Abstract
OBJECTIVE
To determine long-term changes in pharmacological treatment of type 2 diabetes after primary Roux-en-Y gastric bypass (RYGB) surgery, in patients with and without pharmacological treatment of diabetes preoperatively.
SUMMARY BACKGROUND DATA
Several studies have shown that gastric bypass has good effect on diabetes, at least in the short-term. This study is a nationwide cohort study using Swedish registers, with basically no patients lost to follow-up during up to 7-years after surgery.
METHODS
The effect of RYGB on type 2 diabetes drug treatment was evaluated in this nationwide matched cohort study. Participants were 22,047 adults with BMI ≥30 identified in the nationwide Scandinavian Surgical Obesity Registry, who underwent primary RYGB between 2007 and 2012. For each individual, up to 10 general population comparators were matched on birth year, sex, and place of residence. Prescription data were retrieved from the nationwide Swedish Prescribed Drug Register through September 2015. Incident use of pharmacological treatment was analyzed using Cox regression.
RESULTS
67% of patients with pharmacological treatment of type 2 diabetes before surgery were not using diabetes drugs 2 years after surgery and 61% of patients were not pharmacologically treated up to 7 years after surgery. In patients not using diabetes drugs at baseline, there were 189 new cases of pharmacological treatment of type 2 diabetes in the surgery group and 2319 in the matched general population comparators during a median follow-up of 4.6 years (incidence: 21.4 vs. 27.9 per 10,000 person-years; adjusted hazard ratio 0.77, 95%CI 0.67–0.89; P<0.001).
CONCLUSIONS
Gastric bypass surgery not only induces remission of pharmacological treatment of type 2 diabetes, but also protects from new onset of pharmacological diabetes treatment. The effect seems to persist in most, but not all, patients over 7 years of follow-up.
BACKGROUND
Obesity is increasing worldwide[1, 2], and consequently so are co-morbid diseases associated with obesity, such as type 2 diabetes mellitus[3]. Roux-en-Y gastric bypass (RYGB) surgery has well documented effects on type 2 diabetes, with initial remission rates between 38–75% after surgery[4–9], mainly depending on which criteria are used to define remission. Some of these patients will later relapse[5, 8]. Other studies present reduced diabetes drug use after RYGB when comparing to an obese control group[10, 11]. However, many previous studies are based on data from centers of excellence or investigate surgical procedures no longer in use.
In recent years, several randomized trials have shown that bariatric surgery is more effective than pharmacological treatment of type 2 diabetes[8, 9, 12, 13], even in patients with moderate obesity[14]. Remission rates in these studies range from 38–75%, with a follow-up time of up to 5 years. In a review of the literature with regard to the effects of bariatric surgery on type 2 diabetes, remission rates of 66.7% 2 years after surgery were reported but considerable losses to follow-up were found in most reviewed studies[6]. The SOS study found that after 15 years of follow-up only 30.4% of the participants were in remission compared to 72.3% at 2 years after surgery[15]. Yet, the SOS study is mainly based on gastric banding and vertical banded gastroplasty, procedures that are no longer used.
There are also studies suggesting that bariatric surgery prevent the development of type 2 diabetes. At the 15-year follow-up, the SOS study reported a 76% reduction of incident type 2 diabetes in patients without diabetes at the time of surgery[16]. A population-based matched cohort study from the United Kingdom demonstrated a lower incidence of type 2 diabetes after surgery (4.3%) compared to the obese controls (16.2%) over 7 years follow-up[17] and a very recent study demonstrates even lower rates of de novo diabetes with an incidence of 0.4% with a median follow-up of 9 years[18]. The SOS study found that this diabetes protective effect of bariatric surgery was not correlated to BMI. However, patients with impaired fasting glucose at the time of surgery had a greater protective effect than patients with normoglycemia (number needed to treat 1.3 and 7, respectively)[19] and a recent report from the SOS study show that long-term microvascular disease can be reduced in patients after surgery, especially in patients with prediabetes.[20]
The aim of this study was to assess the impact of RYGB on diabetes type 2 drug treatment. Using Swedish nationwide registry data with close to 100% follow-up, remission and recurrence was studied over up to 7 years of follow-up. We also assessed the preventive effect of RYGB on type 2 diabetes drug treatment among patients without such treatment at baseline versus matched general population comparators.
RESEARCH DESIGN & METHODS
This study was conducted using a register linkage based on the Scandinavian Obesity Surgery Registry (SOReg)[21] linked to nationwide health registers, using the unique personal identity number assigned to each Swedish resident. Comparators from the general population were sampled and matched to the bariatric surgery patients.
Study Population & Intervention
We retrieved 29,432 adults with BMI ≥30kg/m2 from SOReg who underwent primary RYGB at some point between 2007 and 2012. SOReg has been estimated to cover 98.5% of all bariatric surgery procedures in Sweden, including data from 40 surgical units. During the study period, 97% of all bariatric procedures were RYGB. Data are stored electronically and recorded as part of clinical practice.
Other Registers
The nationwide Swedish Prescribed Drug Register was established in 2005 and includes all dispensed prescription drugs classified according to the World Health Organization Anatomical Therapeutic Chemical (ATC) classification system. The register is updated monthly.
The National Patient Register is a nationwide register that all hospitals are obliged to report to and it contains inpatient and outpatient hospital care data. The inpatient component of the register attained national coverage in 1987 and covers nearly 100% of all hospital admissions in public healthcare. The outpatient component was started in 2001 and initially covered about 75% of outpatient visits in specialized health care, and today it covers about 96%.
The Total Population Register provides data on emigration/immigration, marriage/divorce, dates of birth/death for each individual in Sweden[22]. This register is complete and continually updated by Statistics Sweden. Data on education level were retrieved from the Education Register also kept by Statistics Sweden.
Inclusion and Exclusion Criteria
There were no mandatory national eligibility criteria for bariatric surgery during the study period, although most counties in Sweden used BMI≥35 kg/m2 with or without obesity-related comorbidity. Adults ≥18 years of age with a BMI ≥30 kg/m2 were included. Patients without a registered preoperative HbA1c (n=5592; 19.0%), as well as patients with an in- or outpatient visit listing type 1 diabetes mellitus (ICD10: E10; without a diagnosis of type 2 diabetes [ICD10: E11]) in the National Patient Register were excluded (n=141; 0.5%).
Cohorts
The patients were divided into two cohorts, one to study the remission of pharmacological diabetes treatment in patients with type 2 diabetes (referred to as the remission cohort), and the second cohort to study the incidence of pharmacological treatment of diabetes after surgery in patients without diabetes drug treatment before surgery (referred to as the incidence cohort).
Remission Cohort:
The remission cohort consisted of all patients with at least one prescription of diabetes drugs the year before surgery (n=3629). The remission cohort was divided into 2 subgroups based on duration of pharmacological diabetes treatment (<2 years and ≥2 years).
Incidence Cohort:
The incidence cohort consisted of all patients without any sign of diabetes at baseline, more specifically patients without preoperative pharmacological treatment of diabetes, no preoperative diabetes diagnosis and HbA1c<6.5% (48mmol/mol) before surgery (n=18,418). Patients in this cohort were categorized according to the American Diabetes Association guidelines[23] guidelines into patients with euglycemia (HbA1c<5.7% (39mmol/mol)) and prediabetes (HbA1c 5.7 to <6.5% (39 to <48mmol/mol)) based upon the HbA1c level before surgery.
Matched Comparators from the General Population
For each patient who had bariatric surgery, up to 10 comparators from the general population were matched by birth year, calendar year (comparators required to be alive the year of surgery for the matched surgery case), sex, and place of residence. BMI and glycemic status were not available for the general population comparators.
Covariates
Age, sex and educational level were retrieved from the Total Population Register[22] and the Education Register at Statistics Sweden. Baseline BMI and HbA1c were retrieved from SOReg for the bariatric surgery patients, while such data were not available for the matched general population comparators. Dispensed prescription drugs were retrieved from the Prescribed Drug Register and hospital visits from the National Patient Register for both surgery patients and general population comparators.
Outcome & Follow-Up
In the remission cohort with baseline pharmacological treatment of type 2 diabetes, the outcome was remission of pharmacological diabetes treatment defined as no prescribed diabetes drugs for one entire year. In the incidence cohort without pharmacological treatment of diabetes before surgery, the outcome was incident use of pharmacological treatment of type 2 diabetes after surgery.
Prescription drug data were retrieved from the Prescribed Drug Register through September 2015. Participants were followed from the surgery date (or matching date for general population comparators) until emigration, death, or end of follow-up, whichever came first. Emigration and death dates were retrieved from the Total Population Register, respectively. During follow-up 124 participants from the surgery cohort and 2650 participants from the general population comparators emigrated, making register-based follow-up complete for 99.4% (21923/22047) in the surgery cohort and 98.5% (172488/175138) among the comparators.
Statistical analysis
For patients in the remission cohort, the annual proportion of diabetes drug treatment was calculated for up to 5 years preoperatively and up to 7 years postoperatively.
For patients in the incidence cohort, the prevalence of diabetes drug treatment for each 1-year interval from the date of surgery up to 7 years after surgery, was calculated for the total surgery group, as well as for the subgroups with euglycemia and prediabetes.
For the incidence cohort, incident use of pharmacological diabetes treatment was also analyzed using survival analysis. Adjusted hazard ratios were estimated using conditional Cox regression (conditioned on the matching set with each set containing 1 surgery patient and up to 10 general population comparators). Robust confidence intervals were estimated and the proportional hazard assumption was evaluated by interacting time and treatment.
Statistical analyses were performed using SAS (version 9.4) and Stata (version 14).
RESULTS
Baseline Characteristics
Remission Cohort:
A total of 3629 patients with pharmacological treatment of type 2 diabetes in the year before surgery were included for analysis (mean age 49 years; 60.7% women; mean preoperative BMI 42.2; Table 1). Patients in the remission cohort with a longer duration of diabetes (≥2 years) were on average older, had higher baseline HbA1c value and their BMI was lower than patients with a shorter duration of diabetes (<2 years). The patients with a longer duration of pharmacologically treated diabetes were more likely to be pharmacologically treated with anti-hypertensive and lipid-lowering medication than those with a shorter duration of pharmacological diabetes treatment (Table 1).
Table 1.
Patient characteristics at baseline in the remission cohort
| Patients with Drug-Treated Diabetes1 before Surgery | |||
|---|---|---|---|
| Diabetes duration < 2 years |
Diabetes duration ≥ 2 years |
All | |
| N | 1139 | 2490 | 3629 |
| Age (Years), Mean (SD) | 46 (9.9) | 50 (9.5) | 49 (9.8) |
| Women, n (%) | 734 (64.4) | 1469 (59.0) | 2203 (60.7) |
| University Education, n (%) | 229 (20.1) | 487 (19.6) | 716 (19.7) |
| Married, n (%) | 508 (44.6) | 1195 (48.0) | 1703 (47.0) |
| Body-Mass Index (kg/m2), Mean (SD) | 43.0 (5.9) | 41.8 (5.7) | 42.2 (5.8) |
| 30 to <35, n (%) | 43 (3.8) | 163 (6.5) | 206 (5.7) |
| 35 to <40, n (%) | 357 (31.3) | 897 (36.0) | 1254 (34.6) |
| 40 to <50, n (%) | 609 (53.5) | 1196 (48.0) | 1805 (49.7) |
| ≥50, n (%) | 130 (11.4) | 234 (9.4) | 364 (10.0) |
| HbA1c (%) | |||
| Mean (SD) | 7.1 (1.3) | 8.1 (1.6) | 7.8 (1.6) |
| Median (25th-75th percentile) | 6.8 (6.2–7.8) | 7.8 (6.8–9.1) | 7.5 (6.5–8.7) |
| Antihypertensive Therapy2, n (%) | 658 (57.8) | 1921 (77.1) | 2579 (71.1) |
| Lipid Modifying Therapy3, n (%) | 470 (41.3) | 1654 (66.4) | 2124 (58.5) |
| Cardiovascular Disease4, n (%) | 454 (39.9) | 1457 (58.5) | 1911 (52.7) |
Insulin (A10A), oral diabetes drugs (A10B)
ARB (ATC C02), thiazide diuretics (C03A, C03EA01), beta blockers (C07 [excl. C07AA07]), vessel selective calcium antagonists (C08C), ACE inhibitors (C09A, C09B), alfa blockers and other centrally acting drugs (C09C, C09D), renin inhibitors (C09X)
ATC code C10
Defined as history of hospitalization listing cardiovascular disease
Incidence Cohort:
A total of 18,418 patients without previous diabetes diagnosis, HbA1c<6.5% at baseline and no history of pharmacological treatment (ever) of type 2 diabetes were included for analysis (mean age 39 years; 78.8% women; mean preoperative BMI 42.7; Table 2). Compared to the general population, gastric bypass patients had a lower education level, while the proportion married was similar. In terms of physical health, gastric bypass patients had several-fold higher prevalence of treatment for hypertension and dyslipidemia, as well as history of hospitalization for cardiovascular disease. The subgroups with euglycemia and prediabetes differed in the sense that age and BMI were higher in the group with prediabetes than in patients with euglycemia. Patients with prediabetes also in higher degree had pharmacological treatment for hypertension and dyslipidemia and were more likely to have a history of hospitalization listing cardiovascular disease.
Table 2.
Patient characteristics at baseline in the incidence cohort
| Euglycemia | Prediabetes | All | ||||
|---|---|---|---|---|---|---|
| Surgery | Matched Comparators | Surgery | Matched Comparators | Surgery | Matched Comparators | |
| N | 12,407 | 118,132 | 6011 | 57,006 | 18,418 | 175,138 |
| Age (Years), Mean (SD) | 37 (10.0) | 37 (10.0) | 44 (10.2) | 44 (10.1) | 39 (10.5) | 39 (10.5) |
| Women, n (%) | 9974 (80.4) | 94,929 (80.4) | 4544 (75.7) | 43,153 (75.7) | 14,518 (78.8) | 138,082 (78.8) |
| University Education, n (%) | 2727 (22.0) | 43,627 (36.9) | 1299 (21.6) | 20,924 (36.7) | 4026 (21.9) | 64,551 (36.9) |
| Married, n (%) | 4964 (40.0) | 47,456 (40.2) | 2623 (43.7) | 26,816 (47.1) | 7587 (41.2) | 74,272 (42.4) |
| Body-Mass Index (kg/m2), Mean (SD) | 42.4 (5.3) | - | 43.2 (5.7) | - | 42.7 (5.5) | - |
| 30 to <35, n (%) | 470 (3.8) | - | 162 (2.7) | - | 632 (3.4) | - |
| 35 to <40, n (%) | 3902 (31.4) | - | 1709 (28.4) | - | 5611 (30.5) | - |
| 40 to <50, n (%) | 6994 (56.4) | - | 3435 (57.1) | - | 10429 (56.6) | - |
| ≥50, n (%) | 1041 (8.4) | - | 705 (11.7) | - | 1746 (9.5) | - |
| Hba1c | ||||||
| Mean (SD) | 5.3 (0.3) | - | 6.0 (0.2) | - | 5.5 (0.4) | - |
| Median (25th-75th percentile) | 5.4 (5.2–5.5) | - | 5.9 (5.8–6.1) | - | 5.5 (5.3–5.8) | - |
| Antihypertensive Therapya, n (%) | 2560 (20.6) | 9272 (7.8) | 2210 (36.8) | 7355 (12.9) | 4770 (25.9) | 16,627 (9.5) |
| Lipid Modifying Therapyb, n (%) | 667 (5.4) | 2436 (2.1) | 819 (13.6) | 2513 (4.4) | 1486 (8.1) | 4949 (2.8) |
| Cardiovascular Diseasec, n (%) | 1516 (12.2) | 4400 (3.7) | 1476 (24.6) | 3399 (6.0) | 2992 (16.2) | 7799 (4.5) |
ARB (ATC C02), thiazide diuretics (C03A, C03EA01), beta blockers (C07 [excl. C07AA07]), vessel selective calcium antagonists (C08C), ACE inhibitors (C09A, C09B), alfa blockers and other centrally acting drugs (C09C, C09D), renin inhibitors (C09X)
ATC code C10
Defined as history of hospitalization listing cardiovascular disease
Remission of Pharmacological Treatment of Type 2 Diabetes After Gastric Bypass
In Figure 1, the proportion of pharmacological treatment of type 2 diabetes is shown for each 1-year interval from 5 years before and up to 7 years after surgery in the remission cohort. 67% of the surgery patients were in remission of pharmacological diabetes treatment 2 years after surgery, and after 7 years follow-up 61% were without pharmacological diabetes treatment.
Figure 1.

Annual proportion of pharmacological diabetes treatmenta in the remission cohort up to 5 years before and up to 7 years after gastric bypass surgery. A: total cohort; B: patients with diabetes treatment duration <2 years; C: patients with diabetes treatment duration ≥ 2years.
Patients in the remission cohort with a shorter duration (<2 years) of pharmacological diabetes treatment had a higher rate of treatment remission than those with longer duration (≥2 years). In patients with shorter duration of pharmacological diabetes treatment, 88% had pharmacological treatment remission after 2 years follow-up and after 7 years follow-up 86% were without pharmacological diabetes treatment. In patients with longer duration of pharmacological diabetes treatment, 58% have pharmacological diabetes treatment remission after 2 years and 46% were without pharmacological diabetes treatment after 7 years follow-up.
New Onset of Pharmacological Treatment of Type 2 Diabetes After Gastric Bypass
In Figure 2, the annual proportion of pharmacological treatment of type 2 diabetes is shown for the incidence cohort and general population comparators (patients and comparators without previous pharmacological diabetes treatment; matched by age, sex and place of residence but not weight or glycemic status).
Figure 2.
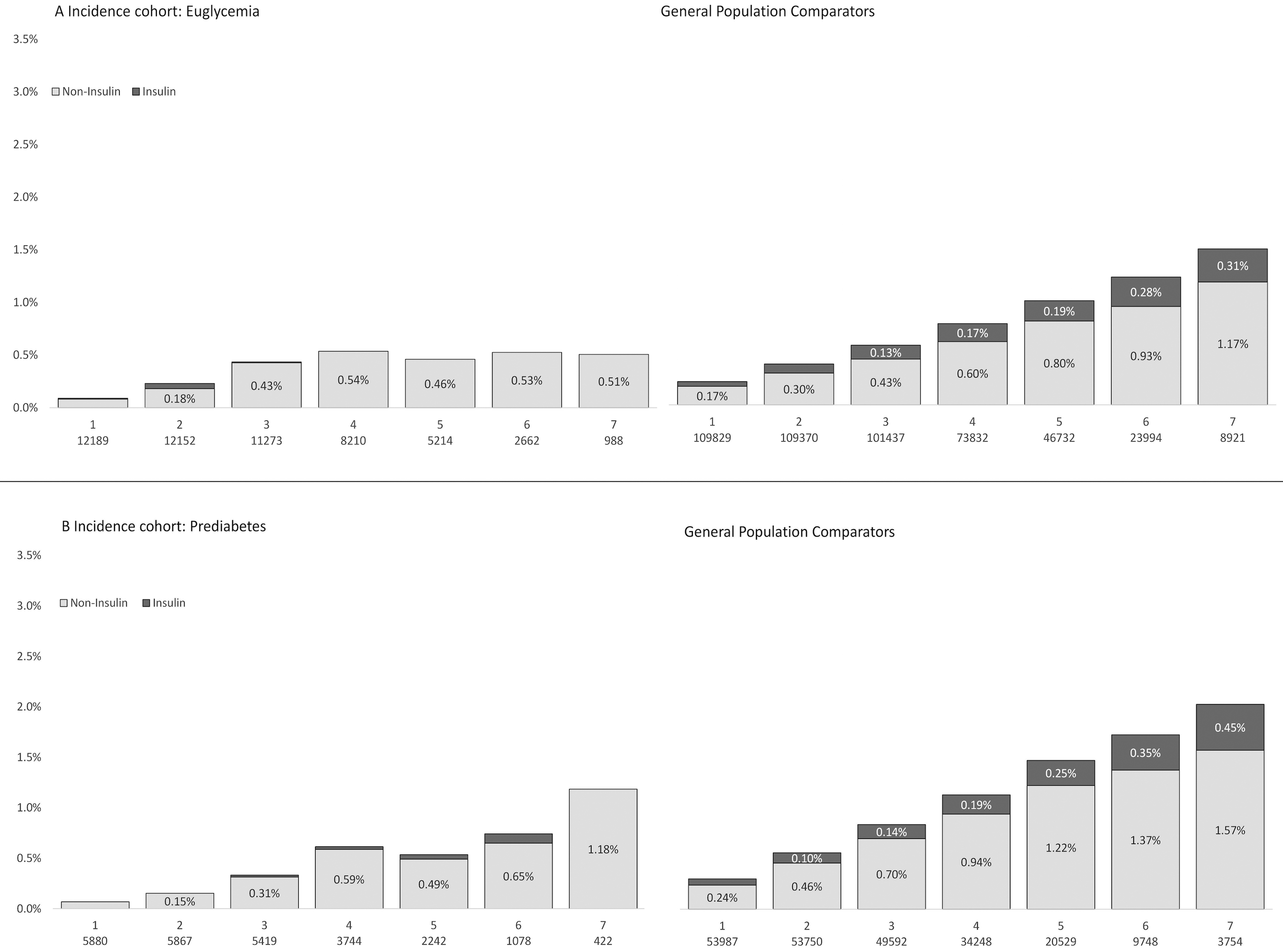
Annual proportion of pharmacological diabetes treatmentb after gastric bypass surgery in the incidence cohort (left column) and matched general population controls (right column) by glycemic status. A: euglycemia; B: prediabetes.
Matched general population comparators: Matched by age, sex and place of residence. Not matched by BMI or glycemic status.
In Figure 3, the result from the survival analysis is presented in the subgroups with euglycemia and prediabetes. Each subgroup has its own matched control group.
Figure 3.
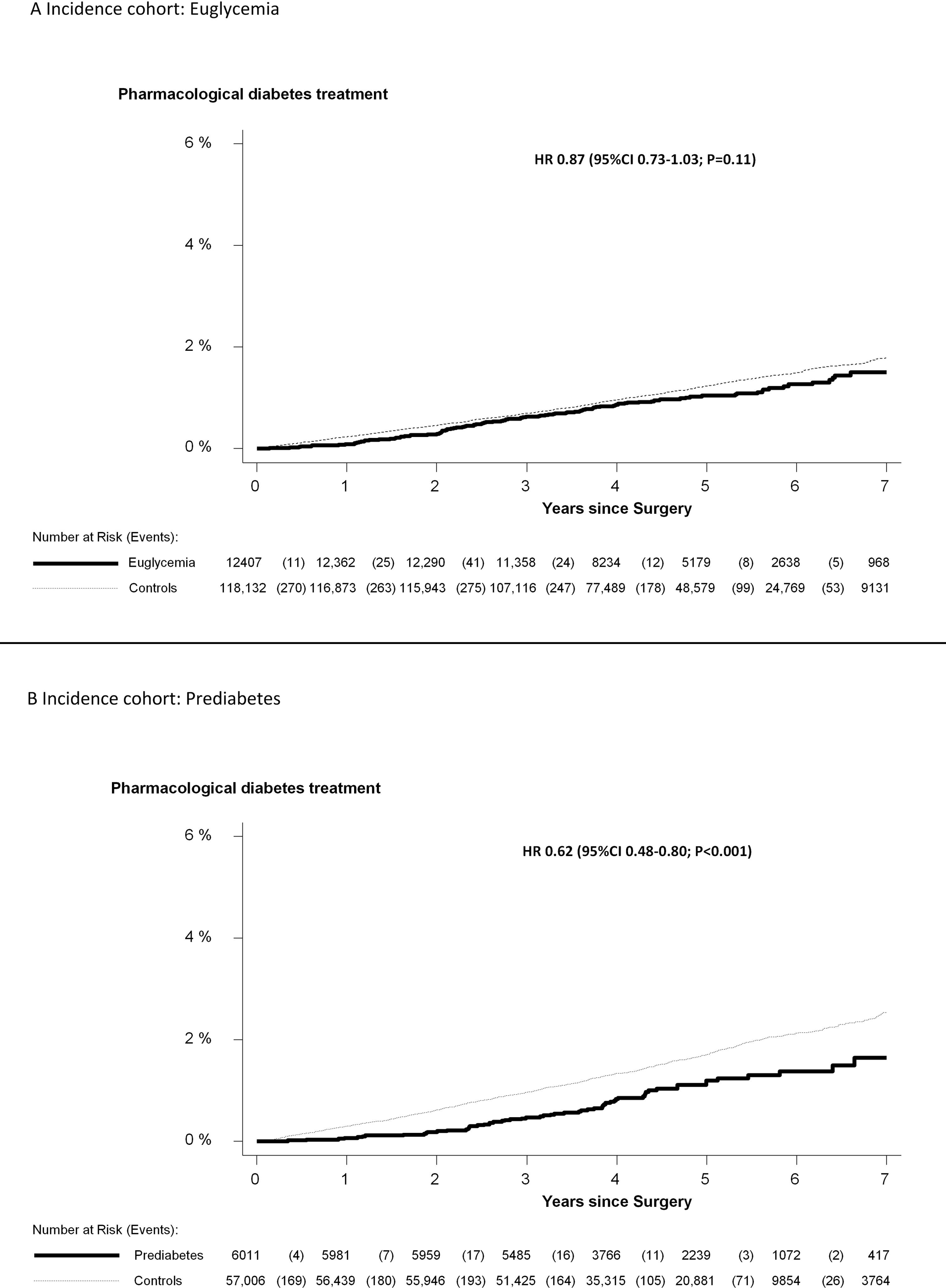
Cumulative incidence of pharmacological diabetes treatmentc in the incidence cohort in individuals without diabetes at baseline. The incidence cohort is divided by glycemic status at baseline and the groups are presented with their respective control group. A: euglycemia B: prediabetes
Euglycemia=HbA1c<5.7%; Prediabetes=HbA1c 5.7 to <6.5%
Controls: Matched comparators from the general population (matched for age, sex and place of residence)
Total group
During a median follow-up of 4.6 years, there were 189 incident cases of pharmacological diabetes treatment in the total incidence cohort and 2319 in the matched general population comparators (21.4 vs. 27.9 cases per 10,000 person-years; adjusted hazard ratio 0.77 (95%CI 0.67–0.89; P<0.001).
Euglycemia
In RYGB patients with an HbA1c value indicating euglycemia at baseline, none were on insulin treatment at 7 years postoperatively, while 0.5% were on any pharmacological diabetes treatment at that time. In the matched general population 0.3% were on insulin treatment, while1.5% were on any pharmacological diabetes treatment at 7-year follow-up (Figure 2)
Adjusted hazard ratio were 0.87 (95%CI 0.73–1.03; P=0.11) for patients with euglycemia.
Prediabetes
In RYGB patients with prediabetes (based on HbA1c values) at baseline, none were on insulin treatment 7 years postoperatively, while 1.2% were on any pharmacological diabetes treatment. In the matched general population, 0.4% were on insulin treatment and 2% were on any pharmacological diabetes treatment (Figure 2).
Adjusted hazard ratio were 0.62 (95%CI 0.48–0.80; P<0.001) for patients with prediabetes.
DISCUSSION
We conducted a nationwide cohort study with more than 20,000 cases and almost 200,000 matched comparators from the general population to investigate the effect of RYGB surgery on type 2 diabetes treatment, and if RYGB has a protective effect on type 2 diabetes in patients without pharmacological diabetes treatment before surgery. Our study demonstrates that more than 60% of patients who were pharmacologically treated for type 2 diabetes prior to RYGB surgery managed without medication up to 7 years after surgery. The need to reinstate treatment for diabetes was low and with up to 7 years of follow-up the rate of de novo cases was similar or lower compared with the level observed in the general population, despite the fact that most patients remain obese after RYGB surgery.
Our nationwide data on remission of diabetes drug treatment after RYGB, assuming that pharmacological diabetes treatment is an indication of patients’ actual diabetes status, is similar to other large studies on diabetes remission after bariatric surgery. Yet, the present study is unique in that it has near 100% follow-up and covers the whole Swedish population. The SOS study demonstrated a diabetes remission (defined as normal fasting glucose) rate of 72% at the 2-year follow-up which decreased to 36% at the 10-year follow-up[24]. However, it is important to recognize that the SOS study is mainly comprised of restrictive surgical procedures not in use today. The Utah Obesity Study on health benefits of RYGB demonstrated a remission rate (defined as normal fasting glucose and HbA1c without medical treatment) of 75% at 2 years, and 62% at 6 years of follow-up after RYGB[4] and the LABS2 study 67% remission at 3-year follow-up after RYGB[25].
We demonstrate a lower rate of recurrence of drug treated diabetes compared to previous studies, with a remission rate of 67% 2 years after surgery and 61% 7 years after surgery. This recurrence is slightly lower than in the earlier mentioned Utah Obesity Study[4]. That study had a follow-up rate of more than 90%, which is comparable to our follow-up rate of close to 100%. The reason behind this discrepancy might be their stricter definition of remission (normal fasting glucose and HbA1c versus free of diabetes drug treatment).
The cumulative incidence of new cases with pharmacological treatment for type 2 diabetes after surgery was less than 2% over the 7-year follow-up period. Our results are comparable to 2% in the Utah Obesity Study[4] with 6-year follow-up and 0.9% in LABS2[25] with 3-year follow-up. Our data compare favorably to a recent publication from the United Kingdom where the incidence of new cases after bariatric surgery was 4.2% after 7 years of follow-up[26], all these studies use a definition of diabetes based on diabetes treatment and glycemic status. Another reason for this difference could be that the United Kingdom study included sleeve gastrectomy and gastric banding in addition to RYGB. The authors reported that the lowest number of new cases came from patients who had undergone RYGB. A lower de novo diabetes incidence of 0.4% during a median of 9 years of follow-up was presented in a recent retrospective single-center study from Cleveland, but as the authors point out in the discussion the true number could possibly be a little higher because of potential missing data during follow-up[18].
Both the Utah Obesity Study[4] and the study from the United Kingdom[26] demonstrate that the incidence of new cases of type 2 diabetes after surgery is lower than for obese matched controls. We show that the incidence of new cases of diabetes treatment after RYGB in Sweden is at par or lower than that seen in the age- and sex-matched general population. This is true for patients with euglycemia as well as prediabetes at baseline. We also demonstrated that longer duration of pharmacological diabetes treatment is associated with lower likelihood of remission. This is in line with previous data demonstrating that duration of diabetes is a predictor of remission[27]. Thus obese patients with short disease duration should consider having early surgery and obese patients with prediabetes could consider surgery before diabetes develops.
Strengths of the present study are the long and near complete follow-up in a large nationwide cohort, which should allow for good generalizability. Weaknesses include lack of follow-up data on plasma glucose and HbA1c values, which resulted in a less strict outcome definition than in previous studies (diabetes drug treatment rather than abnormal glycemic status) and the use of administrative rather than clinical data. Furthermore, we can not exclude that some patients and controls were prescribed metformin for other reasons than type 2 diabetes such as prophylaxis of diabetes remission or for polycystic ovarian syndrome (PCOS). However, in the years covered by this study the use of metformin as prophalaxis of diabetes remission was uncommon in Sweden.
One might argue that the use of general population comparators can be seen as both a strength and weakness. Previous studies have used obese matched control groups. We utilized a matched control group from the general population with near complete follow-up regarding diabetes drug use, but the comparator group likely has a lower risk of developing type 2 diabetes due to the low prevalence of morbid obesity. Another weakness is that we did not have baseline HbA1c data from the general population. It is likely that some individuals among the general population comparators had undetected diabetes or prediabetes, increasing the risk of becoming an incident diabetes drug user.
In conclusion this large nationwide study has demonstrated that RYGB results in durable remission of type 2 diabetes treatment over up to 7 years follow-up, especially in patients with a short duration of diabetes treatment. The incidence of new diabetes drug treatment in patients without diabetes at baseline is similar or lower than the level seen in the general population, even in patients with prediabetes.
ACKNOWLEDGEMENTS
Ethics
The register linkage was approved by the regional ethical committee of Stockholm. All analyses were conducted on de-identified data.
Funding
Research reported in this publication was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under award number R01DK105948. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funders were not involved in the design and conduct of the study; collection, management, analysis, or interpretation of the data; and preparation, review, or approval of the manuscript.
Disclaimer
The authors of this article are responsible for its contents. Olof Backman is guarantor of the content in this article.
Potential Conflicts of Interest
Dr. Ingmar Näslund reports personal fees from Baricil Bariatrics AB, Sweden, outside the submitted work.
Dr. Neovius reports grants from NIH, during the conduct of the study. The funders were not involved in the design and conduct of the study or approval of the manuscript.
All other authors declare no conflict of interest.
Footnotes
Insulin (A10A), oral diabetes drugs (A10B)
Insulin (A10A), oral diabetes drugs (A10B)
Insulin (A10A), oral diabetes drugs (A10B)
REFERENCES
- 1.Collaboration NCDRF, Di Cesare M, Bentham J et al. Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet 2016; 387: 1377–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flegal KM, Kruszon-Moran D, Carroll MD et al. Trends in Obesity Among Adults in the United States, 2005 to 2014. JAMA 2016; 315: 2284–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collaboration NCDRF. Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet 2016; 387: 1513–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adams TD, Davidson LE, Litwin SE et al. Health benefits of gastric bypass surgery after 6 years. JAMA 2012; 308: 1122–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sjostrom L, Lindroos AK, Peltonen M et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 2004; 351: 2683–2693. [DOI] [PubMed] [Google Scholar]
- 6.Puzziferri N, Roshek TB 3rd, Mayo HG et al. Long-term follow-up after bariatric surgery: a systematic review. JAMA 2014; 312: 934–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dicker D, Yahalom R, Comaneshter DS, Vinker S. Long-Term Outcomes of Three Types of Bariatric Surgery on Obesity and Type 2 Diabetes Control and Remission. Obes Surg 2016; 26: 1814–1820. [DOI] [PubMed] [Google Scholar]
- 8.Mingrone G, Panunzi S, De Gaetano A et al. Bariatric–metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. The Lancet 2015; 386: 964–973. [DOI] [PubMed] [Google Scholar]
- 9.Courcoulas AP, Belle SH, Neiberg RH et al. Three-Year Outcomes of Bariatric Surgery vs Lifestyle Intervention for Type 2 Diabetes Mellitus Treatment. JAMA Surgery 2015; 150: 931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keating C, Neovius M, Sjoholm K et al. Health-care costs over 15 years after bariatric surgery for patients with different baseline glucose status: results from the Swedish Obese Subjects study. Lancet Diabetes Endocrinol 2015; 3: 855–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neovius M, Narbro K, Keating C et al. Health care use during 20 years following bariatric surgery. JAMA 2012; 308: 1132–1141. [DOI] [PubMed] [Google Scholar]
- 12.Cummings DE, Arterburn DE, Westbrook EO et al. Gastric bypass surgery vs intensive lifestyle and medical intervention for type 2 diabetes: the CROSSROADS randomised controlled trial. Diabetologia 2016; 59: 945–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schauer PR, Bhatt DL, Kirwan JP et al. Bariatric surgery versus intensive medical therapy for diabetes--3-year outcomes. N Engl J Med 2014; 370: 2002–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gloy VL, Briel M, Bhatt DL et al. Bariatric surgery versus non-surgical treatment for obesity: a systematic review and meta-analysis of randomised controlled trials. BMJ 2013; 347: f5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sjostrom L, Peltonen M, Jacobson P et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA 2014; 311: 2297–2304. [DOI] [PubMed] [Google Scholar]
- 16.Carlsson LM, Peltonen M, Ahlin S et al. Bariatric surgery and prevention of type 2 diabetes in Swedish obese subjects. N Engl J Med 2012; 367: 695–704. [DOI] [PubMed] [Google Scholar]
- 17.Booth H, Khan O, Prevost T et al. Incidence of type 2 diabetes after bariatric surgery: population-based matched cohort study. The Lancet Diabetes & Endocrinology 2014; 2: 963–968. [DOI] [PubMed] [Google Scholar]
- 18.Nor Hanipah Z, Punchai S, Brethauer SA et al. Development of De Novo Diabetes in Long-Term Follow-up After Bariatric Surgery. Obes Surg 2018. March 9. doi: 10.1007/s11695-018-3194-z. [DOI] [PubMed]
- 19.Sjostrom L Review of the key results from the Swedish Obese Subjects (SOS) trial - a prospective controlled intervention study of bariatric surgery. J Intern Med 2013; 273: 219–234. [DOI] [PubMed] [Google Scholar]
- 20.Carlsson LM, Sjoholm K, Karlsson C et al. Long-term incidence of microvascular disease after bariatric surgery or usual care in patients with obesity, stratified by baseline glycaemic status: a post-hoc analysis of participants from the Swedish Obese Subjects study. Lancet Diabetes Endocrinol 2017; 5: 271–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hedenbro JL, Naslund E, Boman L et al. Formation of the Scandinavian Obesity Surgery Registry, SOReg. Obes Surg 2015; 25: 1893–1900. [DOI] [PubMed] [Google Scholar]
- 22.Ludvigsson JF, Almqvist C, Bonamy AK et al. Registers of the Swedish total population and their use in medical research. Eur J Epidemiol 2016; 31: 125–136. [DOI] [PubMed] [Google Scholar]
- 23.American Diabetes A 2. Classification and Diagnosis of Diabetes. Diabetes Care 2017; 40: S11–S24. [DOI] [PubMed] [Google Scholar]
- 24.Sjöström L, Lindroos AK, Peltonen M et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 2004; 351: 2683–2693. [DOI] [PubMed] [Google Scholar]
- 25.Courcoulas AP, Christian NJ, Belle SH et al. Weight change and health outcomes at 3 years after bariatric surgery among individuals with severe obesity. JAMA 2013; 310: 2416–2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Booth H, Khan O, Prevost T et al. Incidence of type 2 diabetes after bariatric surgery: population-based matched cohort study. Lancet Diabetes Endocrinol 2014; 2: 963–968. [DOI] [PubMed] [Google Scholar]
- 27.Panunzi S, Carlsson L, De Gaetano A et al. Determinants of Diabetes Remission and Glycemic Control After Bariatric Surgery. Diabetes Care 2016; 39: 166–174. [DOI] [PubMed] [Google Scholar]


