Abstract
Background and purpose:
The use of vancomycin, as a key therapeutic choice for treatment of hazardous infections, may be associated with nephrotoxicity. The proposed mechanism is the indirect production of reactive oxygen species and oxidative stress. The purpose of this study was to investigate the effect of vitamin E as an antioxidant agent in the prevention of vancomycin-induced nephrotoxicity.
Experimental approach:
In a matched-groups interventional study, patients who received vancomycin for any indication were assigned to vitamin E (n = 30) and control (n = 60) groups. The patients in experimental group received 400 units of oral vitamin E per day for 10 days started concurrently with vancomycin, while the patients in control group received vancomycin alone. Serum level of creatinine, blood urea nitrogen (BUN), creatinine clearance (CrCl), and 24-h urine output were determined and recorded before the start of interventions, every other day during therapy, and 12 h after the last dose of vancomycin in 10th day of therapy for all patients. Also, the rate of acute kidney injury (AKI) in the two groups was recorded. Finally, the mean values of the measured parameters were compared between the groups.
Findings / Results:
Treatment with vitamin E for 10 days resulted in a significant reduction of BUN (from 17.5 ± 7.8 mg/dL at baseline to 11.4 ± 4.8 mg/dL at the end; P < 0.001) along with slightly non-significant increase of CrCl (from 84.7 ± 18.9 mL/min at baseline to 91.3 ± 19.5 mL/min at the end; P = 0.301) in comparison to the control group. However, CrCl decreased significantly in the control group. Vitamin E had no significant effect on 24-h urine output. Regarding vancomycin-induced AKI, 12 cases were observed in the control group, while no case was reported in experimental group (P = 0.041).
Conclusion and implications:
This study showed the beneficial effect of add-on therapy of vitamin E besides vancomycin in reducing AKI, which could be considered as a new potential prophylactic therapy for vancomycin-induced nephrotoxicity.
Keywords: Acute kidney injury, Nephrotoxicity, Vancomycin, Vitamin E
INTRODUCTION
Vancomycin is a glycopeptide antibiotic that is mostly effective against gram positive bacteria. This antibiotic plays a major role in the treatment of infections caused by methicillin-resistant Staphylococcus aureus (1). This antibiotic has bactericidal activity against growing sensitive bacteria through inhibition of cell wall synthesis. However, clinical use of this antibiotic has been associated with nephrotoxicity (2). Vancomycin- induced nephrotoxicity has been reported in 5 to 25% of patients as well as 35% of patients receiving concurrent aminoglycosides (3,4). New therapeutic recommendations have suggested higher doses of vancomycin (to attain trough level of 15-20 mg/L) for treatment of methicillin- resistant Staphylococcus aureus complicated infections such as endocarditis, osteomyelitis, meningitis, bacteremia, and hospital-acquired pneumonia (5,6).
This level of vancomycin would be associated with more risk of renal damage. Also, the duration of treatment with vancomycin is important as well; therefore patients receiving this drug for more than 7 days suffer nephrotoxicity more frequently (7,8).
The mechanism of vancomycin nephrotoxicity has not been completely understood. According to several studies, oxygen free radicals may be involved in the pathogenesis of vancomycin nephrotoxicity which is probably a result of superoxide dismutase inhibition (9). Reactive oxygen species have been responsible for cell death in various models of renal insufficiency due to vancomycin, aminoglycosides, lithium, and cisplatin (10,11).
The positive effect of several antioxidant agents such as N-acetylcysteine (12), vitamins E and C (12), curcumin (13), and atorvastatin (14) on the vancomycin-induced nephrotoxicity has been shown in animal models.
Vitamin E (α-tocopherol) is a potent fatsoluble antioxidant. The main function of vitamin E in cell membrane and lipoproteins is to entrap peroxide radicals and stop chain reaction of lipids peroxidation (15). The beneficial effect of vitamin E in prevention of renal damage induced by several substances and drugs including mercury chloride (16), gentamicin (17), cisplatin (18), and vancomycin (12) has been shown in animal models. However, to the best of our knowledge, no clinical study towards the effect of this antioxidant in the prevention of vancomycin- induced nephrotoxicity has yet been reported. Thus, this study aimed to assess the possible protective effect of vitamin E against this adverse effect in patients receiving vancomycin.
MATERIALS AND METHODS
This was a matched-groups interventional study performed in Alzahra hospital affiliated to Isfahan University of Medical Sciences (IUMS), Isfahan, I.R. Iran.
Compliance with ethical standards
The study protocol was approved by the Ethics Committee of IUMS (Project No. 394481) and was done between April, 2016 and March, 2017. Informed consent was obtained from all participants before the study, and the whole study process was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki (19).
Patient population
Patients were selected from those who were admitted to different departments of this hospital. The inclusion criteria were as follow: (1) age > 18 years; (2) receiving vancomycin for at least 5 days; (3) creatinine clearance > 60 mL/min; (4) not having proteinuria and/or hematuria; (5) not having any renal disorder including glomerulonephritis, polycystic kidney disease, and renal artery stenosis; (6) no history of acute kidney injury (AKI); (7) not having sepsis; (8) not using any other nephrotoxic drugs including aminoglycosides, amphotericin B, cyclosporine, tacrolimus, iodinated contrast media, furosemide, and nonsteroidal anti-inflammatory drugs (NSAIDs) currently and within the last 4 days; (9) not using any other antioxidant medication (e.g., vitamin C); and (10) no history of allergic reaction to vitamin E.
Critically ill patients or those with sepsis at the admission time or during hospitalization were excluded from this study. Sepsis was diagnosed by the practitioner based on more recent and admitted definitions (20).
Sample size calculation
Since we planned to investigate the effect of vitamin E on drug-induced nephropathy, we used the data from the same previous study on cisplatin-induced nephrotoxicity to calculate the sample size (21). One of the main outcomes of this study was glomerular filtration rate (GFR) which is a continuous quantitative variable, in order to use this for calculating trustworthy sample size, the t-student equation was applied. The mean significant difference (d parameter in the equation) of GFR between two groups after the completion of the mentioned study was 10 mL/min (84 mL/min in the control group vs 94 mL/min in the target group); considering the standard deviation (SD) of GFR as about 14 according to that study, and considering a = 0.05, and P = 0.20, the sample size (n) was calculated as below:
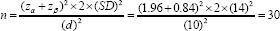
The minimum number of cases needed for each control and vitamin E groups would be 30. Due to the short period of intervention in the present study (10 days), it might be difficult to retrieve considerable results by having 30 patients in each group, therefore to increase the study power to derive minor changes made in such a short period, we decided to recruit patients in the control group two times more than the number of patients in the vitamin E group. Therefore, we were forced to match the patients on recruitment in two groups regarding the baseline characteristics mainly the age and baseline serum creatinine. Also, we tried to allocate almost equal numbers of patients with the same vancomycin therapy indications to two study groups; this helps in matching the groups regarding severity of the baseline conditions. Accordingly, we finally recruited 30 and 60 patients in vitamin E and control groups,respectively.
Study design
In this non-randomized interventional study, patients who received vancomycin for non- critical indications including nosocomial pneumonia, osteomyelitis, and diabetic foot infection and met the inclusion criteria were assigned to one of the two treatment groups of drug (vitamin E) and control.
Vancomycin was initially administered at 15 mg/kg IV every 12 h followed by dose adjustment to obtain a trough concentration of 10-15 mg/L. The blood samples were collected just 30 min before the 4th dose of vancomycin. For each case, 5 mL of whole blood was taken and rapidly transferred to the hospital laboratory in heparinized tube. After centrifugation at 5000 rpm for 5 min, the serum (supernatant) was separated by sampler and used for assay. The drug concentration was determined by fluorescence polarization technique using the COBAS INTEGRA® 400 plus analyzer (Roche Diagnostics International Ltd, Basle, Switzerland). Participants in drug group received 400 units of oral vitamin E per day for 10 days started concurrently with vancomycin initiation, while the patients in control group received vancomycin alone. In case of vancomycin cessation earlier than 10 days due to any reason except for AKI (e.g., thrombocytopenia and hypersensitivity reactions), the patient was excluded from the study. Baseline and follow-up laboratory assessments including serum level of creatinine (SCr) and blood urea nitrogen (BUN) as well as creatinine clearance (CrCl) and 24-h urine volume were recorded before the start of intervention, every other day during the study, and 12 h after the last dose of vancomycin in 10th day of therapy for all patients. The estimated glomerular filtration rate (eGFR) as a magnitude of CrCl was calculated using chronic kidney disease epidemiology collaboration (CKD-EPI) equation (22). Also, the number of cases presented with AKI after vancomycin administration in two study groups was recorded. AKI was defined based on the kidney disease improving global outcomes (KDIGO definition, as an absolute increase of at least 0.3 mg/dL in SCr within 48 h or a 50% increase in SCr within 7 days from baseline value (23). The primary outcomes were the differences in the amount of changes in SCr and BUN concentrations, urine output, and CrCl from the study enrollment to the end of follow-up (day 10) between the vitamin E and control groups. The secondary outcome measure was the differences in the number of AKI cases between the groups.
Statistical analysis
The normality of data distribution with regard to the age and clinical parameters of the study population in two groups was assessed by Kolmogorov-Smirnov test. Since the results were significant for most of these parameters meaning that their distribution was not normal, and considering the small number of included patients, the non-parametric statistical tests were applied to enhance the strength of analysis. The mean values of the measured parameters were finally compared between the two groups. The endpoints of this study were a change in SCr, BUN, CrCl, and urine output in measurements available for each patient after treatment with vancomycin + vitamin E or vancomycin alone. Data are presented as mean ± SD. Changes in parameters within groups from baseline to the end of the study (at the 5 every- other-day assessment points) were analyzed using Friedman test. Differences in these parameters were compared between the two study groups at the initiation of the study and at each study point, using Mann-Whitney U test. The comparison of number of cases with AKI occurrence was performed between groups by Pearson Chi-Square test. Analysis of covariance (ANCOVA) was performed with age, and baseline SCr or BUN as a covariate and the rate of SCr, BUN, CrCl, and urine output changes during the study period as the dependent variables. All analyses were conducted using SPSS for windows (SPSS, Chicago, IL, USA) version 20.0. A level of P < 0.05 was considered statistically significant.
RESULTS
Ninety (68 males and 22 females, aged 18-90 years) patients who met eligibility criteria were assigned to group 1 (vitamin E, 30 patients) or group 2 (control, 60 patients). The two groups were similar with respect to age (P = 0.550), vancomycin serum trough level, and baseline clinical parameters including SCr concentration (P = 0.099) and urine output (P = 0.247), but not serum BUN concentration (P = 0.011) and CrCl (P = 0.045); therefore, there were no statistically significant baseline differences between the two groups across all analyzed variables, except for BUN and CrCl. However, it’s noteworthy to declare that patients in the two groups were matched on recruitment based on the age and baseline SCr. Table 1 shows demographic, clinical, and laboratory characteristics of both groups over the study period.
Table 1.
Laboratory and clinical characteristics of patients in vitamin E and control groups over the study period. Data are presented as mean ± SD (range).
| Parameter (unit) | Time (day) | Vitamin E (n = 30) | Control (n = 60) | P valuea |
|---|---|---|---|---|
| Age (years) | Baseline | 46.6 ± 17.1 (21-85) | 49.2 ± 18.7 (18-90) | 0.550 |
| Vancomycin trough level (μg/mL) | Before the 4th dose | 12.93 ± 1.46 (10-15) | 13.03 ± 1.51 (10-15) | 0.766 |
| 0 | 0.99 ± 0.19 (0.7-1.4) | 0.93 ± 0.16 (0.5-1.5) | 0.099 | |
| 1 | 0.98 ± 0.21 (0.7-1.6) | 0.97 ± 0.20 (0.6-1.9) | 0.707 | |
| Serum Cr (mg/dL) | 2 | 0.97 ± 0.22 (0.6-1.5) | 0.99 ± 0.26 (0.6-2.5) | 0.612 |
| 3 | 0.93 ± 0.16 (0.6-1.2) | 1.01 ± 0.37 (0.6-3.3) | 0.570 | |
| 4 | 0.93 ± 0.17 (0.5-1.3) | 1.04 ± 0.46 (0.6-4.2) | 0.723 | |
| 5 | 0.92 ± 0.17 (0.6-1.3) | 1.09 ± 0.50 (0.6-4.3) | 0.065 | |
| P valueb | 0.210 | < 0.001 | ||
| 0 | 17.5 ± 7.8 (5-33) | 13.6 ± 5.9 (6-38) | 0.011 | |
| 1 | 15.5 ± 7.7 (5-36) | 14.7 ± 6.2 (6-40) | 0.683 | |
| BUN (mg/dL) | 2 | 14.1 ± 7.7 (5-38) | 15.0 ± 7.3 (6-51) | 0.349 |
| 3 | 11.7 ± 5.1 (5-23) | 15.7 ± 8.8 (6-54) | 0.010 | |
| 4 | 11.5 ± 4.1 (6-20) | 15.5 ± 9.9 (5-63) | 0.039 | |
| 5 | 11.4 ± 4.8 (5-22) | 16.7 ± 11.3 (5-83) | 0.003 | |
| P valueb | < 0.001 | < 0.001 | ||
| 0 | 84.7 ± 18.9 (60.7-133.4) | 91.8 ± 20.3 (60.2-143.3) | 0.045 | |
| 1 | 86.1 ± 20.2 (53.3-139.1) | 88.1 ± 21.2 (37.4-147.9) | 0.507 | |
| CrCl (mL/min) | 2 | 89.0 ± 20.1 (56.5-130.0) | 87.2 ± 24.0 (27.5-165.0) | 0.625 |
| 3 | 89.9 ± 19.7 (66.3-128.7) | 88.1 ± 25.7 (19.7-160.1) | 0.801 | |
| 4 | 90.8 ± 22.4 (61.5-136.2) | 87.6 ± 27.0 (15.2-129.5) | 0.650 | |
| 5 | 91.3 ± 19.5 (60.4-132.7) | 81.4 ± 24.4 (14.2-138.2) | 0.080 | |
| P valueb | 0.301 | < 0.001 | ||
| 0 | 1403.7 ± 270.4 (870-2100) | 1494.7 ± 224.5 (680-1660) | 0.247 | |
| 1 | 1297.6 ± 317.7 (730-1940) | 1481.2 ± 342.1 (940-2400) | 0.318 | |
| 24-h urine output (mL/day) | 2 | 1194.8 ± 334.5 (480-2260) | 1362.8 ± 320.1 (1000-1800) | 0.560 |
| 3 | 1668.2 ± 412.9 (660-2450) | 1255.3 ± 247.8 (920-1720) | 0.197 | |
| 4 | 1724.5 ± 198.6 (880-2100) | 1620.6 ± 182.9 (720-2100) | 0.721 | |
| 5 | 1719.6 ± 333.2 (840-1950) | 1722.2 ± 421.6 (830-2640) | 0.690 | |
| P valueb | 0.092 | 0.122 | ||
| Number of cases with AKI occurrence | During the study period | 0 | 12 | 0.041c |
0, Baseline; 1 to 5, every-other-day assessment from the start of intervention till the 10th day of therapy; a, betweengroup comparison at each study point (Mann-Whitney test); b, within-group comparison during the study period (Friedman test); c, Between-group comparison during the study period (Pearson Chi-Square test); Cr, creatinine; BUN, blood urea nitrogen; CrCl, creatinine clearance, calculated by chronic kidney disease epidemiology collaboration equation; AKI, acute kidney injury; SD, standard deviation.
As presented in Table 1, treatment with vitamin E concomitant with vancomycin for 10 days resulted in a slightly decrease of SCr, along with slightly increase of CrCl. Although the increase in CrCl was not significant in the treatment group (P = 0.301), it decreased significantly in the control group (P < 0.001). The same results are obtained for SCr in both groups.
According to the Friedman test, 24-h urine output did not significantly change during the study period in either groups (P = 0.092 in the vitamin E, P = 0.122 in the placebo group). While BUN increased over time in the control group, this parameter tended to decrease significantly in the vitamin E treatment group during the 10-days study period (P < 0.001).
Mann-Whitney U test revealed no significant differences in the amount of changes in SCr, CrCl, and 24-h urine output using vitamin E compared to the control group at midpoints and final analyses (Table 1). As shown, vitamin E had no significant effect on SCr, CrCl, and urine output compared to the control group; however, the difference of BUN between two study groups was evident after the 5th day of treatment with vitamin E.
In order to evaluate the impact of baseline SCr and BUN on the amount of response to the vitamin E treatment, analysis of covariance was performed. For both analyses, the correlation was not significant.
Regarding the rate of AKI incidence in the two groups, 12 cases of vancomycin-induced AKI were observed in the control group, while no case was reported in vitamin E group. The difference was significant (P = 0.041) according to Pearson Chi-Square test.
DISCUSSION
In the present study, vitamin E showed a significant protective effect against vancomycin-induced AKI. Despite some previous animal studies on the use of antioxidants in preventing vancomycin-induced nephrotoxicity, human data in this regard are scarce. Only few trials have directly addressed the protective effect of antioxidants on renal function in patients receiving nephrotoxic drugs. Most of these studies were of small sample, and many did not include a control arm.
Vitamin E is one of the antioxidant agents which prohibits lipid peroxidation (12,15,16,17). In the present study, it was shown that receiving vitamin E concomitantly with vancomycin therapy for 10 days could not create significant changes in terms of SCr concentration and CrCl in the treatment group. However, since these variables showed significant undesirable changes in the control group, it can be concluded that vitamin E may have protective effects against oxidative damages induced by vancomycin when simultaneously administered. In the case of BUN, vitamin E administration could reduce this parameter , while it increased in the control group. A significant difference between the two groups was observed 5 days after the treatment. However, BUN by itself, could not be considered as a specific indicator of renal function to evaluate the effectiveness of an intended drug.
In a study by Celik et al on vancomycin- induced nephrotoxicity in rats, protective effect of different antioxidants (alpha-lipoic acid, Ginkgo biloba extract, and melatonin) was observed in improving renal function compared to the control group. This effect is probably attributed to the prohibition of free radicals production by antioxidants (24). In another study that investigated the effect of 4-week administration of vitamin E on renal damage induced by mercuric chloride in rats, the concentration of serum creatinine in the vitamin E group showed no significant increase compared to the control group; so, it was concluded that vitamin E may have positive effects on attenuating renal damage caused by nephrotoxic agents (25).
In another animal study, the effects of vitamins E and C on cisplatin-induced renal damage in mice were investigated. Vitamin E was administered with the dose of 500 mg/kg and vitamin C with the dose of 250 mg/kg. In this study, admissible effects were observed in preventing the creatinine increase induced by cisplatin (18). In the same study, the protective effect of vitamin E and silymarin was investigated on gentamicin-induced nephrotoxicity in dogs. These agents could also prevent the gentamicin-induced GFR decline (26). The beneficial effect of several antioxidants were studied separately in a small study on rats with vancomycin nephrotoxicity. This effect was significant for vitamins E and C specified by serum BUN reduction (12).
In one human study, the effects of vitamin E and selenium on cisplatin-induced nephrotoxicity was investigated in cancer patients. In this study, 22 patients received 400 IU vitamin E and 200 μg selenium daily, while 24 patients received placebo concomitantly with their cisplatin-based chemotherapy regimen for one month. This therapy resulted in significant differences of GFR between two study groups (21). In that study, the number of patients with AKI incidence was 12 in the control group versus no case of AKI in vitamin E group.
Considering oxidative reactions as a major cause of kidney injury in patients receiving vancomycin, this could be concluded that antioxidants such as vitamin E can prevent this damage probably by attenuating oxidative stress. Vitamin E potentially suppresses the production of toxic free radicals such as hydroxyl and superoxide ions, lipid peroxyl and hydroperoxyl groups, and nitrates; it also can prevent the toxic agents produced by oxidative damage leaking from the biological membranes (15). These mechanisms constitute the antioxidative properties of vitamin E, which potentiate the idea of using it as a protective agent in the harmful conditions known to be induced by oxidative damage, namely kidney injury.
While the present study has some advantages such as including the control arm, evaluating only one antioxidant as the protective agent in this population, and matching the two study groups with regard to the baseline characteristics and clinical parameters, the major limitation of this study was its non-randomized and non-blinded design. Therefore, this beneficial effect should be evaluated in clinical trials with more powerful design. Furthermore, measuring the oxidative markers may be very conclusive, since the effects of antioxidants such as vitamin E would be more explicable accordingly. This objective could be followed in future research in this regard.
CONCLUSION
Our study, as the first one carried out in human, showed that vitamin E may have the potential benefit for preventing vancomycin- induced nephrotoxicity. Furthermore, it can reduce the incidence of vancomycin-induced AKI as well.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest for this study.
AUTHORS’ CONTRIBUTION
R. Soltani and Sh. Badri developed the idea of research, design of study, and performed data analysis. F. Khorvash, M. Meidani, and Sh. Taheri recruited the patients. S. Alaei performed all data gathering and patients’ follow-up. All authors contributed in manuscript preparation and revision.
ACKNOWLEDGEMENTS
The content of this paper is extracted from a Pharm. D. thesis which was financially supported (Grant No. 394481) by the vice chancellery for research and technologyat Isfahan University of Medical Sciences. Authors would like to acknowledge the laboratory department of Alzahra hospital for their assistance.
REFERENCES
- 1.Rybak M, Lomaestro B, Rotschafer JC, Moellering R, Jr, Craig W, Billeter M, et al. Therapeutic monitoring of vancomycin in adult patients:a consensus review of the American society of health-system pharmacists, the infectious diseases society of America, and the society of infectious diseases pharmacists. Am J Health Syst Pharm. 2009;66(1):82–98. doi: 10.2146/ajhp080434. DOI: 10.2146/ajhp080434. [DOI] [PubMed] [Google Scholar]
- 2.Hazlewood KA, Brouse SD, Pitcher WD, Hall RG. Vancomycin-associated nephrotoxicity: grave concern or death by character assassination? Am J Med. 2010;123(2):182. doi: 10.1016/j.amjmed.2009.05.031. e1-7,1-11. DOI: 10.1016/ j.amjmed.2009.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elyasi S, Khalili H, Dashti-Khavidaki S, Mohammadpour A. Vancomycin-induced nephrotoxicity: mechanism, incidence, risk factors and special populations a literature review. Eur J Clin Pharmacol. 2012;68(9):1243–1255. doi: 10.1007/s00228-012-1259-9. DOI: 101007/ s00228-012-1259-9. [DOI] [PubMed] [Google Scholar]
- 4.Iwamoto T, Kagawa Y, Kojima M. Clinical efficacy of therapeutic drug monitoring in patients receiving vancomycin. Biol Pharm Bull. 2003;26(6):876–879. doi: 10.1248/bpb.26.876. DOI: 10.1248/bpb.26.876. [DOI] [PubMed] [Google Scholar]
- 5.Filippone EJ, Kraft WK, Farber JL. The nephrotoxicity of vancomycin. Clin Pharmacol Ther. 2017;102(3):459–469. doi: 10.1002/cpt.726. DOI: 10.1002/cpt.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khoei A, Soltani R, Emami J, Badri S, Taheri S. Therapeutic drug monitoring of vancomycin by AUCT-MIC ratio in patients with chronic kidney disease. Res Pharm Sci. 2019;14(1):84–92. doi: 10.4103/1735-5362.251856. DOI: 10.4103/1735-5362.251856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rybak MJ, Lomaestro BM, Rotschafer JC, Moellering RC, Craig WA, Billeter M, et al. Vancomycin therapeutic guidelines: a summary of consensus recommendations from the infectious diseases society of America, the American society of health-system pharmacists, and the society of infectious diseases pharmacists. Clin Infect Dis. 2009;49(3):325–327. doi: 10.1086/600877. DOI: 10.1086/600877. [DOI] [PubMed] [Google Scholar]
- 8.Elyasi S, Khalili H, Hatamkhani S, Dashti-Khavidaki S. Prevention of vancomycin induced nephrotoxicity: a review of preclinical data. Eur J Clin Pharmacol. 2013;69(4):747–754. doi: 10.1007/s00228-012-1406-3. DOI: 10.1007/s00228-012-1406-3. [DOI] [PubMed] [Google Scholar]
- 9.Parlakpinar H, Ozer MK, Sahna E, Vardi N, Cigremis Y, Acet A. Amikacin-induced acute renal injury in rats: protective role of melatonin? J Pineal Res. 2003;35(2):85–90. doi: 10.1034/j.1600-079x.2003.00059.x. DOI: 10.1034/j.1600-079x.2003.00059.x. [DOI] [PubMed] [Google Scholar]
- 10.Parlakpinar H, Tasdemir S, Polat A, Bay-Karabulut A, Vardi N, Ucar M, et al. Protective role of caffeic acid phenethyl ester (cape) on gentamicin- induced acute renal toxicity in rats. Toxicology. 2005;207(2):169–177. doi: 10.1016/j.tox.2004.08.024. DOI: 10.1016/ j.tox.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 11.Ozen S, Akyol O, Iraz M, Sogut S, Ozugurlu F, Ozyurt H, et al. Role of caffeic acid phenethyl ester, an active component of propolis, against cisplatin- induced nephrotoxicity in rats. J Appl Toxicol. 2004;24(1):27–35. doi: 10.1002/jat.941. DOI: 10.1002/jat.941. [DOI] [PubMed] [Google Scholar]
- 12.Ocak S, Gorur S, Hakverdi S, Celik S, Erdogan S. Protective effects of caffeic acid phenethyl ester, vitamin C, vitamin E and N-acetylcysteine on vancomycin-induced nephrotoxicity in rats. Basic Clin Pharmacol Toxicol. 2007;100(5):328–333. doi: 10.1111/j.1742-7843.2007.00051.x. DOI: 10.1111/j. 1742-7843.2007.00051.x. [DOI] [PubMed] [Google Scholar]
- 13.Ahmida MH. Protective role of curcumin in nephrotoxic oxidative damage induced by vancomycin in rats. Exp Toxicol Pathol. 2012;64(3):149–153. doi: 10.1016/j.etp.2010.07.010. DOI: 10.1016/ j.etp.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 14.Panonnummal R, Varkey J, Dinoop DR. Protective effect of atorvastatin against vancomycin induced nephrotoxicity in albino rats. Pharmacie Globale (IJCP) 2011;8(10):1–6. [Google Scholar]
- 15.Young IS, Woodside JV. Antioxidants in health and disease. J Clin Pathol. 2001;54(3):176–186. doi: 10.1136/jcp.54.3.176. DOI: 10.1136/jcp.54.3.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aslanturk A, Uzunhisarcikli M, Kalender S, Demir F. Sodium selenite and vitamin E in preventing mercuric chloride induced renal toxicity in rats. Food Chem Toxicol. 2014;70:185–190. doi: 10.1016/j.fct.2014.05.010. DOI: 10.1016/j.fct.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 17.Varzi HN, Esmailzadeh S, Morovvati H, Avizeh R, Shahriari A, Givi ME. Effect of silymarin and vitamin E on gentamicin-induced nephrotoxicity in dogs. J Vet Pharmacol Ther. 2007;30(5):477–481. doi: 10.1111/j.1365-2885.2007.00901.x. DOI: 10.1111/j.1365-2885.2007.00901.x. [DOI] [PubMed] [Google Scholar]
- 18.Ajith TA, Usha S, Nivitha V. Ascorbic acid and alpha-tocopherol protect anticancer drug cisplatin induced nephrotoxicity in mice: a comparative study. Clin Chim Acta. 2007;375(1-2):82–86. doi: 10.1016/j.cca.2006.06.011. DOI: 10.1016/j.cca.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 19.World Medical Association. World medical association declaration of Helsinki: ethical principles for medical research involving human subjects. Bull World Health Organ. 2001;79(4):373–374. [PMC free article] [PubMed] [Google Scholar]
- 20.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39(2):165–228. doi: 10.1007/s00134-012-2769-8. DOI: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hemati S, Arbab Jolfaie N, Gookizadeh A, Rafienia M, Ghavamnasiri MR. The effects of vitamin E and selenium on cisplatin-induced nephrotoxicity in cancer patients treated with cisplatin-based chemotherapy: a randomized, placebo-controlled study. J Res Med Sci. 2012;1:S49–S58. [PMC free article] [PubMed] [Google Scholar]
- 22.Levey AS, Stevens LA. Estimating GFR using the CKD epidemiology collaboration (CKD-EPI) creatinine equation: more accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am J Kidney Dis. 2010;55(4):622–627. doi: 10.1053/j.ajkd.2010.02.337. DOI: 10.1053/j.ajkd.2010.02.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2(1):1–138. [Google Scholar]
- 24.Celik I, Cihangiroglu M, Ilhan N, Akpolat N, Akbulut HH. Protective effects of different antioxidants and amrinone on vancomycin-induced nephrotoxicity. Basic Clin Pharmacol Toxicol. 2005;97(5):325–332. doi: 10.1111/j.1742-7843.2005.pto_153.x. DOI: 10.1111/j.1742-7843.2005.pto_153.x. [DOI] [PubMed] [Google Scholar]
- 25.Aslanturk A, Uzunhisarcikli M, Kalender S, Demir F. Sodium selenite and vitamin E in preventing mercuric chloride induced renal toxicity in rats. Food Chem Toxicol. 2014;70:185–190. doi: 10.1016/j.fct.2014.05.010. DOI: 10.1016/j.fct.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 26.Varzi HN, Esmailzadeh S, Morovvati H, Avizeh R, S hahriari A, Givi ME. Effect of silymarin and vitamin E on gentamicin-induced nephrotoxicity in dogs. J Vet Pharmacol Ther. 2007;30(5):477–481. doi: 10.1111/j.1365-2885.2007.00901.x. DOI: 10.1111/j.1365-2885.2007.00901.x. [DOI] [PubMed] [Google Scholar]


