |||
| (−0.065, 0.23) | 0.27 | (0.13, 0.4) | < 0.001 | (0.33, 0.56) | < 0.001 | |
| Asian | −0.19 | −0.16 | −0.035 | |||
| (−0.33, −0.043) | 0.012 | (−0.3, −0.016) | 0.03 | (−0.18, 0.11) | 0.65 | |
| Hispanic | 0.11 | 0.3 | 0.48 | |||
| (−0.033, 0.26) | 0.13 | (0.16, 0.43) | < 0.001 | (0.36, 0.59) | < 0.001 | |
| Public transportation | −0.52 | −0.35 | 0.16 | |||
| (−0.62, −0.4) | < 0.001 | (−0.47, −0.21) | < 0.001 | (0.015, 0.3) | 0.031 | |
| Bachelors or graduate | −0.36 | −0.62 | −0.82 | |||
| (−0.48, −0.22) | < 0.001 | (−0.7, −0.52) | < 0.001 | (−0.87, −0.77) | < 0.001 | |
| Rent ≥50% of income | 0.19 | 0.45 | 0.68 | |||
| (0.048, 0.33) | <0.001 | (0.32, 0.56) | < 0.001 | (0.59, 0.75) | < 0.001 | |
| Non-citizen | −0.25 | −0.031 | 0.37 | |||
| (−0.39, −0.11) | < 0.001 | (−0.18, 0.12) | 0.68 | (0.24, 0.49) | < 0.001 | |
| Poverty | −0.09 | 0.099 | 0.46 | |||
| (−0.23, 0.059) | 0.24 | (−0.049, 0.24) | 0.19 | (0.33, 0.57) | < 0.001 | |
| Uninsured | −0.099 | 0.2 | 0.67 | |||
| (−0.24, 0.049) | 0.19 | (0.049, 0.33) | < 0.001 | (0.58, 0.75) | < 0.001 | |
| Median income | −0.15 | −0.4 | −0.72 | |||
| (−0.29, −0.0035) | 0.045 | (−0.52, −0.27) | < 0.001 | (−0.79, −0.64) | < 0.001 | |
| Public assistance | 0.0026 | 0.23 | 0.57 | |||
| (−0.14, 0.15) | 0.97 | (0.084, 0.36) | < 0.001 | (0.46, 0.66) | < 0.001 | |
4. Discussion
This study sought to characterize the spatial distribution of COVID-19 testing rates, positivity rates, and proportion of positive tests. We identified spatial clusters of high testing rates, high positivity rates, and high proportion of positive tests, as well as low test rate values. Furthermore, associations with contextual factors of vulnerability in space indicate inequities in testing and in disease burden.
In eastern Brooklyn, choropleth maps showed zip codes below the 50th percentile in terms of testing rates but above the 50th percentile in terms of positivity rates, suggesting that testing in this area was mainly performed in more severe cases. A similar pattern can be seen in Flushing, Queens.
The zip codes included in clusters of low testing rates, high testing rates, and high proportion of positive tests showed differing demographic distributions. The results show that areas with lower test rates and lower proportions of those tests being positive are likely the result of less severe illness and track with higher income, education, and white populations. Areas with higher test rates and higher proportions of positive tests point to more severe cases, which were disproportionately in areas of black population, uninsured, and have rent ≥50 percent of income. The third pattern of lower test rate areas coupled with higher positive test proportions may indicate severe illness, but inadequate testing, which appeared among areas with non-citizens and high use of public transportation. The strong inverse association of white race, education, and income with positive test proportion suggests either lower severity, excess testing, or both among this population. Equally strong positive associations with black race, Hispanic ethnicity, poverty, uninsured, and rent ≥50 percent of income may indicate a greater burden in this population. Both the cluster analysis and the correlation analysis indicate greater burden of COVID-19, particularly in terms of positive test proportion, among socially and economically disadvantaged groups, therefore exposing racial, ethnic, and income inequalities regarding the impact of the epidemic. Furthermore, given the context of well-documented undertesting of COVID-19, these associations are likely to be underestimates due to general lack of access to tests as well as high proportions of asymptomatic cases resulting in substantial underreporting (Baird, 2020; Patel et al., 2020).
These results fit into a larger narrative regarding social inequalities and health. Previous research in New York City has found increasing inequality between wealthy and poor neighborhoods in mortality from HIV/AIDS, diabetes, and liver disease between 1989 and 2001 (Karpati et al., 2006). Wide disparities in infant mortality according to income have also been observed across New York zip codes. Lower income was associated with increased infant mortality even after controlling for other socioeconomic indicators (Sohler et al., 2003). In addition to income, disparities in health outcomes by race are present in New York City. Grady and Ramírez found that black racial residential segregation was associated with increased risk of low birthweight infants (Grady and Ramírez, 2008) and Merkin et al. show increased risk of breast cancer diagnosis among black women in New York, which was particularly pronounced among individuals living in low income zip codes, highlighting the importance of intersectionality (Merkin et al., 2002). More recent research showed no change in neighborhood-level disparities by race, ethnicity, and insurance status in avoidable hospital conditions despite an overall 50 percent decrease in avoidable hospital conditions in New York from 1999 to 2013 and city efforts to alleviate those disparities over the same period (Gusmano et al., 2017). This research shows that socioeconomic health inequalities in New York City are pervasive and persistent and lend support to the observed associations reported here showing inequalities in COVID-19 burden by race and socioeconomic status. A recent paper by Bavel et al. highlights how issues of social inequality become magnified when faced with epidemic disease and that the COVID-19 pandemic is no exception (Bavel et al., 2020). The authors discuss the intersection of economic disadvantage with racial inequalities that increases vulnerability in the face of rapidly spreading infectious disease (Bavel et al., 2020). Increases in transmission may be due to disproportionate presence in high public contact jobs coupled with racialized social networks and decreases in the response to public health messaging and the acquisition of appropriate medical care are the result of low levels of trust in institutions that exhibit contemporary and historical systems of discrimination and racism (Bavel et al., 2020). Furthermore, African Americans have been found to have higher COVID-19 infection rates and mortality rates due to higher burden of co-morbidities, higher housing density, and lack of privilege to be able to social distance, which are all compounded by low socioeconomic status (Yancy, 2020).
A major strength of this study is the fine spatial resolution. Analysis at the zip code level allows for a more precise understanding of which neighborhoods have a higher case burden than analyses at courser spatial scales. Furthermore, the rigorous statistical tests of clustering in the COVID-19 test outcomes offers pinpointed analysis for resource distribution by illuminating areas that have low test rates, but likely high case burden with high numbers of positive tests among those who received a test. Correlation results indicate important inequalities related to testing and case severity which may be useful in guiding policy efforts in other large United States cities in planning their responses to COVID-19 as the pandemic unfolds their cities.
This study has some limitations. First, this analysis only describes associations between COVID-19 testing patterns and contextual factors of the zip code. We make no claim for a causal relationship between any of the variables examined. Second, as in any spatial analysis, this study is subject to the modifiable areal unit problem (Fotheringham and Wong, 1991). Zip codes, like any boundaries, are arbitrary units and different associations may have been found using different boundary definitions. However, zip codes do roughly correspond to neighborhoods and can still be used to guide resource allocation and policy planning. Third, given that zip codes are relatively small spatial units, American Community Survey demographic data may have a substantial error. Since this study did not take this error into account, further caution should be taken when evaluating the associations presented in the correlation results. Fourth, it has been noted that many wealthy residents of New York City have left the city and are residing in their second homes (Quealy, 2020), therefore artificially inflating the population denominator in those areas and driving down the test rates in those areas.
As the number of cases and deaths due to COVID-19 increases exponentially, a swift, collaborative effort must be organized to protect those at greatest risk. Clusters of susceptible populations that are forced to go to work may be at greater need of testing resources. While high density cities such as New York are more vulnerable to rapid infectious disease spread, this study shows the COVID-19 epidemic is exposing structural inequalities in the city. We show that testing and health care resources should be directed to eastern Brooklyn centered on the Brownsville neighborhood, which simultaneously has low testing rates and high proportion of positive tests. Use of public transportation, including subways and buses, was also identified to be associated with low testing rates and high proportion of positive tests, suggesting measures such as targeted advertising in these areas may be effective at reaching underserved, high risk groups. This study adds to previous literature that has identified racial/ethnic minorities and those with lower socioeconomic status at increased risk across a range of health outcomes, including previous infectious disease epidemics such as HIV/AIDS (Freeman et al., 2011; Haile et al., 2011; Nanín et al., 2009; Parker et al., 2017; Ransome et al., 2016; Rubin et al., 2010). Therefore, in addition to the likely consequences of COVID-19 to communities, to individual lives, and to the economy, COVID-19 may result in an exacerbation of existing inequalities. Avoiding this is imperative; it is an issue of human rights.
Supporting information
S1 Figure. Spatial distribution of ZCTA population (A) proportion white, (B) proportion black, (C) proportion Asian, (D) proportion Hispanic, (E) proportion non-citizens, (F) proportion without health insurance, (G) proportion using public transportation for work, (H) proportion with a bachelor's or graduate degree, (I) median household income*, (J) proportion of households receiving public assistance, (K) proportion of households with rent ≥50% of income, and (L) proportion of households in poverty. Quantile breaks correspond to the 25th, 50th, and 75th percentiles.
*The color gradient is flipped for median household income to allow comparison with rent ≥50% of income, public assistance, and poverty.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.sste.2020.100355.
Contributor Information
Jack Cordes, Email: jcordes@g.harvard.edu.
Marcia C. Castro, Email: mcastro@hsph.harvard.edu.
Appendix A. Supplementary materials
Supplementary Raw Research Data. This is open data under the CC BY license http://creativecommons.org/licenses/by/4.0/
References
- Anselin L., Syabri I., Kho Y. GeoDa: an introduction to spatial data analysis. In: Fischer M.M., Getis A., editors. Handbook of Applied Spatial Analysis: Software Tools, Methods and Applications. Springer; 2010. pp. 73–89. [DOI] [Google Scholar]
- Arthur R.F., Gurley E.S., Salje H., Bloomfield L.S.P., Jones J.H. Contact structure, mobility, environmental impact and behaviour: the importance of social forces to infectious disease dynamics and disease ecology. Philos. Trans. R. Soc. B. 2017;372(1719) doi: 10.1098/rstb.2016.0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird, R.P. (2020, March 16). What went wrong with coronavirus testing in the U.S.. The New Yorker. https://www.newyorker.com/news/news-desk/what-went-wrong-with-coronavirus-testing-in-the-us.
- Bavel, J.J.V., Baicker, K., Boggio, P., Capraro, V., Cichocka, A., Crockett, M., Cikara, M., Crum, A., Douglas, K., Druckman, J., Drury, J., Dube, O., Ellemers, N., Finkel, E.J., Fowler, J.H., Gelfand, Mi., Han, S., Haslam, S.A., Jetten, J., … Willer, R. (2020). Using social and behavioural science to support COVID-19 pandemic response. 10.31234/osf.io/y38m9. [DOI] [PubMed]
- Brewer C.A., Pickle L. Evaluation of Methods for classifying epidemiological data on choropleth maps in series. Ann. Assoc. Am. Geogr. 2002;92(4):662–681. doi: 10.1111/1467-8306.00310. [DOI] [Google Scholar]
- Brondolo E., Gallo L.C., Myers H.F. Race, racism and health: disparities, mechanisms, and interventions. J Behav Med. 2008;32(1):1. doi: 10.1007/s10865-008-9190-3. [DOI] [PubMed] [Google Scholar]
- Buchanan L., Patel J.K., Rosenthal B.M., Singhvi A. The New York Times; 2020, April 1. A Month of Coronavirus in New York City: See the Hardest-Hit Areas.https://www.nytimes.com/interactive/2020/04/01/nyregion/nyc-coronavirus-cases-map.html [Google Scholar]
- CDC . Centers for Disease Control and Prevention; 2020, February 11. Coronavirus Disease 2019 (COVID-19)https://www.cdc.gov/coronavirus/2019-ncov/faq.html [Google Scholar]
- Charu V., Zeger S., Gog J., Bjørnstad O.N., Kissler S., Simonsen L., Grenfell B.T., Viboud C. Human mobility and the spatial transmission of influenza in the United States. PLoS Comput. Biol. 2017;13(2) doi: 10.1371/journal.pcbi.1005382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinazzi M., Davis J.T., Ajelli M., Gioannini C., Litvinova M., Merler S., Piontti A.P.y, Mu K., Rossi L., Sun K., Viboud C., Xiong X., Yu H., Halloran M.E., Longini I.M., Vespignani A. The effect of travel restrictions on the spread of the 2019 novel coronavirus (COVID-19) outbreak. Science. 2020 doi: 10.1126/science.aba9757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COVID-19: Data—NYC Health. (2020). Retrieved April 12, 2020, fromhttps://www1.nyc.gov/site/doh/covid/covid-19-data.page.
- COVID-19 Map. (2020). Johns Hopkins Coronavirus Resource Center. Retrieved April 12, 2020, from https://coronavirus.jhu.edu/map.html.
- Derose K.P., Escarce J.J., Lurie N. Immigrants and health care: sources of vulnerability. Health Aff. 2007;26(5):1258–1268. doi: 10.1377/hlthaff.26.5.1258. [DOI] [PubMed] [Google Scholar]
- ESRI 2018 . Environmental Systems Research Institute; 2018. ArcGIS Desktop: Release 10.6.1. [Google Scholar]
- Fotheringham A.S., Wong D.W.S. The Modifiable areal unit problem in multivariate statistical analysis. Environ. Plan. A: Econ. Space. 1991;23(7):1025–1044. doi: 10.1068/a231025. [DOI] [Google Scholar]
- Freeman K., Zonszein J., Islam N., Blank A.E., Strelnick A.H. Mortality trends and disparities among racial/ethnic and sex subgroups in New York City, 1990 to 2000. J. Immigr. Minority Health. 2011;13(3):546–554. doi: 10.1007/s10903-010-9345-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Governor Cuomo Signs the “New York State on PAUSE” Executive Order. (2020, March 20). Governor Andrew M. Cuomo. https://www.governor.ny.gov/news/governor-cuomo-signs-new-york-state-pause-executive-order.
- Grady S.C., Ramírez I.J. Mediating medical risk factors in the residential segregation and low birthweight relationship by race in New York City. Health Place. 2008;14(4):661–677. doi: 10.1016/j.healthplace.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Gusmano M.K., Rodwin V.G., Weisz D. Persistent inequalities in health and access to health services: evidence from New York City. World Med. Health Policy. 2017;9(2):186–205. doi: 10.1002/wmh3.226. [DOI] [Google Scholar]
- Haile R., Padilla M.B., Parker E.A. ‘Stuck in the quagmire of an HIV ghetto’: the meaning of stigma in the lives of older black gay and bisexual men living with HIV in New York City. Cult. Health Sex. 2011;13(4):429–442. doi: 10.1080/13691058.2010.537769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpati A.M., Bassett M.T., McCord C. Neighbourhood mortality inequalities in New York City, 1989-1991 and 1999-2001. J. Epidemiol. Community Health. 2006;60(12):1060–1064. doi: 10.1136/jech.2006.047100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger N. Epidemiology and the web of causation: has anyone seen the spider. Soc. Sci. Med. 1994;39(7):887–903. doi: 10.1016/0277-9536(94)90202-X. [DOI] [PubMed] [Google Scholar]
- Kulldorff M. A spatial scan statistic. Commun. Stat. - Theory Methods. 1997;26(6):1481–1496. doi: 10.1080/03610929708831995. [DOI] [Google Scholar]
- Manson, S., Schroeder, J., Van Riper, D., & Ruggles, S. (2019). IPUMS national historical geographic information system: version 14.0 [2018 American community survey: 5-year data [2014-2018, block groups & larger areas]]. 2018 American Community Survey: 5-Year Data [2014-2018, Block Groups & Larger Areas]. 10.18128/D050.V14.0. [DOI]
- McKinley J. The New York Times; 2020, March 22. New York City Region is Now an Epicenter of the Coronavirus Pandemic.https://www.nytimes.com/2020/03/22/nyregion/Coronavirus-new-York-epicenter.html [Google Scholar]
- Merkin S.S., Stevenson L., Powe N. Geographic socioeconomic status, race, and advanced-stage breast cancer in New York City. Am. J. Public Health. 2002;92(1):64–70. doi: 10.2105/AJPH.92.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran P.A.P. Notes on continuous stochastic phenomena. Biometrika. 1950;37(1/2):17–23. doi: 10.2307/2332142. JSTOR. [DOI] [PubMed] [Google Scholar]
- Nanín J., Osubu T., Walker J., Powell B., Powell D., Parsons J. “HIV is still real”: perceptions of HIV testing and HIV prevention among black men who have sex with men in New York City. Am. J. Men’s Health. 2009;3(2):150–164. doi: 10.1177/1557988308315154. [DOI] [PubMed] [Google Scholar]
- Parker C.M., Garcia J., Philbin M.M., Wilson P.A., Parker R.G., Hirsch J.S. Social risk, stigma and space: key concepts for understanding HIV vulnerability among black men who have sex with men in New York City. Cult. Health Sex. 2017;19(3):323–337. doi: 10.1080/13691058.2016.1216604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel I., Goff A., Cho S. The race against COVID-19 in the US. Res. Pharm. Health Sci. 2020;6(1):101–102. [Google Scholar]
- Quealy K. The New York Times; 2020, May 15. The Richest Neighborhoods Emptied Out Most as Coronavirus Hit New York City.https://www.nytimes.com/interactive/2020/05/15/upshot/who-left-new-york-coronavirus.html [Google Scholar]
- Ransome Y., Kawachi I., Braunstein S., Nash D. Structural inequalities drive late HIV diagnosis: the role of black racial concentration, income inequality, socioeconomic deprivation, and HIV testing. Health Place. 2016;42:148–158. doi: 10.1016/j.healthplace.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin M.S., Colen C.G., Link B.G. Examination of inequalities in HIV/AIDS mortality in the United States from a fundamental cause perspective. Am. J. Public Health. 2010;100(6):1053–1059. doi: 10.2105/AJPH.2009.170241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohler N.L., Arno P.S., Chang C.J., Fang J., Schechter C. Income inequality and infant mortality in New York City. J. Urban Health. 2003;80(4):650–657. doi: 10.1093/jurban/jtg071. http://dx.doi.org.ezp-prod1.hul.harvard.edu/10.1093/jurban/jtg071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancy C.W. COVID-19 and African Americans. JAMA. 2020 doi: 10.1001/jama.2020.6548. [DOI] [PubMed] [Google Scholar]
- R Core Team. (2019). R: a language and environment for statistical computing. https://www.R-project.org/.
- U.S. Census Bureau QuickFacts: New York City, New York. (2020). Retrieved May 28, 2020, from https://www.census.gov/quickfacts/newyorkcitynewyork.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Raw Research Data. This is open data under the CC BY license http://creativecommons.org/licenses/by/4.0/