Abstract
Background:
Overexpression of excision repair cross-complementing Group 1 (ERCC-1) is related to cisplatin resistance and defective repair of radiation damage. The purpose of this study was to evaluate the clinical significance of excision (ERCC-1) expression in nasopharyngeal cancer (NPC).
Materials and Methods:
We conducted a retrospective review of patients diagnosed with NPC between 2000 and 2013. The archived tissues were analyzed using immunohistochemistry to determine ERCC-1 expression. The ERCC-1 expression level along with other clinical factors and overall survival (OS) were analyzed. Hazard ratio (HR) with a 95% confidence interval was calculated to assess the risk.
Results:
The analysis of ERCC-1 expression was available in 262 NPC patients who had medical records at our hospital. Among those patients, 221 (84%) were treated with curative radiotherapy (RT)/concurrent chemoradiotherapy, 22 (7%) were treated with palliative RT alone, and 19 (9%) were given best supportive care. There was no correlation between ERCC-1 expression and stage of cancer or OS. No difference in 5-year OS was found between patients with low ERCC-1 expression and high ERCC-1 expression (38% vs. 36%; P = 0.981). The adjusted HR (aHR) of cancer death increased with cancer stage (aHR = 2.93 for advanced Stages III–IV; P = 0.001) and age (aHR = 2.11 for age >55; P ≤ 0.001). ERCC-1 expression exhibited no prognostic significance in our study (aHR = 1).
Conclusion:
In this study, ERCC-1 expression has no statistical significance to be considered a prognostic factor for OS among NPC patients. On the other hand, cancer stage, age, and types of treatment can be prognostic factors in NPC patients.
Keywords: Excision repair cross-complementing Group 1, nasopharyngeal cancer, prognostic factor
INTRODUCTION
The incidence rate of nasopharyngeal cancer (NPC) is high in Southeastern Asia, in both sexes, with the disease being the sixth most common among males in this region.[1] In Thailand, the age-standardized incidence rates of NPC are approximately 2.8 and 0.9 per 100,000 in males and females, respectively.[2] Chiang Mai, a province in the Northern part of Thailand, hasan incidence rate of 2.7 for males and 1.2 for females.[2] Due to anatomical location and extension of disease, as well as a high degree of radiosensitivity, the standard treatment for NPC is definitive radiotherapy (RT) with or without chemotherapy, depending on the stage of the disease. Although the latest evidence has shown a significant improvement in survival with the addition of concurrent platinum-based chemotherapy, a cornerstone chemotherapy regimen, to RT in locoregionally advanced NPC,[3] the treatment outcomes for this group of patients are rather unsatisfactory with the 5-year overall survival (OS) rates of 53%–80% in Stage III and 28%–61% for Stage IV.[4,5,6,7,8,9]
Many studies have suggested a potential use of excision repair cross-complementation Group 1 enzyme (ERCC-1) as a molecular predictor of the treatment outcome of platinum-based chemoradiotherapy in NPC.[10,11,12,13,14] ERCC-1 is an important enzyme in the nucleotide excision repair (NER) pathway which is involved in the DNA repair mechanism in tumor cells damaged by treatment with platinum agents. A study of ERCC-1 in head-and-neck cancer patients treated with RT alone has shown that the high expression of ERCC-1 was associated with poor response to RT, thereby having a role in the repair of RT-induced DNA damage.[15] The objective of this study was to evaluate the clinical significance including treatment outcomes of ERCC-1 expression in NPC patients.
MATERIALS AND METHODS
We considered 382 archived NPC specimens from patients diagnosed between January 2000 and December 2013 at the Faculty of Medicine, Chiang Mai University. The inclusion criteria were having biopsy-proven NPC, having adequate information on staging and treatment, and having archive tissues available for ERCC-1 immunohistochemical (IHC) staining. The data were extracted from the medical records. Among the treatment outcome determining factors and associating factors, age, sex, stage, and type of treatment were included in this study. The cancer stage was determined using the American Joint Committee on Cancer staging system.[16,17,18]
According to clinical practice guidelines for nasopharyngeal cancer, Stage I disease is generally treated by RT alone, while higher nonmetastatic stages are received concurrent chemoradiotherapy (CCRT). In our center, patients who had tumors confined to the nasopharynx without lymph node involvement were given curative RT alone by two-dimensional RT, three-dimensional conformal radiotherapy, or intensity-modulated radiotherapy. Patients who have tumor extension beyond nasopharynx and/or lymph node involvement with good performance status and adequate renal, bone marrow, and liver function received CCRT plus either neoadjuvant or adjuvant platinum-based chemotherapy.
Neoadjuvant or adjuvant chemotherapy consisted of three cycles of cisplatin (100 mg/m2) or carboplatin AUC5 on day 1 plus 5-FU 1000 mg/m2 on days 1–4. Concurrent chemotherapy regimen was either cisplatin 100 mg/m2 every 3 weeks or weekly 40 mg/m2 or weekly carboplatin 100 mg/m2. Patients with advanced disease and poor performance status received either palliative RT or best supportive care.
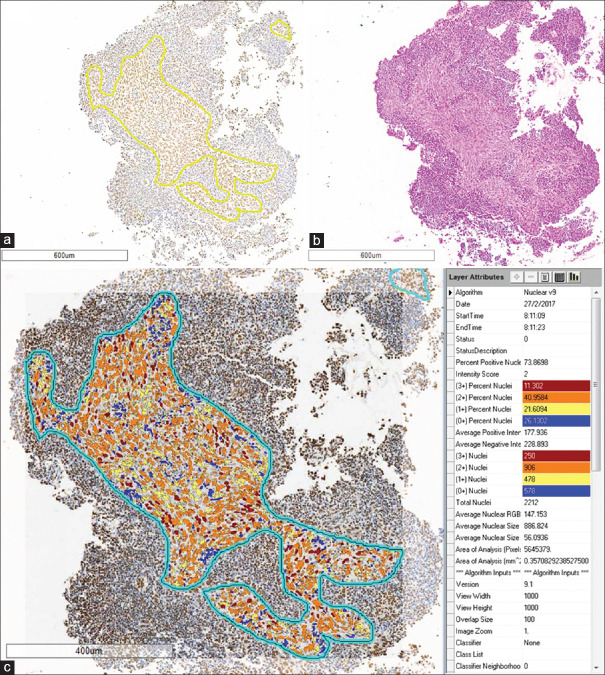
Formalin-fixed, paraffin-embedded tissue sections were stained, using VENTANA BenchMark XT platform, with the antibody against ERCC-1 (rabbit anti-human ERCC-1 monoclonal antibody, Clone SP68, Spring Bioscience, Pleasanton, CA, USA, 1:100), and then, were visualized by the ultraView Universal DAB Detection Kit. ERCC-1 expression was assessed on digital scanned microscopic images (Aperio, Aperio Technologies Inc., Vista, CA, USA) using image analysis (ImageScope software) by a pathologist who was masked to the clinicopathological data. Only well-preserved tumor areas were selected from the whole section for the analysis. Using nuclear count V9 algorithm, the results in percentage were reported as 0 – negative; 1+ – weakly positive; 2+ – moderately positive; and 3+ – strongly positive nuclei staining [Figure 1]. As the result of 1+ nuclei could not be specifically observed at the margin of tissue or at the areas of crushing artifacts, only percentages of 2+ and 3+ nuclei were used to calculate ERCC-1 expression. Patients were then categorized into two groups: “high expression” or “low expression”, using the median as a cut point.
Figure 1.
Image analysis for excision repair cross-complementing Group 1 expression. Tumor areas were selected from excision repair cross-complementing Group 1 immunohistochemical-stained section (a) for image analysis, guided by a H and E-stained section (b). Image analysis results were displayed as shown in panel (c). Only cells with 2 + and 3 + nuclear staining were used for the statistical analysis
Statistical analysis
Data were presented as mean with standard deviation (SD) or median with interquartile range (IQR) as appropriate for continuous variables and as counts and percentages for categorical variables. The proportions of ERCC-1 high expression in categorical variables were compared using Fisher’s exact test or Chi-square test as appropriate. Student’s t-test was used to compare the mean age between high and low expression of ERCC-1. The time to events was measured from the time of diagnosis to the date of events, i.e., death for OS analysis. The survival rate was calculated using the Kaplan–Meier method, and the log-rank test was performed for the significance test. The association between baseline characteristics (sex, age, stage of cancer, type of treatment, and ERCC-1 expression) and events was assessed using univariable and multivariable Cox proportional hazards models. Age and ERCC-1 expression were dichotomized according to the median values. Any variable having a significant univariate test at P value cutoff point of below 0.25 was selected as a candidate for the multivariate analysis. All reported P values are two sided and P < 0.05 was considered statistically significant. All analyses were performed using STATA software version 10.1 (StataCorp, College Station, TX, USA). This study was approved by the Research Ethics Committee of Faculty of Medicine, Chiang Mai University.
RESULTS
Of 382 NPC patients retrieved from the database, only 262 were eligible for the present study. The consort diagram of the study is presented in Figure 2. Among 120 patients who were excluded from the study, eight were with squamous cell carcinoma of primary sites other than nasopharynx, 41 were due to the lack of medical records on their staging or type of treatment, and 71 were due to unavailability of archive tissues for ERCC-1 IHC staining.
Figure 2.
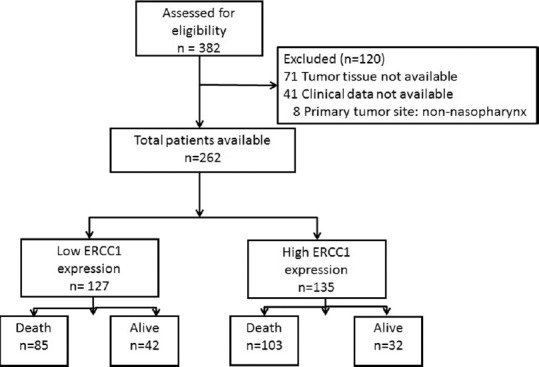
Consort diagram of the study
The mean (SD) age of the study population was 55 years.[13] Most were male (70%) and in advanced stages (87% in Stage III–IV). For the type of treatment, 221 patients (84%) received curative RT/CCRT, while 41 patients (16%) received either palliative RT or best supportive care. Table 1 summarizes the baseline characteristics of a patient by the level of ERCC-1 expression. One hundred and thirty-five patients (52%) had high expression of ERCC-1 and 127 patients (49%) had low expression of ERCC-1. There was no difference in any baseline characteristics between both the groups.
Table 1.
Baseline characteristics and clinicopathological factors by excision repair cross-complementing Group 1 expression
| Variables | All patients (n=262), n (%) | ERCC-1 | ||
|---|---|---|---|---|
| Low expression (n=127), n (%) | High expression (n=135), n (%) | P* | ||
| Sex | ||||
| Female | 79 (30) | 40 (51) | 39 (49) | 0.646 |
| Male | 183 (70) | 87 (48) | 96 (52) | |
| Median age (year), mean (SD) | 55 (13) | 55 (14) | 55 (13) | 0.994† |
| Age groups (years) | ||||
| <55 | 128 (49) | 57 (44) | 71 (56) | 0.212 |
| ≥55 | 134 (51) | 70 (52) | 64 (48) | |
| Stage (n=258) | ||||
| I | 10 (4) | 4 (40) | 6 (60) | 0.591‡ |
| II | 24 (9) | 14 (58) | 10 (42) | |
| III | 80 (31) | 35 (44) | 45 (56) | |
| IV | 144 (56) | 71 (49) | 73 (51) | |
| Treatment | ||||
| Curative RT/CCRT | 221 (84) | 104 (47) | 117 (53) | 0.555 |
| Palliative radiotherapy | 22 (9) | 12 (55) | 10 (45) | |
| Best supportive care | 19 (7) | 11 (58) | 8 (42) | |
*Chi-square test; † Student’s t-test; ‡ Fisher’s exact test. RT=Radiotherapy; CCRT=Concurrent chemoradiotherapy; CI=Confidence interval; SD=Standard deviation; ERCC-1=Excision repair cross-complementing Group 1
The median follow-up time was 2.8 years (IQR: 1.2–5.8). For 5-year survival analysis, 163 patients (62%) had died, with median OS of 33 months (IQR: 15–69). Age, stage of cancer, and type of treatment were significantly predictive of OS using univariable and multivariable Cox regression analyses [Table 2]. However, ERCC-1 expression showed no prognostic significance.
Table 2.
Overall survival: Univariable and multivariable Cox proportional hazard regression analysis
| Covariates | Univariable analysis | Multivariable analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P* | aHR | 95% CI | P* | |
| Sex | ||||||
| Female | 1.00 | 1.00 | ||||
| Male | 1.32 | 0.93-1.86 | 0.117 | 1.33 | 0.93-1.91 | 0.117 |
| Age groups (years) | ||||||
| <55 | 1.00 | 1.00 | ||||
| ≥55 | 1.83 | 1.34-2.51 | <0.001 | 2.11 | 1.50-2.97 | <0.001 |
| Stage | ||||||
| Early (I-II) | 1.00 | 1.00 | ||||
| Advanced (III-IV) | 2.86 | 1.55-5.28 | 0.001 | 2.93 | 1.58-5.46 | 0.001 |
| Treatment | ||||||
| Curative RT/CCRT | 1.00 | 1.00 | ||||
| Palliative radiotherapy | 2.82 | 1.75-4.54 | <0.001 | 2.11 | 1.27-3.49 | 0.004 |
| Best supportive care | 2.86 | 1.68-4.90 | <0.001 | 4.49 | 2.45-8.24 | <0.001 |
| ERCC-1 expression | ||||||
| Low | 1.00 | 1.00 | ||||
| High | 1.00 | 0.74-1.36 | 0.981 | 1.08 | 0.79-1.47 | 0.647 |
*P value from partial likelihood ratio tests. RT=Radiotherapy; CCRT=Concurrent chemoradiotherapy; CI=Confidence interval, HR=Hazard ratio; aHR=Adjusted hazard ratio
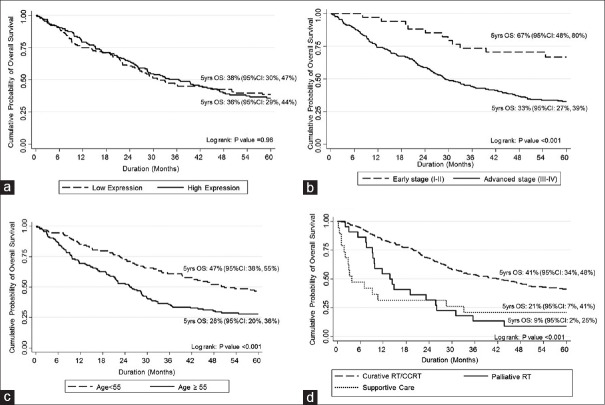
Five-year OS rates were better in age group below 55 years than in older group (47% [95% confidence interval (CI): 38–55] vs. 28% [95% CI: 20–36]; P < 0.001), in early Stages (I–II) than in advanced Stages (III–IV) (67% [95% CI: 48–80] vs. 33% [95% CI: 27–39]; P < 0.001), and in curative RT/CCRT than palliative RT/best supportive care groups (41% [95% CI: 34–48] vs. 9% [95% CI: 2–25]/21% [95% CI: 7–41]; P < 0.001, respectively) [Figure 3]. There was no difference in 5-year OS rate between low and high ERCC-1 expression (38% [95% CI: 30–47] vs. 36% [95% CI: 29–44]; P = 0.98).
Figure 3.
Kaplan–Meier curves of overall survival according to prognostic factors. There is a significant difference in 5-year overall survival between early and advanced stages (b), age group (c) and treatment (d), but not excision repair cross complementing Group 1expression level (a)
DISCUSSION
The outcome of chemoradiotherapy in locoregional advanced NPC patients is still dismal. The 5-year OS rate in this retrospective study is very poor when compared to other studies, as more than half of the patients (56%) included in our study were at Stage IV at diagnosis. Imaging study for tumor evaluation, staging, and RT treatment planning in most of our patients was by CT scanning, while magnetic resonance imaging is better for evaluating intracranial and skull base involvement.[19] This may result in an underestimate of tumor stage and effect treatment decision-making, RT field, and outcomes. However, when we analyzed the OS in each stage, the outcome of the patients in this cohort was in line with other studies.[4,5,6,7,8,9] The 5-year OS of Stage I, II, III, and IV of our patients was 80%, 62%, 42%, and 28%, respectively.
The success of this combination treatment depends on the effectiveness of lethal cell killing and double-strand break by RT and on the synergism effect of cytotoxicity by platinum-based chemotherapy. NER is one of four major pathways to repair damaged DNA.[16] It also plays an important role in identifying and repairing the DNA adducts, particularly those induced by cisplatin.[17] ERCC-1 has a crucial role for the incision step and completion of the NER pathway.[19] Many studies reported ERCC-1 to be involved in the different repair mechanisms such as interstrand cross-link repair and homologous recombination repair.[20,21,22] Overexpression of ERCC-1 has been found in many cancer types, and it has been proposed that ERCC-1 overexpression may serve as a prognostic and/or predictive tumor marker.[23,24,25,26,27,28] Overexpression of ERCC-1 also predicted low sensitivity to the platinum-based regimen for many cancers.[23,24,25] Results from these published data are still inconsistent.[20,21,22]
The prognostic values of ERCC-1 expression have been studied in nasopharyngeal cancer studies,[10,13,29] but the values of it remained controversial. Chan et al.[10] identified that a high ERCC-1 expression predicted a two-fold increase in risk only for locoregional failure but not OS in a retrospective study of NPC. They also concluded that chemotherapy response is not affected by ERCC-1 expression. In line with Chan et al.,[10] our study has not found any differences in OS between the high and low expression of ERCC-1. Even when we focused on the group of patients who received CCRT, the expression of ERCC-1 failed to demonstrate significant OS differences between high and low expression. In contrast with Xu et al.,[29] they reported higher 3-year OS, failure-free survival, locoregional failure-free survival, and distant failure-free survival in the patients with ERCC-1 positive and suggested that ERCC-1 might be a predictor of response to platinum-based chemoradiotherapy. The study by Shen et al.[13] also found that the overall response rate and 5-year distant recurrence risk of the patients with high expression of ERCC-1 are poorer than those of the control patients with low ERCC-1 expression. However, their study did not report on OS. In our study, we found that higher stage, age older than 55, and not receiving chemoradiotherapy were prognostic factors of poor OS but not ERCC-1 expression. We tend to agree with Hayes et al.[30] for their explanations on the lack of the usefulness of ERCC-1 expression as a prognostic factor of survival, i.e., the synergism of concurrent chemotherapy and radiation is able to overcome the relative resistance to platinum conferred by ERCC-1 expression. In our study, most of the patients (80%) received CCRT with platinum based.
As shown in Table 1, we did not find any association between cancer stage and the level of ERCC-1 expression despite using an automated IHC staining platform and objective evaluation. All cases were positively stained for ERCC-1; however, an overall range of expression level was quite narrow (61%, IQR: 40–76). One limitation in the tissue study of nasopharyngeal cancer is that biopsied tissues were usually small, and the crushing artifact was not uncommonly present. This results in a reduction in tissue area for evaluation of ERCC-1 expression. However, in the present study, all well-preserved tumor areas were selected for objective digital image analysis to minimize the bias. Considering the heterogeneity of the interpretation method of ERCC-1 expression, it is difficult to compare our results to others.
CONCLUSION
ERCC-1 expression is not a prognostic factor of OS in patients with nasopharyngeal cancer. Further studies with larger sample sizes are required to investigate whether or not ERCC-1 may use as a prognostic factor for this cancer.
Financial support and sponsorship
This study was financially supported by the Faculty of Medicine, Chiang Mai University, for having a role in data collection.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We would like to thank Faculty of Medicine, Chiang Mai University for having a role in data collection. This study was approved by the Research Ethics Committee of Faculty of Medicine, Chiang Mai University. Ethical approval number is 381/2014.
REFERENCES
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Imsamran W, Chaiwerawattana A, Wiangnon S, Pongnikorn D, Suwanrungruang K, Sangrajrang S, et al. Cancer in Thailand. Vol. 8. Bangkok, Thailand: National Cancer Institute; 2015. [Google Scholar]
- 3.Blanchard P, Lee A, Marguet S, Leclercq J, Ng WT, Ma J, et al. Chemotherapy and radiotherapy in nasopharyngeal carcinoma: An update of the MAC-NPC meta-analysis. Lancet Oncol. 2015;16:645–55. doi: 10.1016/S1470-2045(15)70126-9. [DOI] [PubMed] [Google Scholar]
- 4.Au JS, Law CK, Foo W, Lau WH. In-depth evaluation of the AJCC/UICC 1997 staging system of nasopharyngeal carcinoma: Prognostic homogeneity and proposed refinements. Int J Radiat Oncol Biol Phys. 2003;56:413–26. doi: 10.1016/s0360-3016(02)04610-2. [DOI] [PubMed] [Google Scholar]
- 5.Chua DT, Sham JS, Wei WI, Ho WK, Au GK. The predictive value of the 1997 American joint committee on cancer stage classification in determining failure patterns in nasopharyngeal carcinoma. Cancer. 2001;92:2845–55. doi: 10.1002/1097-0142(20011201)92:11<2845::aid-cncr10133>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 6.Heng DM, Wee J, Fong KW, Lian LG, Sethi VK, Chua ET, et al. Prognostic factors in 677 patients in Singapore with nondisseminated nasopharyngeal carcinoma. Cancer. 1999;86:1912–20. [PubMed] [Google Scholar]
- 7.Lai SZ, Li WF, Chen L, Luo W, Chen YY, Liu LZ, et al. How does intensity-modulated radiotherapy versus conventional two-dimensional radiotherapy influence the treatment results in nasopharyngeal carcinoma patients? Int J Radiat Oncol Biol Phys. 2011;80:661–8. doi: 10.1016/j.ijrobp.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 8.Lee AW, Sze WM, Au JS, Leung SF, Leung TW, Chua DT, et al. Treatment results for nasopharyngeal carcinoma in the modern era: The Hong Kong experience. Int J Radiat Oncol Biol Phys. 2005;61:1107–16. doi: 10.1016/j.ijrobp.2004.07.702. [DOI] [PubMed] [Google Scholar]
- 9.Leung TW, Tung SY, Sze WK, Wong FC, Yuen KK, Lui CM, et al. Treatment results of 1070 patients with nasopharyngeal carcinoma: An analysis of survival and failure patterns. Head Neck. 2005;27:555–65. doi: 10.1002/hed.20189. [DOI] [PubMed] [Google Scholar]
- 10.Chan SH, Cheung FM, Ng WT, Choi CW, Cheung KN, Yiu KH, et al. Can the analysis of ERCC1 expression contribute to individualized therapy in nasopharyngeal carcinoma? Int J Radiat Oncol Biol Phys. 2011;79:1414–20. doi: 10.1016/j.ijrobp.2009.12.072. [DOI] [PubMed] [Google Scholar]
- 11.Hui EP, Ma BB, Chan KC, Chan CM, Wong CS, To KF, et al. Clinical utility of plasma epstein-barr virus DNA and ERCC1 single nucleotide polymorphism in nasopharyngeal carcinoma. Cancer. 2015;121:2720–9. doi: 10.1002/cncr.29413. [DOI] [PubMed] [Google Scholar]
- 12.Liang R, Lin Y, Liu ZH, Liao XL, Yuan CL, Liao SN, et al. Correlation between ERCC1 expression and concurrent chemotherapy and radiotherapy in patients with locally advanced nasopharyngeal cancer. Genet Mol Res. 2015;14:5804–11. doi: 10.4238/2015.May.29.12. [DOI] [PubMed] [Google Scholar]
- 13.Shen C, Chen L, Fu J, Lin H. Expression of excision repair cross-complementation group 1 in locoregionally advanced nasopharyngeal carcinoma treated with cisplatin-based induction chemotherapy. J Cancer Res Ther. 2016;12:72–5. doi: 10.4103/0973-1482.191636. [DOI] [PubMed] [Google Scholar]
- 14.Yang ZH, Dai Q, Kong XL, Yang WL, Zhang L. Association of ERCC1 polymorphisms and susceptibility to nasopharyngeal carcinoma. Mol Carcinog. 2009;48:196–201. doi: 10.1002/mc.20468. [DOI] [PubMed] [Google Scholar]
- 15.Carles J, Monzo M, Amat M, Jansa S, Artells R, Navarro A, et al. Single-nucleotide polymorphisms in base excision repair, nucleotide excision repair, and double strand break genes as markers for response to radiotherapy in patients with stage I to II head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2006;66:1022–30. doi: 10.1016/j.ijrobp.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 16.Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer. 2007;7:573–84. doi: 10.1038/nrc2167. [DOI] [PubMed] [Google Scholar]
- 17.Kirschner K, Melton DW. Multiple roles of the ERCC1-XPF endonuclease in DNA repair and resistance to anticancer drugs. Anticancer Res. 2010;30:3223–32. [PubMed] [Google Scholar]
- 18.McNeil EM, Melton DW. DNA repair endonuclease ERCC1-XPF as a novel therapeutic target to overcome chemoresistance in cancer therapy. Nucleic Acids Res. 2012;40:9990–10004. doi: 10.1093/nar/gks818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glastonbury CM, Salzman KL. Pitfalls in the staging of cancer of nasopharyngeal carcinoma. Neuroimaging Clin N Am. 2013;23:9–25. doi: 10.1016/j.nic.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 20.de Silva IU, McHugh PJ, Clingen PH, Hartley JA. Defining the roles of nucleotide excision repair and recombination in the repair of DNA interstrand cross-links in mammalian cells. Mol Cell Biol. 2000;20:7980–90. doi: 10.1128/mcb.20.21.7980-7990.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niedernhofer LJ, Odijk H, Budzowska M, van Drunen E, Maas A, Theil AF, et al. The structure-specific endonuclease ercc1-xpf is required to resolve DNA interstrand cross-link-induced double-strand breaks. Mol Cell Biol. 2004;24:5776–87. doi: 10.1128/MCB.24.13.5776-5787.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sargent RG, Meservy JL, Perkins BD, Kilburn AE, Intody Z, Adair GM, et al. Role of the nucleotide excision repair gene ERCC1 in formation of recombination-dependent rearrangements in mammalian cells. Nucleic Acids Res. 2000;28:3771–8. doi: 10.1093/nar/28.19.3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ciaparrone M, Caspiani O, Bicciolo G, Signorelli D, Simonelli I, de Campora L, et al. Predictive role of ERCC1 expression in head and neck squamous cell carcinoma patients treated with surgery and adjuvant cisplatin-based chemoradiation. Oncology. 2015;89:227–34. doi: 10.1159/000430447. [DOI] [PubMed] [Google Scholar]
- 24.Deng Q, Yang H, Lin Y, Qiu Y, Gu X, He P, et al. Prognostic value of ERCC1 mRNA expression in non-small cell lung cancer, breast cancer, and gastric cancer in patients from Southern China. Int J Clin Exp Pathol. 2014;7:8312–21. [PMC free article] [PubMed] [Google Scholar]
- 25.Jacobsen F, Taskin B, Melling N, Sauer C, Wittmer C, Hube-Magg C, et al. Increased ERCC1 expression is linked to chromosomal aberrations and adverse tumor biology in prostate cancer. BMC Cancer. 2017;17:504. doi: 10.1186/s12885-017-3489-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klatte T, Seitz C, Rink M, Rouprêt M, Xylinas E, Karakiewicz P, et al. ERCC1 as a prognostic and predictive biomarker for urothelial carcinoma of the bladder following radical cystectomy. J Urol. 2015;194:1456–62. doi: 10.1016/j.juro.2015.06.099. [DOI] [PubMed] [Google Scholar]
- 27.Li S, Wu J, Chen Y, Tang W, Peng Q, Deng Y, et al. ERCC1 expression levels predict the outcome of platinum-based chemotherapies in advanced bladder cancer: A meta-analysis. Anticancer Drugs. 2014;25:106–14. doi: 10.1097/CAD.0000000000000021. [DOI] [PubMed] [Google Scholar]
- 28.Maithel SK, Coban I, Kneuertz PJ, Kooby DA, El-Rayes BF, Kauh JS, et al. Differential expression of ERCC1 in pancreas adenocarcinoma: High tumor expression is associated with earlier recurrence and shortened survival after resection. Ann Surg Oncol. 2011;18:2699–705. doi: 10.1245/s10434-011-1610-x. [DOI] [PubMed] [Google Scholar]
- 29.Xu S, Yu Y, Rong J, Hu D, Zhang L, Fu S, et al. Expression of BRCA1 and ERCC1 as predictive clinical outcome after radiochemotherapy in patients with locoregionally moderate-advanced nasopharyngeal carcinoma. Oncotarget. 2017;8:31355–67. doi: 10.18632/oncotarget.15565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hayes M, Lan C, Yan J, Xie Y, Gray T, Amirkhan RH, et al. ERCC1 expression and outcomes in head and neck cancer treated with concurrent cisplatin and radiation. Anticancer Res. 2011;31:4135–9. [PubMed] [Google Scholar]