Abstract
Exposure to particulate air pollution is a major environmental risk factor for cardiovascular mortality and morbidity, on a global scale. Both acute and chronic cardiovascular impacts have so far been attributed to particulate-mediated oxidative stress in the lung and/or via ‘secondary’ pathways, including endothelial dysfunction, and inflammation. However, increasing evidence indicates the translocation of inhaled nanoparticles to major organs via the circulation. It is essential to identify the composition and intracellular targets of such particles, since these are likely to determine their toxicity and consequent health impacts. Of potential major concern is the abundant presence of iron-rich air pollution nanoparticles, emitted from a range of industry and traffic-related sources. Bioreactive iron can catalyse formation of damaging reactive oxygen species, leading to oxidative stress and cell damage or death.
Here, we identify for the first time, in situ, that exogenous nanoparticles (~15–40 nm diameter) within myocardial mitochondria of young, highly-exposed subjects are dominantly iron-rich, and co-associated with other reactive metals including aluminium and titanium. These rounded, electrodense nanoparticles (up to ~ 10 x more abundant than in lower-pollution controls) are located within abnormal myocardial mitochondria (e.g. deformed cristae; ruptured membranes). Measurements of an oxidative stress marker, PrPC and an endoplasmic reticulum stress marker, GRP78, identify significant ventricular up-regulation in the highly-exposed vs lower-pollution controls. In shape/size/composition, the within-mitochondrial particles are indistinguishable from the iron-rich, combustion- and friction-derived nanoparticles prolific in roadside/urban environments, emitted from traffic/industrial sources. Incursion of myocardial mitochondria by inhaled iron-rich air pollution nanoparticles thus appears associated with mitochondrial dysfunction, and excess formation of reactive oxygen species through the iron-catalyzed Fenton reaction. Ventricular oxidative stress, as indicated by PrPC and GRP78 up-regulation, is evident even in children/young adults with minimal risk factors and no co-morbidities. These new findings indicate that myocardial iron overload resulting from inhalation of airborne, metal-rich nanoparticles is a plausible and modifiable environmental risk factor for cardiac oxidative stress and cardiovascular disease, on an international scale.
Keywords: Particulate air pollution, Nanoparticles, Ultrafine particles, Mitochondrial dysfunction, Iron, Cardiac oxidative stress, Cardiovascular disease, Heart, Mexico City
Highlights
-
•
Air pollution nanoparticles inside human myocardial mitochondria are iron-rich.
-
•
The within-mitochondrial particles match iron-rich particles from traffic/industrial sources.
-
•
Myocardial iron-rich air pollution nanoparticles abundant even in young children.
-
•
Ventricular upregulation of oxidative and ER stress markers in exposed subjects.
-
•
Inhalation of iron-rich air pollution a risk factor for cardiovascular disease.
1. Introduction
Exposure to fine particulate matter (PM2.5, <2.5 μm in aerodynamic diameter) in air pollution is reportedly the largest environmental risk factor contributing to cardiovascular mortality and morbidity, globally (Miller and Newby, 2020; Newby et al., 2015; Pope et al., 2006; Rajagopalan et al., 2018). The most recent exposure-hazard calculations indicate that the annual excess mortality rate from ambient air pollution (mainly PM2.5) in Europe is 790,000 [95% confidence interval (95% CI) 645,000–934,000], of which between 40 and 80% are due to cardiovascular events, which dominate health outcomes (Lelieveld et al., 2019). Short-term PM2.5 exposure raises risk of acute myocardial infarction by up to 5% within a few days (Pope et al., 2006). Longer-term (i.e. several years) exposures incur higher risk (~20%) of cardiovascular events, ascribed partially to development of associated cardiometabolic conditions, e.g. hypertension, diabetes mellitus (Rajagopalan et al., 2018). Exposure to higher ambient PM2.5 concentrations has also been linked specifically with the development of high-risk coronary plaques (Yang et al., 2019).
Traffic-derived PM, arising from both exhaust and non-exhaust emissions (e.g. Gonet and Maher, 2019), may dominate individual exposures and cardiovascular outcomes. In a Dutch cohort, living near major roads was associated with increased cardiopulmonary mortality (relative risk, RR: 1.95, 95% CI: 1.09–3.52) (Hoek et al., 2002). German studies showed a RR of 1.85 (95% CI: 1.21–2.84) in patients living within 150 m of a major road (Hoffmann et al., 2006). The majority of particles emitted from traffic-related PM sources are ultrafine (<100 nm) in size. Most comprise (semi-) volatile carbon-bearing aerosols (some with non-volatile cores). The solid, inorganic fraction is dominated by transition metals, and especially by potentially bioreactive iron oxides, produced in abundance from brake-wear and from exhaust emissions (Gonet and Maher, 2019; Harrison et al., 2004; Sanderson et al., 2016). Despite their toxicity and potential ability to gain access to any organ of the body via ingestion, inhalation and/or the circulation, ultrafine particles are neither monitored nor regulated at the current time. Ultrafine particle numbers show little correlation with measurements of PM2.5 (de Jesus et al., 2019).
So far, the mechanisms linking the statistical associations between exposure to PM2.5 and cardiovascular impacts have been attributed to PM-mediated oxidative stress in the lung and/or more systemically across vascular beds, and/or to ‘secondary’ effector pathways, including endothelial barrier disruption, inflammation, arrhythmogenesis and pro-thrombotic processes (Miller and Newby, 2020; Newby et al., 2015; Rajagopalan et al., 2018). However, major gaps remain in our understanding of the causality underlying the epidemiological associations between PM exposure and CVD, and, critically, of the specific causal components and CV targets of airborne PM. The translocation of air pollution nanoparticles from their portal of entry to remote tissues may constitute the key link between exposure to particulate air pollution and the observed and multiple epidemiological associations (Miller and Newby, 2020; Calderon-Garciduenas et al., 2019; Maher et al., 2016).
1.1. Exogenous nanoparticles in myocardial mitochondria of young urbanites
Recent, landmark studies have demonstrated (post mortem) direct penetration of air pollution nanoparticles into the human brain (Maher et al., 2016) and heart (Calderon-Garciduenas et al., 2019), and (experimentally) the systemic circulation of gold nanoparticles in mice and humans (Miller et al., 2017). These studies indicate that exogenous nanoparticles can be translocated to major organs. Such particle translocation might constitute a key pathway accounting for the associations between exposure to airborne PM and the multiple manifestations of damage to the CV system. Critical, however, is to identify the composition and intracellular location of exogenous particles, since these will influence their reactivity and cytotoxicity in target tissues remote from their portal of entry.
Here, building upon our prior investigation (Calderon-Garciduenas et al., 2019), we identify for the first time, in situ, the composition of exogenous nanoparticles in human myocardial mitochondria. These new findings substantiate our identification of these within-mitochondrial nanoparticles as air pollution-, combustion- and friction-derived nanoparticles, and underline their likely major impacts upon mitochondrial function and cardiac oxidative stress - even in very young cases with minimal conventional risk factors and no co-morbidities.
2. Materials and methods
Here, we examined post mortem left ventricular tissue from 2 cases (3y and 26y) randomly selected from 63 previously-investigated children and young adults. For both the 3 y and 26 y old, there are no potential confounding variables (i.e. no obesity, smoking/alcohol use/drug-taking, occupational hazard, or chronic/ageing-related diseases). Both resided in Metropolitan Mexico City (MMC), where PM2.5 levels frequently exceed US EPA (~40% of days) and WHO (~75% of days) standards (Supplementary Fig. 1). Control samples were also previously obtained from 9 subjects who had resided in less populated and polluted locations (Veracruz city, Veracruz, Mexico). No air quality data are available for Veracruz but emission inventories indicate PM2.5 emissions of ~15,433 ton/y in MMC, compared with 4518 ton/y in Veracruz. Our prior investigations (Calderon-Garciduenas et al., 2019) had identified, by bright field transmission electron microscopy (TEM), the abundant presence of electrodense nanoparticles - of unknown composition - in the myocardium of the 63 exposed MMC cases (~2–10 x the particle numbers in 9 controls). Magnetic measurements of bulk tissue samples, and high-resolution TEM (HRTEM) of magnetic particles extracted from digested tissue samples, had identified that at least some of these myocardial particles consisted of the strongly magnetic minerals, magnetite and/or maghemite (Calderon-Garciduenas et al., 2019). However, until now, no analysis had been made of the elemental composition of the myocardial nanoparticles in situ (reflecting the analytical challenges in acquiring HRTEM data at high spatial resolution in tissue sections vulnerable to electron beam damage).
The Instituto de Ciencias Forenses approved the collection of samples from forensic autopsies. Autopsies were performed 3.7 ± 1.9 h after sudden death (accidents, homicides, suicides); subjects had no pathological evidence of inflammatory processes, CVD, chest trauma, head injury, or stroke. For TEM, heart sections were dissected and cut with ceramic knifes and plastic forceps, free from metal contamination, and fixed in 2% paraformaldehyde and 2% glutaraldehyde in sodium phosphate buffer.
We used TEM (FEI Titan3 Themis 300, X-FEG S/TEM with S-TWIN objective lens, monochromator (energy spread approx. 0.25 eV) and multiple HAADF/ADF/BF STEM detectors, operated at 300 kV) and energy dispersive X ray analysis (EDX, FEI Super-X 4-detector system) to examine the intra-cellular location of electrodense nanoparticles, their shape, size, and, perhaps most critically in terms of toxicity and biological impact, their elemental composition. To limit beam-induced damage, a probe current of 60 pA was used to acquire the elemental maps. To ensure that the nanoparticles identified and analysed can be considered representative of the samples, samples were analysed blind to case, and grids/tissue sections and grid areas were randomly selected and methodically scanned.
3. Results
Whereas left ventricular tissue and mitochondria from a control subject were normal (Fig. 1 A), TEM imaging in the Mexico City 3y old (Fig. 1B) revealed the abundant presence of rounded, electron-dense nanoparticles, mostly ~15–40 nm, most frequently inside mitochondria.
Fig. 1.
A. Control (low pollution exposure) 17y male left ventricle with intact mitochondria (m) and absence of nanoparticles. B. Three year old MMC boy with mitochondria (m) containing electrodense, rounded nanoparticles (black arrows) and fibrillary matrix material (* in insert).
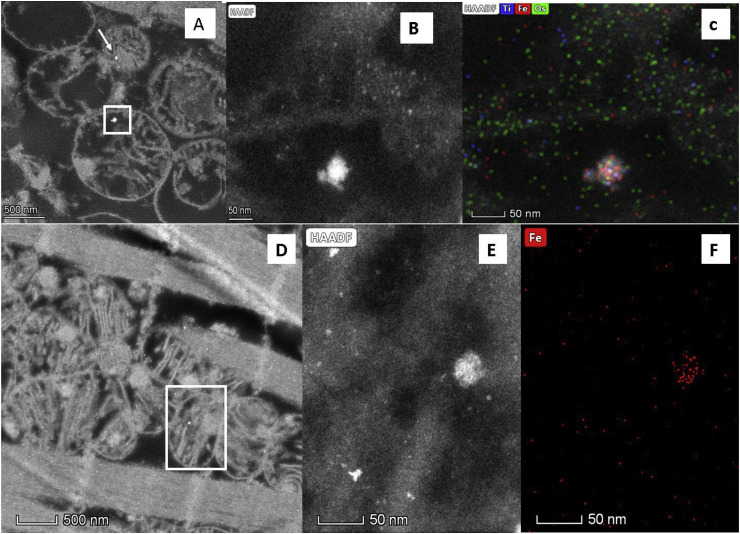
The HRTEM/EDX data identify that the composition of these electrodense, rounded, within-mitochondrial nanoparticles (Fig. 2 A, B, D and E) is metal-rich, and dominated by iron (oxides) (Fig. 2C and F). Additional, trace exogenous metal species co-associated with the iron-rich nanoparticles include aluminium and titanium (Fig. 2C). The mitochondria containing these iron-rich nanoparticles appear damaged, with deformed, fragmented or missing cristae and altered, sometimes ruptured membrane structures (Fig. 1B).
Fig. 2.
High-angle annular dark-field scanning/transmission electron microscopy (HAADF-STEM) of left ventricle tissue, 3 y old (A–C) and 26 y old (D–F) MMC cases. A, B, D and E: Bright, electrodense NPs (white arrow and boxes), between ~15 and 40 nm, inside mitochondria; C and F: Elemental maps showing the iron-rich composition of the NPs shown in white boxes in A and D. (The smallest electrodense particles, < 10 nm, comprise osmium tetroxide used to stain the tissue).
Even in the very young Mexico City cases examined here, cardiac oxidative stress is evident, with significant up-regulation of the normal cellular isoform of the prion protein, PrPC, which acts as a key oxidative stress response. PrPC values are 3.16 (3 y old) and 9.25 (26 y old), against an average of 1.06 (variance 0.004) for 9 controls (the mean of the 63 exposed cases = 8.7 x mean 9 controls, p = 0.0003)13. Significant up-regulation of GRP78, a marker of endoplasmic reticulum stress, is also evident in the Mexico City cases: 1.485 (3 y old) and 4.227 (26 y old) versus an average of 1.05 (variance 0.00489) for 9 controls, (mean, 63 exposed cases = 3.2 x mean 9 controls, p = 0.0001)13. These data are shown in Supplementary Table 1.
4. Discussion
4.1. Impacts of iron overload in mitochondria
That the composition of these within-mitochondrial nanoparticles is dominated by the presence of iron is likely to have major implications for mitochondrial function and cardiac oxidative stress. Mitochondria are particularly abundant in ventricular tissue, driving energy supply, and modifying cell signalling by continuous production of reactive oxygen species (ROS), such as the superoxide anion (O2(−)) and hydrogen peroxide (H2O2). But with iron overload, the iron-catalyzed Fenton reaction can transform these less oxidising products into the highly reactive hydroxyl radical (HO·) which can attack proteins, lipids and DNA, leading to cell and tissue damage. Indeed, chronic and acute increases in myocardial mitochondrial ROS production can lead to a catastrophic cycle of mitochondrial DNA damage, functional decline, and further oxygen radical generation (Manickam et al., 2017; Nemmar et al., 2016; Tsutsui et al., 2006). Resultant cellular injury can include myocyte hypertrophy, endothelial dysfunction, apoptosis, and thrombosis (Tsutsui et al., 2006), and progressive modification of myocardial gene responses to oxidative and endoplasmic reticulum stress (Manickam et al., 2017; Villarreal-Calderon et al., 2013). Thus, iron-driven myocardial mitochondrial dysfunction and uncontrolled ROS production can play a major role in initiation and progression of cardiovascular disease. That cardiac myopathy can result from mitochondrial iron overload is evident, for example, in Friedreich's ataxia, an inherited neurological disease associated with iron dysregulation (Martelli and Puccio, 2014). For exposed urbanites, excess mitochondrial iron may be an environmental, rather than a genetic, risk factor; iron-rich air pollution particles readily available in acute and/or chronic doses via inhalation.
4.2. The link with air pollution: combustion- and friction-derived iron-rich nanoparticles
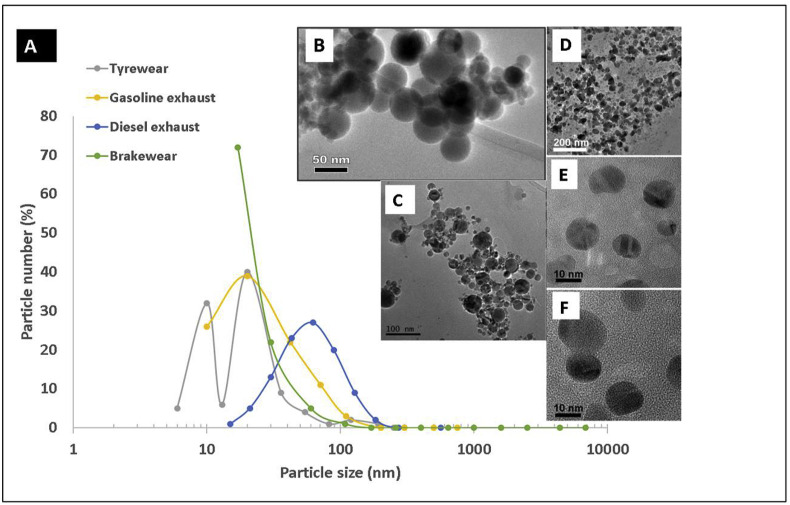
In size, shape and composition, the within-mitochondrial, iron-dominated nanoparticles identified here in the Mexico City cases (Fig. 2) are indistinguishable from the iron-rich nanoparticles so abundant and pervasive in urban and roadside air pollution (Fig. 3 B − F). Of key potential concern for cardiovascular outcomes is that, almost invariably, iron is the most abundant metal in the solid (non-volatile) fraction of the ultrafine particles present in urban air pollution (Fig. 3A). Fig. 3B − F shows iron-rich nanoparticles collected at urban roadsides (Maher, 2019), demonstrating their abundance, nanoscale dimensions, and often rounded shape (reflecting their formation as ‘molten’ droplets, which cool and crystallise rapidly upon emission into the air). Outdoors, ultrafine iron-rich air pollution nanoparticles are emitted, mostly as combustion- and friction-derived nanoparticles, from many sources, including industry (e.g. power generation, incinerators, steelworks), non-exhaust vehicle sources – especially brake-wear - and exhaust (fuel, oil, catalyst and engine wear) emissions (see review by Gonet and Maher, 2019). Indoors, iron-rich nanoparticles can be emitted from sources as diverse as open fires, and office printers (Gminski et al., 2011).
Fig. 3.
A. The particle size distribution of air pollution particles emitted from different road traffic sources (calculated as a % of the total emitted from each separate source). The majority of particles (a mixture of volatile carbon-bearing and solid metal-bearing particles) emitted from each individual source occur as nanoparticles (<100 nm). N.B. Since each measurement of particle numbers emitted from each source (diesel, gasoline, brake wear and tyre wear) was made under different conditions (e.g. different dynamometer cycles, sampling techniques etc), it is not possible currently to compare the absolute numbers of particles emitted by any particular source (especially in the cases of tyre and brake wear, where no ‘standard’ sampling/measurement procedure has yet been established). B–F: Transmission electron micrographs of iron- (and magnetite-) rich air pollution nanoparticles: B: from diesel exhaust (Liati et al., 2015); C: from roadside particles collected in Birmingham, U.K. (Sanderson et al., 2016); D–F: from roadside particles collected in Lancaster, U.K. (Maher, 2019).
Hence, airborne iron-rich pollution nanoparticles, often co-associated with transition metals (including Ni, Cu, Co, Pt, Ti), are abundant and pervasive in the urban environment, especially along heavily-trafficked roads. They occur as a range of iron and iron oxide phases, the latter including strongly magnetic magnetite (Fe3O4, a mixed ferrous/ferric iron oxide) and maghemite (γ Fe2O3), and the most oxidised form, haematite (α Fe2O3). In exhaust emissions (Fig. 3B), these iron phases are formed as primary particles of incombustible ash, forming from iron impurities in fuel (Liati et al., 2015; Abdul-Razzaq and Gautam, 2001), in-cylinder melting of engine fragments (Liati et al., 2015), and use of iron-rich lubricating oils and fuel additives (e.g. ferrocene) (Wakefield et al., 2008).
The strongly magnetic, magnetite-like phases are formed and emitted in particular abundance from vehicle brake wear. Frictional heat and pressure upon braking causes the release, melting, condensation and partial oxidation of iron from the brake pad and, possibly, the brake disk (Gonet and Maher, 2019; Kukutschova et al., 2011). In some commercially-available brake pads, up to 50% by wt magnetite powder is added as a lubricant/filler. Brake wear thus provides a substantial source of iron- and magnetite-rich nanoparticles to the roadside air (Gonet and Maher, 2019). Tyre-wear also contributes iron-rich particles, especially in the fine and ultrafine particle size fractions (Gustafsson et al., 2008). The number of just the strongly magnetic iron-rich nanoparticles (i.e. not including the weakly magnetic iron oxide particles, like haematite) in roadside air pollution can be estimated by magnetic remanence measurements of pumped air samples. In Lancaster, U.K. (a moderate-sized city, population ~ 138,000), for example, magnetic nanoparticle number concentrations reach ~4 × 108/m3 of roadside air. Strongly magnetic nanoparticles thus comprise ~1% of the total roadside particle numbers (much of the remainder being composed of elemental carbon and volatile organic carbon compounds) and ~10% of the primary (non-volatile) nanoparticles (Maher, 2019).
It is likely that many components of airborne PM can elicit oxidative stress, whether singly and/or synergistically with other components. In the case of iron, it is also likely that the particle size, specific iron phase, and co-associated metal species, together with any surface corona formed upon interaction with the biological substrates encountered, will play important roles in governing subsequent impacts in the intra-cellular environment. Because iron can have many different particulate sources (including natural, windblown dust), it may be that some previous studies have not discriminated between them, producing an ‘umbrella’ analysis of total iron, rather than of its more bioreactive, ultrafine components. (In fact, there are numerous studies of PM composition which do not provide Fe content, presumably because of the catholic nature of its sources). In the increasing number of studies examining the impacts of iron oxide nanoparticles on cells, it is clear that those impacts vary widely depending on both the properties of the iron oxide particles used, and the types of cell lines and antioxidants used. Overall, more, and more specific, information and data are necessary in order to resolve the question of the relative potential impacts of different PM species. Evident from our current findings, however, is that iron dominates the composition of the within-mitochondria nanoparticles; perhaps unsurprising since iron is frequently the dominant metal species in the solid fraction of the ultrafine particles produced and emitted in urban airborne pollution.
The abundance of iron-rich mitochondrial air pollution nanoparticles in myocardial cells, even in a 3 y old, indicates their direct translocation to the heart following repeated inhalation exposure in urban environments. The myocardial iron-rich nanoparticles are co-associated with other potentially bioreactive, exogenous metals (e.g. Al (Hashimoto et al., 2016), Ti (Wang et al., 2015), Fig. 2). Such metal mixtures are typical of airborne combustion- and friction-derived nanoparticles; the former also often bear surface-adsorbed organic species, including carcinogenic and ROS-active polyaromatic hydrocarbons (Maher, 2019). Notwithstanding that the composition and concentrations of air pollution nanoparticles may differ between urban locations (e.g. depending on size/mix/age of vehicle fleet, subway materials, industry sources etc), contributing to differential health impacts across the globe, these findings are directly relevant to heavily-trafficked urban environments, on an international scale. They may bear further significance in light of the current Covid-19 pandemic, with particulate air pollution and prior health damage (especially CV disease and diabetes) linked to increased Covid-19 mortality rates (Wu et al., 2020).
Finally, given that many of the iron-rich nanoparticles in the left ventricular tissues of the exposed cases are strongly magnetic (magnetite and/or maghemite) (Calderon-Garciduenas et al., 2019), then such particles may respond to external magnetic fields (e.g. from household appliances, like hair dryers (Kirschvink et al., 1992) and mobile phones, and from occupational exposures, such as those reported for welders, and power line engineers (Bowman et al., 2007)). Depending on their location and ferrimagnetic response (the latter determined by magnetite particle size, concentration and inter-particle interactions), magnetic pollution particles translocated to the heart might feasibly induce heart electrical dysfunction, and cell damage, whether by magnetic rotation or hyperthermia.
5. Conclusions
In conclusion, these findings indicate that acute and chronic exposures specifically to iron-rich airborne nanoparticles, which are abundant in the urban environment, constitute a plausible, pervasive risk factor for cardiac mitochondrial dysfunction and subsequent CVD development, from earliest childhood. Critically, exposure to such air pollution represents a modifiable risk factor for CVD, on a global scale, reinforcing the urgent need for individual and government actions not just to reduce PM2.5 but to monitor, regulate and reduce emissions of these specific, ultrafine components of the urban air pollution ‘cocktail’. Given that exposure to air pollution is estimated to cause >3 million premature, CVD-related deaths annually (more than arise from conventional cardiac risk factors such as smoking, obesity or diabetes) (Hadley et al., 2018), it seems essential that health care professionals assess cardiovascular risk from iron-rich air pollution exposure at every life stage, including (and perhaps especially) the pre-natal and childhood phases.
Credit author statement
Barbara Maher and Lilian Calderón-Garcidueñas contributed equally to this study, designing the study and writing the paper. Barbara Maher also performed the HRTEM/EDX analyses; Lilian Calderón-Garcidueñas, Angelica González-Maciel and Rafael Reynoso-Robles prepared the samples and performed the PrPC and GRP78 analyses. Ricardo Torres-Jardón contributed information on the Mexico City PM2.5 data.
Declaration of competing interest
B. Maher receives funding for a PhD Studentship from JaguarLandRover, UK. The remaining authors have nothing to disclose.
Acknowledgements
The authors thank Zabeada Aslam and Mark S'Ari of Leeds University's EPSRC Nanoscience and Nanotechnology Facility (LENNF) and Partha S. Mukherjee from the Indian Statistical Institute, Kolkata, India for their support and assistance in this work. This work was partially supported by E022 Instituto Nacional de Pediatria, Mexico City, Mexico.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envres.2020.109816.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Abdul-Razzaq W., Gautam M. Discovery of magnetite in the exhausted material from a diesel engine. Appl. Phys. Lett. 2001;78(14):2018–2019. [Google Scholar]
- Bowman J.D., Touchstone J.A., Yost M.G. A population-based job exposure matrix for power-frequency magnetic fields. J. Occup. Environ. Hyg. 2007;4(9):715–728. doi: 10.1080/15459620701528001. [DOI] [PubMed] [Google Scholar]
- Calderon-Garciduenas L., Gonzalez-Maciel A., Mukherjee P.S., Reynoso-Robles R., Perez-Guille B., Gayosso-Chavez C., Torres-Jardon R., Cross J.V., Ahmed I.A.M., Karloukovski V.V., Maher B.A. Combustion- and friction-derived magnetic air pollution nanoparticles in human hearts. Environ. Res. 2019;176 doi: 10.1016/j.envres.2019.108567. [DOI] [PubMed] [Google Scholar]
- de Jesus A.L., Rahman M.M., Mazaheri M., Thompson H., Knibbs L.D., Jeong C., Evans G., Nei W., Ding A.J., Qiao L.P., Li L., Portin H., Niemi J.V., Timonen H., Luoma K., Petaja T., Kulmala M., Kowalski M., Peters A., Cyrys J., Ferrero L., Manigrasso M., Avino P., Buonano G., Reche C., Querol X., Beddows D., Harrison R.M., Sowlat M.H., Sioutas C., Morawska L. Ultrafine particles and PM2.5 in the air of cities around the world: are they representative of each other? Environ. Int. 2019;129:118–135. doi: 10.1016/j.envint.2019.05.021. [DOI] [PubMed] [Google Scholar]
- Gminski R., Decker K., Heinz C., Seidel A., Könczöl M., Goldenberg E., Grobéty B., Ebner W., Gieré R., Mersch‐Sundermann V. Genotoxic effects of three selected black toner powders and their dimethyl sulfoxide extracts in cultured human epithelial A549 lung cells in vitro. Environ. Mol. Mutagen. 2011;52(4):296–309. doi: 10.1002/em.20621. [DOI] [PubMed] [Google Scholar]
- Gonet T., Maher B.A. Airborne, vehicle-derived Fe-bearing nanoparticles in the urban environment: a review. Environ. Sci. Technol. 2019;53(17):9970–9991. doi: 10.1021/acs.est.9b01505. [DOI] [PubMed] [Google Scholar]
- Gustafsson M., Blomquist G., Gudmundsson A., Dahl A., Swietlicki E., Bohyard M., Lindbom J., Ljungman A. Properties and toxicological effects of particles from the interaction between tyres, road pavement and winter traction material. Sci. Total Environ. 2008;393(2-3):226–240. doi: 10.1016/j.scitotenv.2007.12.030. [DOI] [PubMed] [Google Scholar]
- Hadley M.B., Baumgartner J., Vedanthan R. Developing a clinical approach to air pollution and cardiovascular health. Circulation. 2018;137(7):725–742. doi: 10.1161/CIRCULATIONAHA.117.030377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison R.M., Jones A.M., Lawrence R.G. Major component composition of PM10 and PM2.5 from roadside and urban background sites. Atmos. Environ. 2004;38(27):4531–4538. [Google Scholar]
- Hashimoto M., Sasaki J.I., Imazato S. Investigation of the cytotoxicity of aluminum oxide nanoparticles and nanowires and their localization in L929 fibroblasts and RAW264 macrophages. J. Biomed. Mater. Res. B Appl. Biomater. 2016;104(2):241–252. doi: 10.1002/jbm.b.33377. [DOI] [PubMed] [Google Scholar]
- Hoek G., Brunekreef B., Goldbohm S., Fischer P., van den Brandt P.A. Association between mortality and indicators of traffic-related air pollution in The Netherlands: a cohort study. Lancet. 2002;360(9341):1203–1209. doi: 10.1016/S0140-6736(02)11280-3. [DOI] [PubMed] [Google Scholar]
- Hoffmann B., Moebus S., Stang A., Beck E.-M., Dragano N., Moehlenkamp S., Schmermund A., Memmesheimer M., Mann K., Erbel R., Joeckel K.-H., Heinz Nixdorf R.S.I. Residence close to high traffic and prevalence of coronary heart disease. Eur. Heart J. 2006;27(22):2696–2702. doi: 10.1093/eurheartj/ehl278. [DOI] [PubMed] [Google Scholar]
- Kirschvink J.L., Kobayashikirschvink A., Diazricci J.C., Kirschvink S.J. Magnetite IN human tissues - a mechanism for the biological effects OF weak ELF magnetic-fields. Bioelectromagnetics. 1992:101–113. doi: 10.1002/bem.2250130710. [DOI] [PubMed] [Google Scholar]
- Kukutschova J., Moravec P., Tomasek V., Matejka V., Smolik J., Schwarz J., Seidlerova J., Safarova K., Filip P. On airborne nano/micro-sized wear particles released from low-metallic automotive brakes. Environ. Pollut. 2011;159(4):998–1006. doi: 10.1016/j.envpol.2010.11.036. [DOI] [PubMed] [Google Scholar]
- Lelieveld J., Klingmuller K., Pozzer A., Poschl U., Fnais M., Daiber A., Munzel T. Cardiovascular disease burden from ambient air pollution in Europe reassessed using novel hazard ratio functions. Eur. Heart J. 2019;40(20):1590–1596. doi: 10.1093/eurheartj/ehz135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liati A., Pandurangi S.S., Boulouchos K., Schreiber D., Dasilva Y.A.R. Metal nanoparticles in diesel exhaust derived by in-cylinder melting of detached engine fragments. Atmos. Environ. 2015;101:34–40. [Google Scholar]
- Maher B.A. Airborne magnetite- and iron-rich pollution nanoparticles: potential neurotoxicants and environmental risk factors for neurodegenerative disease, including alzheimer's disease. J. Alzheim. Dis. 2019;71(2):361–375. doi: 10.3233/JAD-190204. [DOI] [PubMed] [Google Scholar]
- Maher B.A., Ahmed I.A., Karloukovski V., MacLaren D.A., Foulds P.G., Allsop D., Mann D.M., Torres-Jardón R., Calderon-Garciduenas L. Magnetite pollution nanoparticles in the human brain. Proc. Natl. Acad. Sci. Unit. States Am. 2016;113(39):10797–10801. doi: 10.1073/pnas.1605941113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manickam V., Periyasamy M., Dhakshinamoorthy V., Panneerselvam L., Perumal E. Recurrent exposure to ferric oxide nanoparticles alters myocardial oxidative stress, apoptosis and necrotic markers in male mice. Chem. Biol. Interact. 2017;278:54–64. doi: 10.1016/j.cbi.2017.10.003. [DOI] [PubMed] [Google Scholar]
- Martelli A., Puccio H. Dysregulation of cellular iron metabolism in Friedreich ataxia: from primary iron-sulfur cluster deficit to mitochondrial iron accumulation. Front. Pharmacol. 2014;5 doi: 10.3389/fphar.2014.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M.R., Newby D.E. Air pollution and cardiovascular disease: car sick. Cardiovasc. Res. 2020;116(2):279–294. doi: 10.1093/cvr/cvz228. [DOI] [PubMed] [Google Scholar]
- Miller M.R., Raftis J.B., Langrish J.P., McLean S.G., Samutrtai P., Connell S.P., Wilson S., Vesey A.T., Fokkens P.H.B., Boere A.J.F., Krystek P., Campbell C.J., Hadoke P.W.F., Donaldson K., Cassee F.R., Newby D.E., Duffin R., Mills N.L. Inhaled nanoparticles accumulate at sites of vascular disease (vol 11, pg 4542, 2017) ACS Nano. 2017;11(10):10623–10624. doi: 10.1021/acsnano.6b08551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemmar A., Beegam S., Yuvaraju P., Yasin J., Tariq S., Attoub S., Ali B.H. Ultrasmall superparamagnetic iron oxide nanoparticles acutely promote thrombosis and cardiac oxidative stress and DNA damage in mice. Part. Fibre Toxicol. 2016;13 doi: 10.1186/s12989-016-0132-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newby D.E., Mannucci P.M., Tell G.S., Baccarelli A.A., Brook R.D., Donaldson K., Forastiere F., Franchini M., Franco O.H., Graham I., Hoek G., Hoffmann B., Hoylaerts M.F., Kuenzli N., Mills N., Pekkanen J., Peters A., Piepoli M.F., Rajagopalan S., Storey R.F., European Assoc C., Assoc E.S.C.H.F. Expert position paper on air pollution and cardiovascular disease. Eur. Heart J. 2015;36(2) doi: 10.1093/eurheartj/ehu458. 83-U28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope C.A., Muhlestein J.B., May H.T., Renlund D.G., Anderson J.L., Horne B.D. Ischemic heart disease events triggered by short-term exposure to fine particulate air pollution. Circulation. 2006;114(23):2443–2448. doi: 10.1161/CIRCULATIONAHA.106.636977. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S., Al-Kindi S.G., Brook R.D. Air pollution and cardiovascular disease JACC state-of-the-art review. J. Am. Coll. Cardiol. 2018;72(17):2054–2070. doi: 10.1016/j.jacc.2018.07.099. [DOI] [PubMed] [Google Scholar]
- Sanderson P., Su S., Chang I., Saborit J.D., Kepaptsoglou D., Weber R., Harrison R.M. Characterisation of iron-rich atmospheric submicrometre particles in the roadside environment. Atmos. Environ. 2016;140:167–175. [Google Scholar]
- Tsutsui H., Ide T., Kinugawai S. Mitochondrial oxidative stress, DNA damage, and heart failure. Antioxidants Redox Signal. 2006;8(9-10):1737–1744. doi: 10.1089/ars.2006.8.1737. [DOI] [PubMed] [Google Scholar]
- Villarreal-Calderon R., Franco-Lira M., Gonzalez-Maciel A., Reynoso-Robles R., Harritt L., Perez-Guille B., Ferreira-Azevedo L., Drecktrah D., Zhu H.T., Sun Q., Torres-Jardon R., Aragon-Flores M., Calderon-Garciduenas A., Diaz P., Calderon-Garciduenas L. Up-regulation of mRNA ventricular PRNP prion protein gene expression in air pollution highly exposed young urbanites: endoplasmic reticulum stress, glucose regulated protein 78, and nanosized particles. Int. J. Mol. Sci. 2013;14(12):23471–23491. doi: 10.3390/ijms141223471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakefield G., Wu X., Gardener M., Park B., Anderson S. Envirox fuel-borne catalyst: developing and launching a nano-fuel additive. Technol. Anal. Strat. Manag. 2008;20(1):127–136. [Google Scholar]
- Wang Y.R., Cui H.Y., Zhou J.P., Li F.J., Wang J.J., Chen M.H., Liu Q.D. Cytotoxicity, DNA damage, and apoptosis induced by titanium dioxide nanoparticles in human non-small cell lung cancer A549 cells. Environ. Sci. Pollut. Control Ser. 2015;22(7):5519–5530. doi: 10.1007/s11356-014-3717-7. [DOI] [PubMed] [Google Scholar]
- Wu X., Nethery R.C., Sabath B.M., Braun D., Dominici F. Exposure to air pollution and COVID-19 mortality in the United States: a nationwide cross-sectional study. medRxiv. 2020;2020 doi: 10.1126/sciadv.abd4049. 04.05.20054502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S., Lee S.P., Park J.B., Lee H., Kang S.H., Lee S.E., Kim J.B., Choi S.Y., Kim Y.J., Chang H.J. PM2.5 concentration in the ambient air is a risk factor for the development of high-risk coronary plaques. Eur. Heart J. Cardiovasc. Imaging. 2019;20(12):1355–1364. doi: 10.1093/ehjci/jez209. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.