Abstract
Feline calicivirus (FCV) is a highly contagious pathogen that causes acute upper respiratory infections and oral disease in cats, thus seriously endangering feline health. Recently, there have been outbreaks of particularly virulent variant strains of FCV, which can cause both acute symptoms and fatal systemic disease. The discovery of effective antiviral agents to treat FCV infection is, therefore, gradually assuming increased importance. In this study, we showed that both nitazoxanide and mizoribine had antiviral activity in F81 cells infected with different strains of FCV and also demonstrated a synergistic effect between the two drugs. Experiments in cats challenged with FCV showed that nitazoxanide significantly reduced the clinical symptoms of FCV infection, reduced viral load in the trachea and lungs, and reduced viral shedding. Our results showed that nitazoxanide and mizoribine could potentially be used as therapeutic agents to treat FCV infection.
Keywords: Feline calicivirus, Nitazoxanide, Mizoribine, Antiviral, In vivo
Highlights
-
•
Mizoribine had antiviral activity against FCV.
-
•
Nitazoxanide and mizoribine had synergistic anti-FCV effects.
-
•
Nitazoxanide alleviated clinical symptoms in FCV-infected kittens.
-
•
Nitazoxanide significantly reduced viral titers in lungs and trachea of kittens infected with FCV.
1. Introduction
Feline calicivirus (FCV), the major representative of the family Caliciviridae, primarily causes upper respiratory tract disease in cats. Highly virulent strains of FCV (VS-FCV) can cause systemic diseases, including subcutaneous edema, necrosis in multiple organs (liver, spleen, pancreas) and interstitial pneumonia (Desselberger, 2019). FCV vaccines have been commercially available for 30 years but FCV remains highly prevalent among cats, and none of the vaccines currently on sale can protect cats from all strains of FCV (Sato et al., 2017). In the past decade, severe systemic diseases caused by VS-FCV have been reported in many countries (Caringella et al., 2019; Guo et al., 2018; Radford and Gaskell, 2011; Schulz et al., 2011). One reason for the incomplete protection afforded by vaccines may be that the broad spectrum of genetic and antigenic variability of FCV results in different strains with little cross-reactivity (Bergmann et al., 2019). Some cats also occasionally spread the virus after being inoculated with live viruses (Afonso et al., 2017).
The cat is a companion animals of humans and, in recent years, the development of companion animal culture has aroused interest in treating diseases caused by FCV. A variety of drugs have been shown to have antiviral effects against FCV, but most of these were tested only in in vitro studies (Aboubakr et al., 2016; Tian et al., 2017; Wu et al., 2016). Whether these compounds can also be used in vivo to treat FCV infection is unknown. Nitazoxanide (NTZ, 2-[(5-nitro-1,3-thiazol-2-yl) carbamoyl] phenyl acetate) was initially identified as an anti-parasitic agent and is approved by the US Food and Drug Administration (FDA) as an orally active treatment for protozoal diarrhea. NTZ has since been shown to have antiviral effects against a variety of RNA and DNA viruses (Dang et al., 2017; Jasenosky et al., 2019; Zhou et al., 2019), including norovirus, a member of the Caliciviridae family, which is a major cause of human gastroenteritis (Dang et al., 2017). Mizoribine (MZR, 1-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-5-hydroxy-1H-imidazole-4-carboxamide) is as an imidazole nucleoside that has anti-proliferative activity against some immune cells. MZR has been used in several countries or regions as an immunosuppressant to treat autoimmune diseases and steroid-resistant nephrotic syndrome after renal transplantation (Ishikawa, 1999). Recently, MZR has been shown to have antiviral activity against cytomegalovirus, respiratory syncytial virus, severe acute respiratory syndrome-associated coronavirus, bovine viral diarrhea virus, and foot-and-mouth disease virus (Li et al., 2019; Saijo et al., 2005; Shigeta, 2000; Shiraki et al., 1990; Stuyver et al., 2002).
Here, we showed first that NTZ and MZR had low cytotoxicity in transformed feline kidney fibroblast (F81) cells. We then evaluated the antiviral effects of the two compounds against FCV. Both compounds were found to be effective against several strains of FCV and the antiviral effects were found to be dose-dependent. There was also a synergistic effect between MZR and NTZ in vitro. Animal experiments showed that NTZ significantly reduced viral load in the trachea and lungs, and also reduced viral shedding. In cats, both clinical score and mortality decreased on administration of NTZ, and blood biochemistry and immunohistochemistry showed that NTZ may be an effective clinical treatment for cats infected with FCV.
2. Materials and methods
2.1. Cells, virus strains and compounds
Feline kidney fibroblast-like monolayer cells (F81) and the CH-SH strain of FCV were provided by The Institute of Military Veterinary Medicine (Changchun, China). CH-JL1, CH-JL2, CH-JL3 and CH-JL4 strains of FCV were isolated and stored in our laboratory (Wang et al., 2017; Zhao et al., 2017). MZR (#M129842) and NTZ (#N159057) were purchased from Aladdin, China. FCV strain CH-JL2 was used for all experiments unless otherwise stated.
2.2. Cytotoxicity of nitazoxanide and mizoribine
F81 cells were seeded into a 96-well plate and grown in minimum essential medium (MEM; Gibco, USA) containing 8% fetal bovine serum (FBS). When the cells had formed monolayers, the medium was replaced by MEM containing 2% FBS and different concentrations of MZR (20, 40, 60, 80, 100, and 200 μM) or NTZ (20, 40, 60, 80, 100, and 200 μM) and 2% FBS. MEM containing 0.4% DMSO was used as the blank control. The cells were incubated for 24 or 72 h at 37 °C under an atmosphere containing 5% CO2 and then washed twice with phosphate-buffered saline (PBS). FBS-free MEM (180 μL) and CCK8 (20 μL, Biosharp, China) were then added and the cells were incubated at 37 °C for 1–2 h. A Cmax Plus plate reader (Molecular Devices, USA) was used to read the optical density (OD) at 450 nm. Cell viability was calculated using the following equation:
| Cell viability = [OD (Compound) - OD (blank)] / [OD (control) - OD (blank)] × 100%. |
2.3. Antiviral effect of mizoribine and nitazoxanide against FCV in vitro
FCV (100 × half-tissue culture infectious dose (TCID50), 200 μL) and different concentrations of MZR or NTZ were added to 96-well plates containing monolayers of F81 cells and the cells were incubated at 37 °C under an atmosphere containing 5% CO2 for 28 h. Each drug concentration was tested in three replicate wells and 0.4% DMSO was used as the blank control. TCID50 values were determined and reverse transcription-quantitative polymerase chain reaction (RT-qPCR) of FCV was carried after three freeze-thaw cycles to evaluate the antiviral effects of MZR and NTZ against FCV. Half-maximum inhibitory concentrations (IC50) were determined, and the results were plotted using GraphPad Prism 8. An indirect immunofluorescence assay (IFA) was performed to observe the antiviral effects against FCV more directly. After immobilization with cold acetone and washing, anti-FCV antibodies (1:300, VMRD, USA) were used as primary antibodies and FITC-labeled rabbit anti-cat IgG antibodies (1:200; Bioss Antibodies, China) were used as secondary antibodies (Cui et al., 2019). Fluorescence was observed using a Leica Dmi8 inverted fluorescence microscope (Leica, Germany).
2.4. Determination of viral titer and RNA expression level
A solution of virus was diluted in a 10-fold gradient. Aliquots (100 μL per well) of solutions with different concentrations of virus, together with MEM containing 2% FBS (100 μL), were added to each plate column. The blank control wells contained no virus. The plates were cultured 28 h at 37 °C under an atmosphere containing 5% CO2 and virus TCID50 values were then calculated using the Reed and Muench formula. Relative RT-qPCR was used to evaluate FCV gene expression. Briefly, viral RNA was extracted using a Simply P total RNA extraction kit (Bioflux, China), retranslated into cDNA using RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher, USA), followed by RT-qPCR using TB Green Premix Ex Taq II (Tli RNaseH Plus, TaKaRa, China). The upstream and downstream primers were: FCV 5′-GCAGGTTGGGATAAACATGGA-3′ and 5′-CACGAGGCGATTGAGTTGAG-3′; GAPDH 5′-TGGAAAGCCCATCACCATC-3′, and 5′-ACTCCACAACATACTCAGCACCA-3′.
2.5. Antiviral effects of mizoribine and nitazoxanide against other FCV strains in vitro
The antiviral effects of MZR and NTZ against other stains of virus (CH-JL1, CH-SH, CH-JL3 and CH-JL4) were determined as described in Section 2.3. Briefly, solutions of virus were diluted to 100 × TCID50 and added, together with MZR or NTZ, to 96-well plates containing monolayers of F81 cells. TCID50 and RNA expression levels were determined after incubation at 37 °C under an atmosphere containing 5% CO2 for 28 h, followed by three freeze-thaw cycles.
2.6. Efficacy of combinations of mizoribine and nitazoxanide in vitro
The checkerboard method, with serial dilutions and mixtures of the two compounds, was used to determine the effect of combinations of MZR and NTZ. TCID50 values were determined and SynergyFinder was used to evaluate the effects of the combinations (Ianevski et al., 2017). The zero interaction efficacy (ZIP) model was used to calculate the synergistic combination score of different concentrations of the drugs (Yadav et al., 2015).
2.7. Antiviral effect of nitazoxanide on FCV in vivo
All animals conformed to the general requirements for animal experiments in China (GB/T 35823-2018). Healthy female cats (5–7 weeks old, 0.5–0.7 kg) were randomly assigned to eight groups (four animals per group) once they had been confirmed to be virus-free by ELSA and PCR detection of FCV RNA and antibodies. The prophylactic effect of NTZ was investigated first. On day −1 (−1 dpi, the day before viral infection) animals in the treatment groups received oral doses of NTZ (20 mg/kg, 10 mg/kg or 5 mg/kg) in PBS (500 μL). These were designed groups A, B and C, respectively. The control group received the same volume of PBS. On day 0, the cats were infected intranasally with FCV (108.22 × TCID50) in MEM (500 μL). The therapeutic effect of NTZ after onset of infection was examined next. On day 3, days post-infection (dpi), infected cats were treated orally with NTZ (20 mg/kg, 10 mg/kg or 5 mg/kg) in PBS (500 μL). These were designated groups D, E and F, respectively. The control group received an equal volume of PBS. Clinical signs and survival rates of the animals were monitored as described previously, and cat health was assessed on days 0 and 7 (see Table 1 for details) (Cui et al., 2019). Virus titers were measured in oral swabs and different tissues by quantitative RT-qPCR. The sequences of the FCV primers were the same as those used for the relative RT-qPCR.
Table 1.
The scoring system for the health and clinical status of cats. Body weight loss (%) = 100 × [(7d weight)] − (0d weight)]/[0d weight].
| Clinical symptoms | Description | Score |
|---|---|---|
| Rectal temperature (°C) | 37.1–39.4 | 0 |
| ≥39.5 | 1 | |
| ≤37.0 | 2 | |
| Body weight loss (%) | Gain or loss of <3% | 0 |
| Loss of ≥3% | 2 | |
| Ulcers (oral and/or nasal) | Absence | 0 |
| Small and few | 1 | |
| Large or numerous | 3 | |
| Nasal discharge | Absence | 0 |
| Slight | 1 | |
| Copious | 2 | |
| Ocular discharge | Absence | 0 |
| Presence | 1 |
2.8. Complete blood count, biochemical analysis and immunohistochemistry
Blood samples were collected from the cats. A complete blood count was carried out using a hematology analyzer (PE-6800VET, Prokan, China) and biochemical analysis was carried out using a VetTest plasma chemistry analyzer (IDEXX, USA). Immunohistochemistry tests were performed as described before (Cui et al., 2019). Briefly, Paraffin sections were prepared after incubation with anti-FCV antibody (1:500, VMRD) for 24 h followed by treatment with HRP-labeled rabbit anti-cat secondary antibody (Gibco).
3. Results
3.1. Antiviral effects of nitazoxanide and mizoribine against FCV in vitro
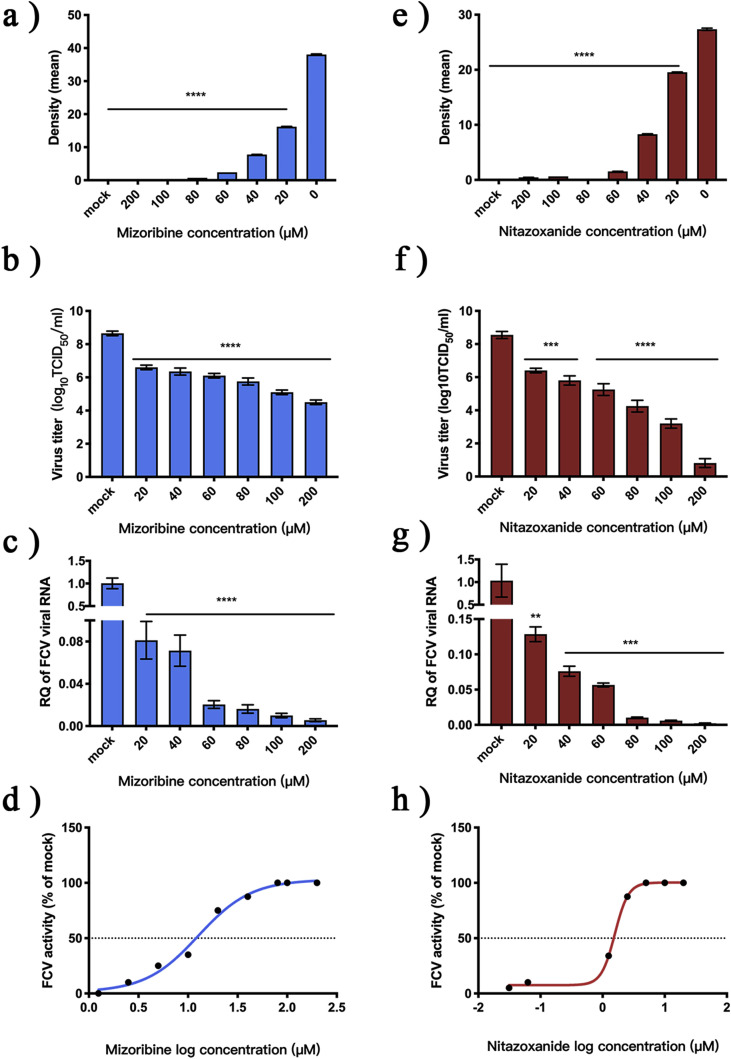
A CCK8 kit was used to test the cytotoxicity of the two compounds in F81 cells. CC50 values of both compounds were >1000 μM (results not shown). IFA was then used to measure the antiviral activity of the two compounds against FCV (Supplementary Material S1) and the results were analyzed using Image J software (Fig. 1 A and E). At a concentration of 20 μM, the optical densities of samples containing the two compounds were significantly different to the control group (p < 0.0001). FCV TCID50 values and levels of gene expression at different compound concentrations were compared with values for the control group. The TCID50 values of FCV was significantly reduced (p < 0.0002) after treatment (Fig. 1 B and F) and gene levels of FCV were significantly downregulated (p < 0.0021) (Fig. 1 C and G). The EC50 of NTZ was 1.53 μM and the EC50 of MZR was 12.68 μM (Fig. 1 D and H).
Fig. 1.
Evaluation of antiviral effects of nitazoxanide and mizoribine against FCV.
(A and E) Solutions of MZR (100 mM) and NTZ (100 mM) in DMSO were diluted with MEM to provide test solutions of different concentration. The solutions were mixed with virus (100 × TCID50); the fluorescence was measured and the optical density was determined statistically. The mock group contained 0.4% DMSO. (B and F) The TCID50 of FCV was measured after incubation with different concentrations of MZR and NTZ for 28 h. (C and G) Relative RT-qPCR was used to measure the expression of FCV RNA. (D and H) EC50 curves for MZR and NTZ. Unless otherwise stated, all experiments were simulated with 0.4% DMSO and 100 × TCID50 FCV, and all were repeated three times. *p < 0.0332; **p < 0.0021; ***p < 0.0002; ****p < 0.0001.
3.2. Antiviral effects against other FCV strains
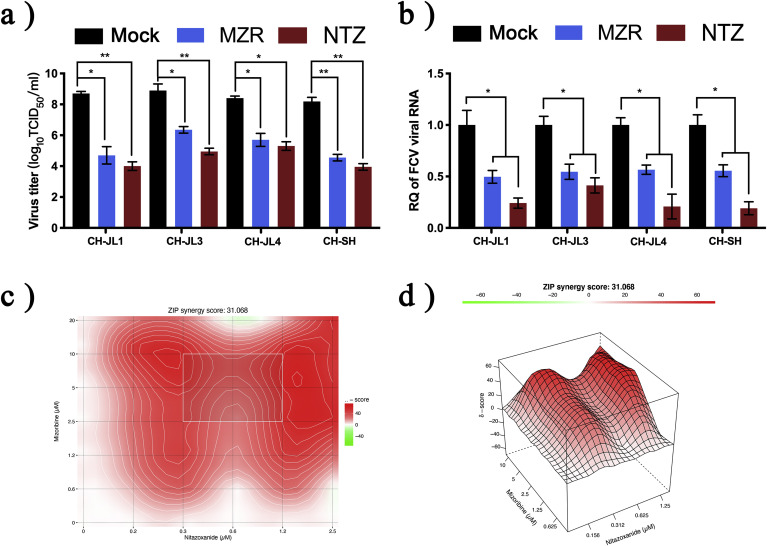
NTZ and MZR not only had antiviral efficacy against the FCV CH-JL2 strain, but the TCID50 values of the CH-JL1, CH-JL3, CH-JL4 and CH-SH strains were also significantly different after drug treatment (p < 0.0332) (Fig. 2 A). FCV gene expression was also significantly downregulated by treatment with the two compounds (p < 0.0332) (Fig. 2B).
Fig. 2.
Evaluation of antiviral effects of nitazoxanide and mizoribine against other FCV strains and their effects in combination.
(A) Once the antiviral activity of NTZ and MZR against the FCV CH-JL2 strain had been established, CH-JL1, CH-JL3, CH-JL4 and CH-SH strains (diluted to 100 × TCID50) were treated with NTZ (20 μM) or MZR (40 μM). TCID50 values were measured after 28 h. (B) Relative RT-qPCR was used to assess changes in gene levels of different FCV strains. (C and D) Virus TCID50 values were calculated to determine the combined effect of different concentrations of NTZ (0–2.5 μM) and MZR (0–20 μM) on FCV. The ZIP model in SynergyFinder was used to analyze the combined effects of the drugs and plot the results. The synergy score for the ZIP model was expressed as the average of all δ scores in the dose-response landscape, and the red portion of the graph indicates synergy. All experiments were repeated three times. *p < 0.0332; **p < 0.0021.
3.3. Synergistic effect of nitazoxanide and mizoribine
The checkerboard method was used to determine the combined effects of NTZ and MZR. Drug solutions were diluted and the ZIP model was used to calculate the scores of different drug dose combinations. The average synergy score was 31.07 (Fig. 2D) and the most synergistic area score was 45.5 (Fig. 2C), indicating that NTZ and MZR had synergistic effects over the concentration range tested.
3.4. Nitazoxanide relieves clinical symptoms in cats infected with FCV
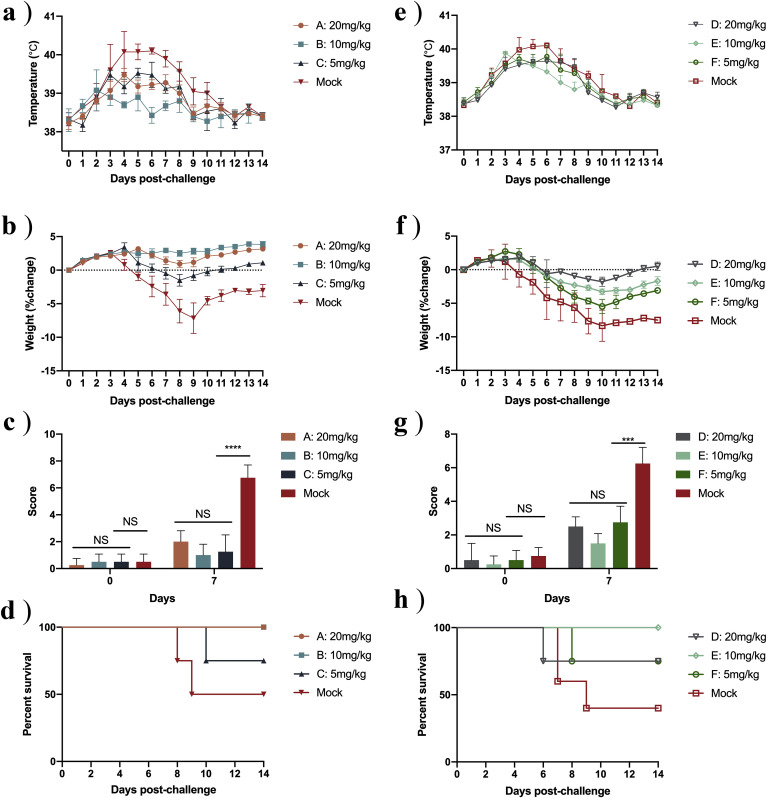
Once we had established that NTZ and MZR both have antiviral activity against FCV, we chose the FDA-approved oral medication NTZ for subsequent animal experiments. We found that, at −1 dpi, rectal temperature was not significantly increased in Group B (Fig. 3 A). The maximum average temperature of groups A, B and C was significantly lower than the control group (p < 0.0021) (Fig. 3A). The rate of weight change was not significantly reduced in groups A and B and the difference was significant at day 7, compared with the control groups (p < 0.0021) (Fig. 3B). When different doses of NTZ were administered orally at 3 dpi (groups D, E and F), there was little difference in rectal temperature of any of the groups, compared with the control group (p < 0.0332) (Fig. 3E). The rate of weight change, however, was significantly lower in groups D and E than in the control group (Fig. 3F). We also calculated clinical scores for all groups on days 0 and 7. On 7 dpi, the average scores of the control groups (treatment at −1 dpi and treatment at 3 dpi) were 5.75 and 5.25, which were significantly different to the scores of the other groups (p < 0.0002) (Fig. 3 C and G). The survival rate of group C was 80%, and that of the relevant control group was 50%. The survival rate of groups E and F was 80% and that of the relevant control group was 50% (Fig. 3 D and H).
Fig. 3.
Nitazoxanide reduces clinical symptoms in cats infected with FCV.
Data are shown for the three oral treatment groups (A, B and C) used to test the prophylactic effect of NTZ (dosing one day before infection, −1 dpi) and the three groups (D, E and F) used to test the therapeutic effect of NTZ (dosed three days post infection, 3 dpi), together with the respective control groups. (A and E) Rectal temperature measured every 2 days from 0 dpi. (B and F) Daily changes in body weight, converted to rate of weight change compared with 0 dpi. (C and G) Clinical scores at 0 dpi and 7 dpi, calculated using Clinical Scores Table. (D and H) Survival rates for different groups. n = 4 cats/group; NS p > 0.1234; ***p < 0.0002; ****p < 0.0001.
3.5. Antiviral effects of nitazoxanide on FCV in vivo
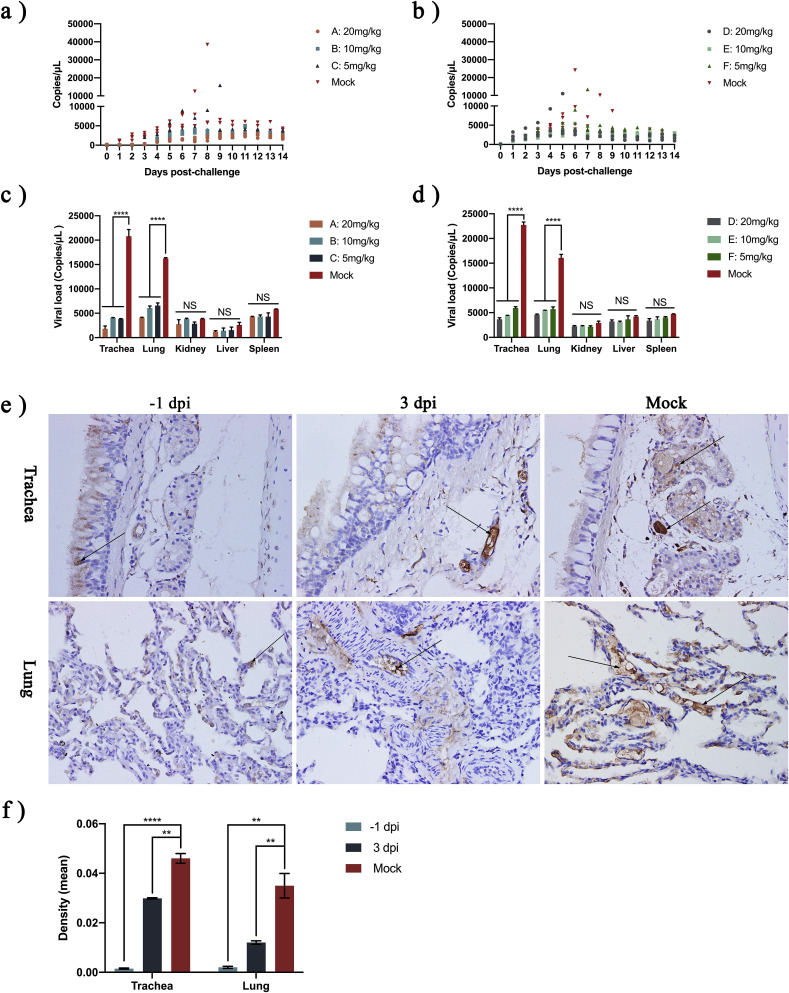
In the −1 dpi oral NTZ cats, one cat in group C died on day 10; viral shedding was 15871 copies/μL on the ninth day (Fig. 4 A). In the 3 dpi oral NTZ cats, one cat in group D died on day 6 and one cat in group F died on day 8; viral shedding at day 5 and day 7 was 11017 copies/μL and 13392 copies/μL, respectively (Fig. 4B). As expected, NTZ reduced viral load in both trachea and lungs (Fig. 4E). After oral NTZ treatment, either on −1 dpi or 3 dpi, levels of FCV in the trachea and lungs were significantly lower than in the control group (p < 0.0002) (Fig. 4F).
Fig. 4.
Nitazoxanide reduces virus shedding and viral load.
(A and B) FCV viral shedding in cat's mouth and nose test paper on 0 dpi, measured by RT-qPCR. (C and D) At 14 dpi, one cat was euthanized in each group, the trachea, lung, spleen, liver and kidney were collected, and shedding of FCV from the different tissues was measured. (E) Cats' tracheas and lungs were sectioned (400-fold) and the sections were treated with cat anti-FCV primary antibody and HRP-labeled rabbit anti-cat secondary antibody, followed by color development. Brown areas indicated by black arrows are FCV-positive. (F) Image-Pro Plus 6.0 was used to analysis optical density in two selected slices (400-fold); n = 4 cats/group; −1 dpi, 10 mg/kg NTZ; 3 dpi, 10 mg/kg NTZ; Control, 500 μL PBS (to simulate 10 mg/kg NTZ orally); NS p > 0.1234; *p < 0.1234; **p < 0.0021; ***p < 0.0002; ***p < 0.0001.
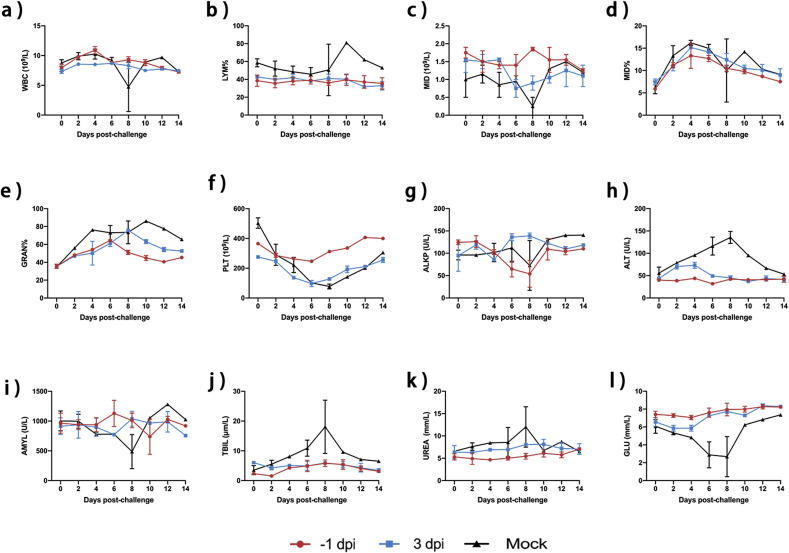
3.6. Complete blood count and biochemical analysis
WBC counts and LYM percentages fluctuated little in cats treated orally with NTZ. MID counts fluctuated, with both MID and GRAN firstly increasing and then decreasing (Fig. 5 A–F). PLT counts showed a slight downward trend but the PLT counts of cats in the −1 dpi oral drug group were within the normal range (Fig. 5F). One cat in the control group did not recover from the infection and died on day 8. The biochemical results showed that there were no significant fluctuations in ALT, AMYL, TBIL, UREA or GLU in cats that received NTZ orally at −1 dpi or 3 dpi, indicating no significant organ damage in these animals compared with the control group (Fig. 5G–L).
Fig. 5.
Complete blood count and biochemical analysis.
After oral NTZ treatment, two cats in groups B and E were selected randomly and venous blood samples were collected every two days for complete blood count and biochemical analysis. (A) White Blood Cell (WBC) counts. (B) Lymphocyte (LYM) percentages. (C) MID cells (MID) counts. (D) MID percentages. (E) Granulocytes (GRAN) percentages. (F) Platelets (PLT) counts. (G) Alkaline phosphatase (ALKP) levels. (H) Alanine transaminase (ALT) levels. (I) Amylase (AMYL) levels. (J) Total bilirubin (TBIL) levels. (K) Urea (UREA) levels. (L) Glucose (GLU) levels.
4. Discussion
There is currently little choice of drugs for the treatment of FCV and, in the clinic, broad-spectrum antiviral drugs, such as famciclovir and ganciclovir, remain the first choice. Recent research has shown, however, that famciclovir has limited efficacy against FCV (Fumian et al., 2018). NITD008, fexaramine and 2CMC are also candidate drugs to treat FCV infection. NTZ and 2CMC have been shown to have synergistic anti-FCV activity, and have been used to treat FCV-infected cats (Enosi Tuipulotu et al., 2019; Fumian et al., 2018; Kim and Chang, 2018). MZR has been shown to have antiviral activity against caprine alpha herpes virus 1 (CpHV-1), but it had not been determined whether it also has activity against FCV (Camero et al., 2017).
In our study, we found that NTZ and MZR have low cytotoxicity. The EC50 values of NTZ and MZR against FCV CH-JL2 were 1.53 μM, and 12.68 μM, respectively, and both compounds showed activity against other FCV strains. We also showed that NTZ and MZR act synergistically, with the most synergistic area scoring 45.5. To our knowledge, this is the first report of the antiviral activity of MZR against FCV. We chose the FDA-approved orally active medication NTZ for animal experiments. Previous studies found that NTZ caused diarrhea and vomiting (Siddiq et al., 2011), but we did not observe these side effects in our study. When we treated cats with different doses of NTZ, either at −1 dpi or 3 dpi, viral shedding and mortality were both reduced. The cats did not show significant changes in body temperature after oral NTZ at 3 dpi, but weight loss was reduced compared with the control group. There was significant relief of the symptoms of oral ulcers, especially in cats who received NTZ at 3 dpi, (Supplementary Material S2). The amount of FCV in the trachea and lungs, which are easily infected by FCV (Pesavento et al., 2011), was significantly reduced after treatment with different doses of NTZ, and this result was confirmed by immunohistochemistry. By analyzing complete blood counts and biochemical results, we found that systemic symptoms were alleviated in cats that received NTZ.
In conclusion, we have confirmed the antiviral activity of NTZ and MZR against FCV in vitro, and shown that the compounds acted synergistically. NTZ was shown to reduce viral load in the trachea and lungs and still had a therapeutic effect on FCV-infected cats when administered at 3 dpi. The synergy between NTZ and and MZR in vivo should now be evaluated and drug resistance studies should be carried out. These studies will provide further evidence that NTZ and MZR can be used as therapeutic agents for diseases caused by FCV.
Author contributions
Zhanding Cui, Dengliang Li, Yinli Xie, and Guixue Hu conceived and designed the experiments. Zhanding Cui, Dengliang Li and Yinli Xie performed the experiments. Zhanding Cui, Dengliang Li and Yinli Xie analyzed the data. Ying Zhang, Guohua Li, Qian Zhang, Xiaoxueying Chen, Yue Teng, Kai Wang, Shihui Zhao, Jiang Shao, Fan Xingmeng, Yanli Zhao, Dongju Du, Yanbing Guo, Hailong Huang and Hao Dong contributed reagents/materials/analysis tools. Zhanding Cui, Dengliang Li and Yinli Xie wrote the paper. Guixue Hu, Yongkun Zhao and Shuang Zhang requested financial support. All authors read and approved the manuscript.
Declaration of competing interest
The authors declare no conflicts of interest.
Acknowledgment
The present work was supported by the National Key R&D Program for the 13th Five-Year Plan, the Ministry of Science and Technology of China and the National Key R&D Program (2016YFD0501002).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.antiviral.2020.104827.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
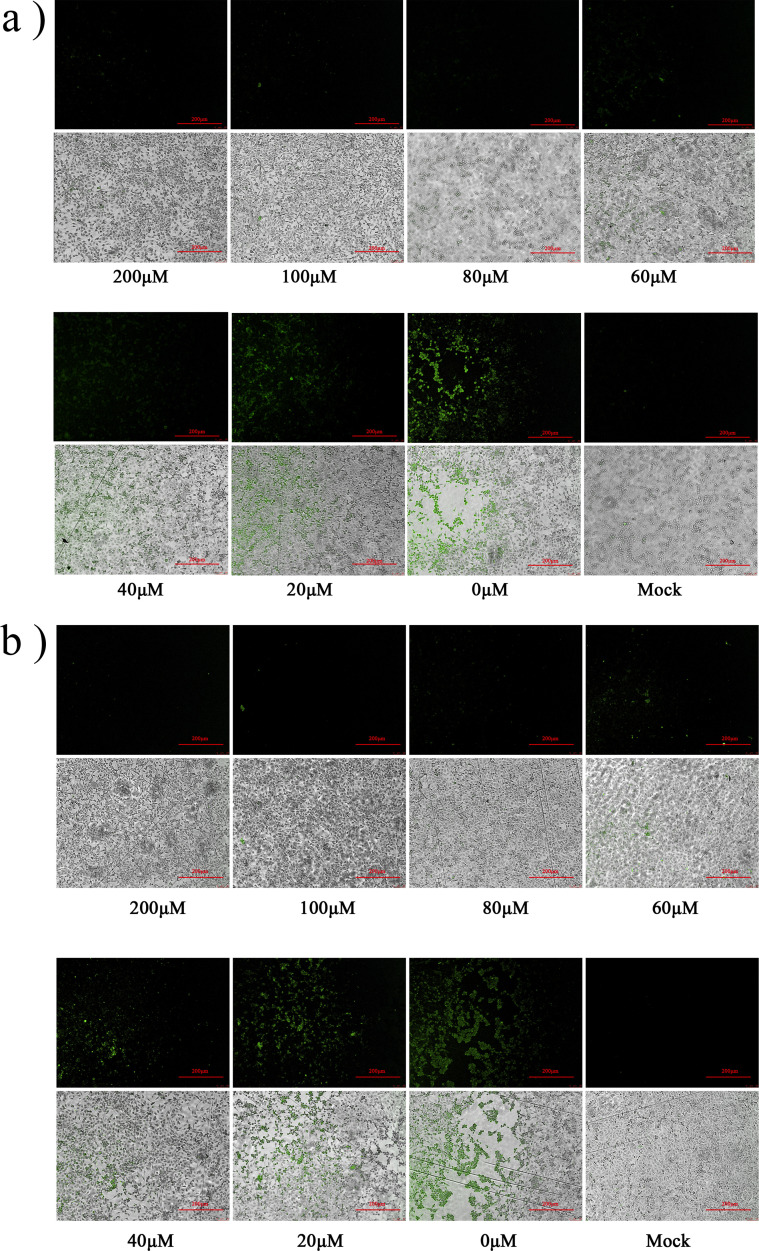
S1.
Nitazoxanide and Mizoribine IFA results. A solution of NTZ in DMSO (100 mM) was diluted to different concentrations (200 μM, 100 μM, 80 μM, 60 μM, 40 μM, 20 μM, 0 μM) with MEM. Solutions with different concentrations were then added to cells, together with 100 × TCID FCV. The mock group was treated with the same volume of MEM containing 0.4% DMSO. (A) NTZ (B) MZR.
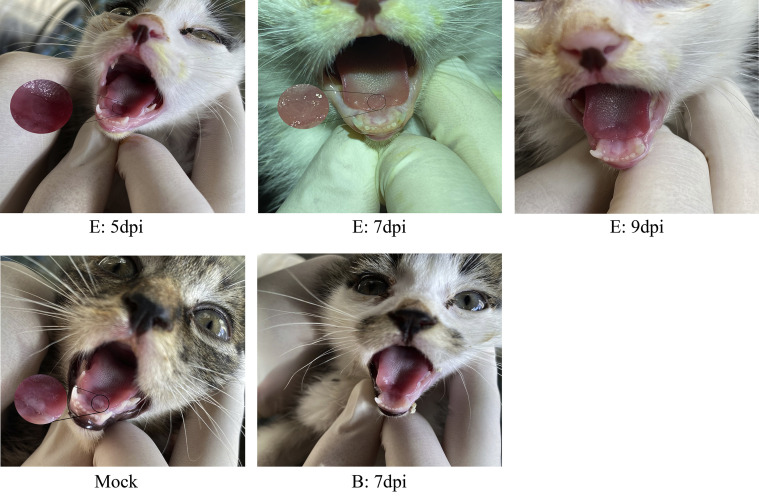
S2.
Reduction of cat oral ulcers.A cat in group E that started oral NTZ at 3 dpi developed a large area of oral ulcers at 3 dpi but the ulcerated area gradually decreased with time. A cat in group B that started oral NTZ at −1 dpi had no visible oral ulcers at 7 dpi. A cat in the control group had a mouth ulcer at 3 dpi.
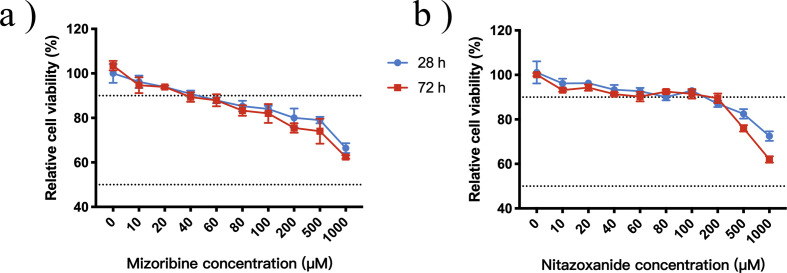
S3.
Nitazoxanide and Mizoribine cytotoxicity results A solution of NTZ and MZR in DMSO (100 mM) was diluted to different concentrations (1000 μM, 500 μM, 200 μM, 100 μM, 80 μM, 60 μM, 40 μM, 20 μM, 0 μM) with MEM. Solutions with different concentrations were then added to cells. The cells were incubated for 28 or 72 h at 37°C under an atmosphere containing 5% CO2. Cell viability was calculated using the following equation:Cell viability = [OD (Compound) - OD (blank)] / [OD (control) - OD (blank)] × 100%. (a) MZR (b) NTZ.
References
- Aboubakr H.A., Nauertz A., Luong N.T., Agrawal S., El-Sohaimy S.A.A., Youssef M.M., Goyal S.M. In vitro antiviral activity of clove and ginger aqueous extracts against feline calicivirus, a surrogate for human norovirus. J. Food Protect. 2016;79:1001–1012. doi: 10.4315/0362-028X.JFP-15-593. [DOI] [PubMed] [Google Scholar]
- Afonso M.M., Pinchbeck G.L., Smith S.L., Daly J.M., Gaskell R.M., Dawson S., Radford A.D. A multi-national European cross-sectional study of feline calicivirus epidemiology, diversity and vaccine cross-reactivity. Vaccine. 2017;35:2753–2760. doi: 10.1016/j.vaccine.2017.03.030. [DOI] [PubMed] [Google Scholar]
- Bergmann M., Speck S., Rieger A., Truyen U., Hartmann K. Antibody response to feline calicivirus vaccination in healthy adult cats. Viruses. 2019:11. doi: 10.3390/v11080702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camero M., Buonavoglia D., Lucente M.S., Losurdo M., Crescenzo G., Trerotoli P., Casalino E., Martella V., Elia G., Tempesta M. Enhancement of the antiviral activity against caprine herpesvirus type 1 of Acyclovir in association with Mizoribine. Res. Vet. Sci. 2017;111:120–123. doi: 10.1016/j.rvsc.2017.02.012. [DOI] [PubMed] [Google Scholar]
- Caringella F., Elia G., Decaro N., Martella V., Lanave G., Varello K., Catella C., Diakoudi G., Carelli G., Colaianni M.L., Bo S., Buonavoglia C. Feline calicivirus infection in cats with virulent systemic disease, Italy. Res. Vet. Sci. 2019;124:46–51. doi: 10.1016/j.rvsc.2019.02.008. [DOI] [PubMed] [Google Scholar]
- Cui Z., Li D., Yi S., Guo Y., Dong G., Niu J., Zhao H., Zhang Y., Zhang S., Cao L., Wang K., Zhao Y., Hu G. Equine immunoglobulin F(ab’)2 fragments protect cats against feline calicivirus infection. Int. Immunopharm. 2019;75:105714. doi: 10.1016/j.intimp.2019.105714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang W., Yin Y., Peppelenbosch M.P., Pan Q. Opposing effects of nitazoxanide on murine and human norovirus. J. Infect. Dis. 2017;216:780–782. doi: 10.1093/infdis/jix377. [DOI] [PubMed] [Google Scholar]
- Desselberger U. Caliciviridae other than noroviruses. Viruses. 2019;11:286. doi: 10.3390/v11030286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enosi Tuipulotu D., Fumian T.M., Netzler N.E., Mackenzie J.M., White P.A. The adenosine analogue NITD008 has potent antiviral activity against human and animal caliciviruses. Viruses. 2019:11. doi: 10.3390/v11060496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumian T.M., Tuipulotu D.E., Netzler N.E., Lun J.H., Russo A.G., Yan G.J.H., White P.A. Potential therapeutic agents for feline calicivirus infection. Viruses. 2018:10. doi: 10.3390/v10080433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H., Miao Q., Zhu J., Yang Z., Liu G. Isolation and molecular characterization of a virulent systemic feline calicivirus isolated in China. Infect. Genet. Evol. 2018;65:425–429. doi: 10.1016/j.meegid.2018.08.029. [DOI] [PubMed] [Google Scholar]
- Ianevski A., He L., Aittokallio T., Tang J. SynergyFinder: a web application for analyzing drug combination dose-response matrix data. Bioinformatics. 2017;33:2413–2415. doi: 10.1093/bioinformatics/btx162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H. Mizoribine and mycophenolate mofetil. Curr. Med. Chem. 1999;6:575–597. [PubMed] [Google Scholar]
- Jasenosky L.D., Cadena C., Mire C.E., Borisevich V., Haridas V., Ranjbar S., Nambu A., Bavari S., Soloveva V., Sadukhan S., Cassell G.H., Geisbert T.W., Hur S., Goldfeld A.E. The FDA-approved oral drug nitazoxanide amplifies host antiviral responses and Inhibits ebola virus. iScience. 2019;19:1279–1290. doi: 10.1016/j.isci.2019.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y., Chang K.-O. Fexaramine as an entry blocker for feline caliciviruses. Antivir. Res. 2018;152:76–83. doi: 10.1016/j.antiviral.2018.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S.-F., Gong M.-J., Sun Y.-F., Shao J.-J., Zhang Y.-G., Chang H.-Y. In vitro and in vivo antiviral activity of mizoribine against foot-and-mouth disease virus. Molecules. 2019;24 doi: 10.3390/molecules24091723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesavento P.A., Stokol T., Liu H., van der List D.A., Gaffney P.M., Parker J.S. Distribution of the feline calicivirus receptor junctional adhesion molecule a in feline tissues. Vet. Pathol. 2011;48:361–368. doi: 10.1177/0300985810375245. [DOI] [PubMed] [Google Scholar]
- Radford A.D., Gaskell R.M. Dealing with a potential case of FCV-associated virulent systemic disease. Vet. Rec. 2011;168:585–586. doi: 10.1136/vr.d3511. [DOI] [PubMed] [Google Scholar]
- Saijo M., Morikawa S., Fukushi S., Mizutani T., Hasegawa H., Nagata N., Iwata N., Kurane I. Inhibitory effect of mizoribine and ribavirin on the replication of severe acute respiratory syndrome (SARS)-associated coronavirus. Antivir. Res. 2005;66:159–163. doi: 10.1016/j.antiviral.2005.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H., Sehata G., Okada N., Iwamoto K., Masubuchi K., Kainuma R., Noda T., Igarashi T., Sawada T., Noro T., Oishi E. Intranasal immunization with inactivated feline calicivirus particles confers robust protection against homologous virus and suppression against heterologous virus in cats. J. Gen. Virol. 2017;98:1730–1738. doi: 10.1099/jgv.0.000827. [DOI] [PubMed] [Google Scholar]
- Schulz B.S., Hartmann K., Unterer S., Eichhorn W., Majzoub M., Homeier-Bachmann T., Truyen U., Ellenberger C., Huebner J. Two outbreaks of virulent systemic feline calicivirus infection in cats in Germany. Berl. Münchener Tierärztliche Wochenschr. 2011;124:186–193. [PubMed] [Google Scholar]
- Shigeta S. Recent progress in antiviral chemotherapy for respiratory syncytial virus infections. Expet Opin. Invest. Drugs. 2000;9:221–235. doi: 10.1517/13543784.9.2.221. [DOI] [PubMed] [Google Scholar]
- Shiraki K., Ishibashi M., Okuno T., Kokado Y., Takahara S., Yamanishi K., Sonoda T., Takahashi M. Effects of cyclosporine, azathioprine, mizoribine, and prednisolone on replication of human cytomegalovirus. Transplant. Proc. 1990;22:1682–1685. [PubMed] [Google Scholar]
- Siddiq D.M., Koo H.L., Adachi J.A., Viola G.M. Norovirus gastroenteritis successfully treated with nitazoxanide. J. Infect. 2011;63:394–397. doi: 10.1016/j.jinf.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuyver L.J., Lostia S., Patterson S.E., Clark J.L., Watanabe K.A., Otto M.J., Pankiewicz K.W. Inhibitors of the IMPDH enzyme as potential anti-bovine viral diarrhoea virus agents. Antivir. Chem. Chemother. 2002;13:345–352. doi: 10.1177/095632020201300602. [DOI] [PubMed] [Google Scholar]
- Tian J., Hu X., Liu D., Wu H., Qu L. Identification of Inonotus obliquus polysaccharide with broad-spectrum antiviral activity against multi-feline viruses. Int. J. Biol. Macromol. 2017;95:160–167. doi: 10.1016/j.ijbiomac.2016.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Pei Z., Dong H., Yang S., Dong G., Hu G. Isolation, genomic characterization and pathogenicity of a feline calicivirus strain Ch-Jl4 from Chinese stray cats. Pak. Vet. J. 2017;4 [Google Scholar]
- Wu H., Liu Y., Zu S., Sun X., Liu C., Liu D., Zhang X., Tian J., Qu L. In vitro antiviral effect of germacrone on feline calicivirus. Arch. Virol. 2016;161:1559–1567. doi: 10.1007/s00705-016-2825-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav B., Wennerberg K., Aittokallio T., Tang J. Searching for drug synergy in complex dose-response landscapes using an interaction potency model. Comput. Struct. Biotechnol. J. 2015;13:504–513. doi: 10.1016/j.csbj.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Chen X., Ying Y., Wang K., Dong H., Gao C., Yang S., Hu G. Isolation and phylogenetic analysis of three feline calicivirus strains from domestic cats in Jilin Province, China. Arch. Virol. 2017;162:2579–2589. doi: 10.1007/s00705-017-3392-3. [DOI] [PubMed] [Google Scholar]
- Zhou H., Su X., Lin L., Zhang J., Qi Q., Guo F., Xu F., Yang B. Inhibitory effects of antiviral drug candidates on canine parvovirus in F81 cells. Viruses. 2019 doi: 10.3390/v11080742. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]