Abstract
Sodium-bicarbonate cotransport in retinal glial cells was studied in the everted eyecup preparation of the rat. Intracellular pH was monitored with the indicator dye BCPCF and fluorescence confocal microscopy. Raising the K+ concentration from 3 to 12 mM in HCO3−-buffered perfusate evoked an intracellular alkalinization in both astrocytes and Müller cells. The alkalinization developed more rapidly and was larger in astrocytes. The K+-induced alkalinization was HCO3−-dependent; it was reduced by 33% in astrocytes and 71% in Müller cells when HCO3− was removed from the perfusate. The alkalinization was effectively blocked by addition of 0.5 mM 4,4″-diisothiocyanato-stilbene-2,2′-disulfonic acid (DIDS). Removal of Na+ from the perfusate evoked a rapid acidification in both types of glial cells. The results indicate that astrocytes and Müller cells in situ in the rat retina possess an electrogenic Na+/HCO3− cotransporter.
Keywords: glial cells, retina, pH, imaging, BCPCF, pH regulation
INTRODUCTION
Regulation of pH within the nervous system is essential for maintaining normal neuronal function. Ion channels (Barnes et al., 1993; Harsany and Mangel, 1993; Tombaugh and Somjen, 1996), neurotransmitter receptors (Traynelis and Cull-Candy, 1990; Tang et al., 1990; Vyklicky et al., 1990), and gap junctions (Spray and Bennett, 1985; Kettenmann et al., 1990) are all, to varying degrees, modulated by extracellular or intracellular pH, and variations in extracellular pH can lead to substantial changes in neuronal behavior (Balestrino and Somjen, 1988; Taira et al., 1993; Barnes et al., 1993; Gottfried and Chesler, 1994).
Neuronal activity causes characteristic changes in extracellular pH (pHo) (Chesler and Kaila, 1992). In many brain regions, activity generates a transient alkalinization (reflecting synaptic activity), followed by a slower acidification (Chen and Chesler, 1992; Tong and Chesler, 1998). In the retina, alkaline shifts in pHo predominate (Borgula et al,. 1989; Yamamoto et al., 1992). Glial cell activity also contributes to variations in pHo (Grichtchenko and Chesler, 1994a; Newman, 1996) and may serve to counterbalance the pH changes generated by neurons (Ransom, 1992). This pH regulatory flux is mediated by the electrogenic Na+/HCO3− cotransport system of glial cells.
The Na+/HCO3− cotransporter has been described in many glial cells, including astrocytes in brain slices (Grichtchenko and Chesler, 1994a; Grichtchenko and Chesler, 1994b), in the optic nerve (Astion and Orkand, 1988), and in culture (Boyarsky et al., 1993; O’Connor et al., 1994; Brune et al., 1994; Brookes and Turner, 1994), oligodendrocytes in culture (Kettenmann and Schlue, 1988; Boussouf et al., 1997), and in leech glial cells (Deitmer and Schlue, 1989). In the retina, the Na+/HCO3− cotransporter has been studied in dissociated amphibian (Newman, 1991; Newman, 1996) and elasmobranch (Newman, 1990) Müller cells.
The goal of the current study was two-fold. First, to confirm that the Na+/HCO3− cotransporter is present in glial cells in intact, acutely isolated tissue; second, to determine whether the cotransporter is present in mammalian retinal glial cells. Glial cells were studied in the intact rat retina, rather than in dissociated cell or culture preparations, as employed in earlier studies. The results indicate that both astrocytes and Müller cells of the intact mammalian retina possess a Na+/HCO3− cotransport system.
MATERIALS AND METHODS
Everted Eyecup Preparation
Male Long-Evans rats (300–400 gms) were killed with an overdose of sodium pentobarbital (200 mg/kg) injected intraperitoneally and the eyes removed. A small portion of the eye, cut from the back of the eyeball, was everted over a Plexiglas dome by lowering a sheet of Plexiglas with a hole (centered over the dome) cut into it. The Plexiglas sheet served to hold the eye in place, prevented the retina from detaching, and formed a seal preventing perfusate from leaking under the tissue.
The eyecup was incubated for 12 min at room temperature in collagenase/dispase (2 mg/ml) and DNase (0.1 mg/ml) in bicarbonate-buffered Ringer’s solution to digest the basal lamina at the inner surface of the retina and the vitreous humor, which were then removed by suction applied through a 28 gauge hypodermic needle. Following thorough rinsing in Ringer’s solution, the eyecup was incubated for 12 min in the pH indicator dye BCPCF-AM (50 µg/ml; Molecular Probes, Eugene, OR) and pluronic acid (1.75 mg/ml; Molecular Probes). Both astrocytes and Müller cells, but not retinal neurons, were well labeled with BCPCF. A similar labeling pattern had been observed previously with another membrane permeant dye (Newman and Zahns, 1998). In preliminary experiments, the isolated retina of the rat was used instead of the everted eyecup. Similar results were obtained using both preparations.
During experiments, the eyecup was perfused with oxygenated Ringer’s solution at 24°C. The preparation was viewed with a video rate confocal scanner (Noran Odyssey; Middleton, WI) and upright microscope (Olympus BX60), with a 40× water immersion objective (0.8 NA).
Intracellular pH Measurements
Intracellular glial cell pH (pHi) was monitored with BCPCF, a derivative of BCECF modified to function as an emission ratio indicator dye (Liu et al., 1997). BCPCF was excited by the 488 nm argon laser line. Fluorescence emission was monitored at 500 nm (near the isosbestic emission wavelength) and at wavelengths longer than 515 nm. Images were acquired simultaneously at the two emission wavelengths. MetaMorph software (Universal Imaging, West Chester, PA) was used to capture and store images and to calculate ratio images.
Measurements of glial pHi in the intact retina proved more difficult to obtain than similar measurements made in freshly-isolated or cultured cells. Due to the inherent inefficiency of confocal imaging, the indicator dye bleached rapidly. Light exposure was kept to a minimum to prevent bleaching and cell damage, limiting the frequency of pHi measurements.
BCPCF Calibration
Intracellular pH measurements were calibrated using the nigericin-high K+ technique (Chaillet and Boron, 1985). Calibration curves were obtained by perfusing eyecups in a series of HEPES-buffered nigericin solutions (pH 6.0 to 8.0). The resulting BCPCF ratios were fit by the equation (Newman, 1994),
| (1) |
where I>515/I500 represents the fluorescence ratio at the two emission wavelengths, normalized to the ratio at pH 7.0.
Reliable calibration curves were obtained for astrocytes imaged at the retinal surface, but not for Müller cells, which were imaged in the inner plexiform layer. It is likely that the calibration solutions could not penetrate the retina rapidly enough to permit Müller cell pHi to equilibrate with perfusate pH. (The lipophilic nature of nigericin presumably limited its diffusion into the retina.) For purposes of this study, BCPCF calibration in Müller cells was assumed to be the same as that in astrocytes. In astrocytes, the BCPCF fluorescence-pHi relation is described by equation (1) with b = 1.28 ± 0.14 and pK = 7.22 ± 0.07 (19).
Ideally, indicator dye calibration curves should be normalized by obtaining a nigericin calibration at a single pHi value on each cell monitored during a study (Newman, 1994). It was not possible to obtain these single-point calibrations, however, as BCPCF bleaching during experiments was severe. Rather, single-point calibrations were run on a population of cells to obtain values for mean steady-state pHi. All other cells were then assumed to have a steady-state pHi equal to the mean value. Due to the near-linear BCPCF calibration relation close to steady-state pHi, small errors in steady-state pHi result in only minor errors in computed ΔpHi values.
Whole-Cell Recording
The membrane potential of astrocytes and Müller cells was monitored with whole-cell patch-clamp recording. Everted eyecups were prepared using the same procedure employed for pHi imaging except that BCPCF labeling was omitted and the preparation was incubated in collagenase/dispase and DNase for an additional 8 min following vitreous removal. Glial cells were identified by filling with Lucifer Yellow.
Solutions
Bicarbonate-buffered Ringer’s contained (in mM): NaCl, 117.0; KCl, 3.0; CaCl2, 2.0; MgSO4, 1.0; NaH2PO4, 0.5; dextrose, 15.0; NaHCO3, 26. It was equilibrated with 5% CO2 in O2 and had a pH of ~7.4 at 24°C. HEPES-buffered Ringer’s contained: NaCl, 135.0; KCl, 3.0; CaCl2, 2.0; MgSO4, 1.0; NaH2PO4, 0.5; dextrose, 15.0; HEPES, 10. It was adjusted to pH 7.4 with NaOH and equilibrated with 100% O2. In 12 mM K+ solutions, KCl was substituted for NaCl. In zero Na+ solutions, N-methyl-D-glucamine chloride was substituted for NaCl and choline bicarbonate for NaHCO3. The nigericin-high K+ calibration solution contained: N-methyl-d-glucamine, 18.5; KCl, 105.0; CaCl2, 2.0; MgSO4, 1.0; NaH2PO4, 0.5; dextrose, 15.0; HEPES, 30.0; nigericin, 20µM, and it was titrated with KOH. DIDS (Sigma, St. Louis, MO) was added to solutions immediately before use. The pipette solution for whole-cell recording contained: NaCl, 25; KCl, 112; CaCl2, 1; MgCl2, 7; Na2ATP, 5; EGTA, 5; HEPES, 1; Lucifer Yellow CH, 0.1%.
Statistics
Results are given as means ± S.D., with number of samples, n, in parentheses. The number of samples represents the number of experimental trials rather than the total number of cells. For each trial, measurements from 3 to 7 astrocytes and 75 to 200 Müller cells were averaged. Statistical significance was assessed using the Student’s t-test (unpaired samples).
RESULTS
Measurement of pHi in Retinal Glial Cells
Glial cells were identified by their morphology and by their location within the retina. Astrocytes were restricted largely to the nerve fiber layer at the vitreal surface of the retina and had multiple processes radiating from their somata, many of which contacted blood vessels (Fig. 1A). For pHi measurements, individual astrocyte somata were imaged. Müller cells were labeled throughout their length and could be followed from the vitreal surface to past their somata in the inner nuclear layer. For pHi measurements, Müller cell primary processes were imaged in the mid-inner plexiform layer, 20 to 30 µm beneath the retinal surface (Fig. 1C). Measurements were made from regions encompassing 75 to 200 Müller cell processes. In BCPCF calibration experiments, Müller cell processes surrounding neuronal somata in the ganglion cell layer were imaged (Fig. 1B). pH within Müller cells equilibrates rapidly and even when Na+/HCO3− cotransporters are localized to a specific cellular region, pHi is essentially uniform throughout the cell (Newman, 1996). pHi measurements were made simultaneously from astrocytes and Müller cells in experimental trials by switching the plane of focus alternately between the retinal surface and the inner plexiform layer.
Fig. 1.
Fluorescence confocal images of the everted rat eyecup labeled with the pH indicator dye BCPCF. A: Astrocytes (arrows) at the vitreal surface of the retina. Astrocyte processes terminating on blood vessels (arrowheads) are visible. B: Müller cell processes (arrows) surrounding the unlabeled somata of neurons in the ganglion cell layer. C: Müller cell processes (small, brightly labeled spots) in the inner plexiform layer. An astrocyte process surrounding a blood vessel (arrow) is also visible. Scale bar = 25 µm.
Steady-State Intracellular pH
Steady-state pHi was not stable in astrocytes and Müller cells, but rather drifted slowly in an acid direction over a number of hours (see Fig. 2). In general, the drift was more severe in Müller cells than it was in astrocytes. This drift contrasts with the stable steady-state pHi observed in dissociated Müller cells (Newman, 1996), and it may be due to the slow recovery of the retina following the trauma of enucleation and the cutting of the eyecup. Alternately, the drift may reflect the slow run-down of the preparation, perhaps due to lack of adequate perfusion.
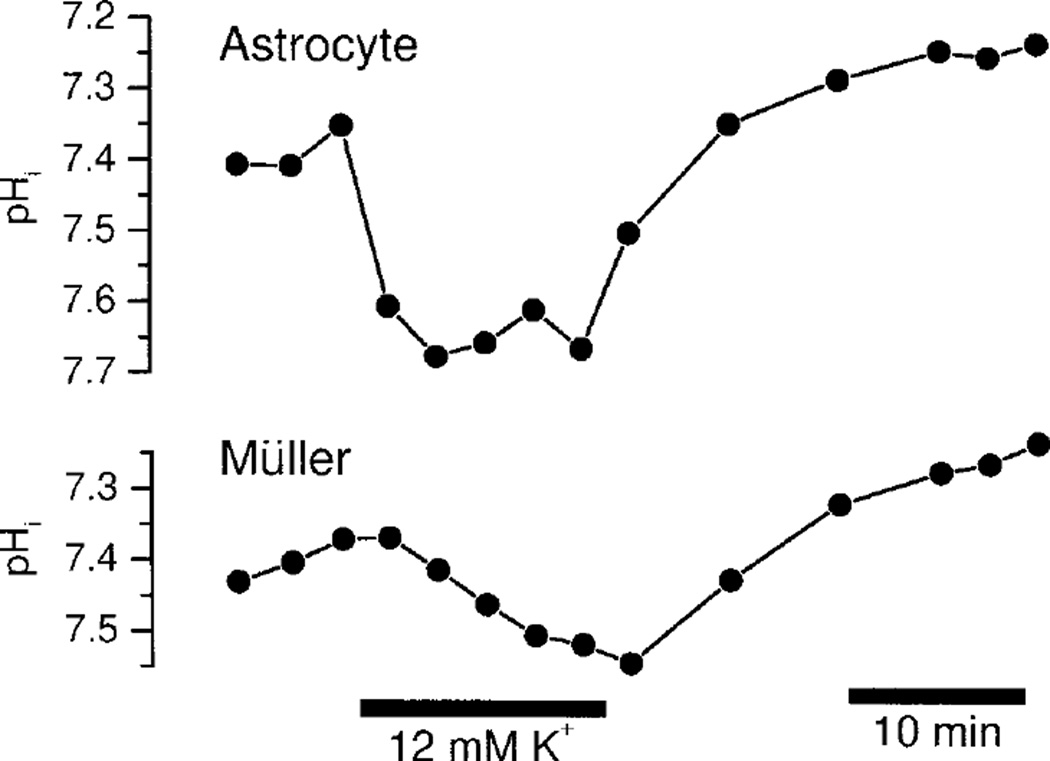
Fig. 2.
K+-induced alkalinization in retinal glial cells. Application of 12 mM K+ in HCO3− perfusate evokes a rapid alkalinization in astrocytes and a slower, smaller alkalinization in Müller cells. The records also show the slow acid drift of pHi present in many preparations.
Steady-state pHi was measured in retinas 1 to 1.5 h after perfusion of the preparation had commenced. pHi in astrocytes equaled 7.27 ± 0.13 (26). pHi in Müller cells, measured within the ganglion cell layer (6 to 8 µm beneath the surface) equaled 7.28 ± 0.06 (5). Müller cell pHi measured in the inner plexiform layer, equaled 7.51 ± 0.22 (5). The large variability in pHi measurements in the inner plexiform layer is most likely due to the difficulty of obtaining accurate nigericin calibrations deep within the retina. Müller cell pHi calibrations within the superficial ganglion cell layer were judged to be more accurate. For the purposes of calibration (see Methods), both astrocytes and Müller cells were assumed to have a steady-state pHi of 7.25. This value is somewhat higher than pHi measured in astrocytes in the brain (Chesler and Kraig, 1989; Grichtchenko and Chesler, 1994b).
K+-Induced Intracellular Alkalinization
An alkalinization was evoked within both astrocytes and Müller cells when the K+ concentration ([K+]) in the perfusate was raised from 3 to 12 mM (Fig. 2). Raising [K+] induced a rapid alkalinization in astrocytes, averaging 0.30 ± 0.07 (8) pH units, measured 12 min after switching to 12 mM K+ solution. The alkalinization was normally complete within 5 min after switching solutions.
In contrast, the alkalinization evoked in Müller cells was substantially slower and smaller. Raising [K+] to 12 mM induced an alkalinization averaging 0.17 ± 0.04 (8) pH units at 12 min. In every instance, the Müller cell alkalinization had not reached a steady-state at 12 min.
The difference in the time course of K+-induced alkalinization in astrocytes and Müller cells could be due to an intrinsic difference in acid-base transport within these cells. Alternately, it could arise because of the longer time it takes for the high K+ perfusate to reach the Müller cells within the retina.
Changes in cell membrane potential in response to raising perfusate [K+] were monitored in order to distinguish between these two possibilities. In both astrocytes and Müller cells, raising [K+] from 3 to 12 mM resulted in a rapid depolarization (Fig. 3). Although depolarization was somewhat slower in Müller cells, the depolarization was essentially complete within 1 min in both types of glial cells. Infiltration of the perfusate into the retina thus does not appear to limit the pHi response time of Müller cells. In mammalian Müller cells of species with vascularized retinas, including rat, most membrane K+ conductance is localized near the soma (Newman, 1987). Thus, monitoring K+-evoked depolarization serves as a good measure of K+ diffusion into the retina.
Fig. 3.
K+-induced depolarization in retinal glial cells. Application of 12 mM K+ evokes a rapid increase in cell membrane potential (Em) in both astrocytes and Müller cells. The two traces were recorded from the same preparation, but not simultaneously.
Bicarbonate Dependence of Intracellular Alkalinization
The K+-induced alkalinization in retinal glial cells could be generated by a depolarization-evoked activation of the Na+/HCO3− cotransport system, as it is in other glial cell preparations. This possibility was tested by determining the HCO3−-dependence and the stilbene sensitivity of the alkalinization.
The K+-induced alkalinization was reduced, but not eliminated, when HCO3− was removed from the perfusate (Fig. 4). In astrocytes, the alkalinization was reduced from 0.30 ± 0.07 (8) pH units in HCO3−-buffered perfusate to 0.20 ± 0.05 (5) pH units in HEPES-buffered perfusate (P < 0.01). Similarly, in Müller cells, the alkalinization was reduced from 0.17 ± 0.04 (8) to 0.05 ± 0.03 (5) pH units (P < 0.001). The K+-induced alkalinization in HEPES was determined 1 to 1.5 h after switching from HCO3− perfusate.
Fig. 4.
Bicarbonate-dependence of K+-induced alkalinization. pHi records on the left were obtained in HEPES-buffered perfusate. The records on the right were obtained in the same preparation (but different cells) following substitution of HCO3− -buffered perfusate. In both astrocytes and Müller cells, application of 12 mM K+ induces a larger alkalinization in the presence of HCO3−.
Substituting HEPES- for HCO3− -buffered perfusate produced a rapid glial cell alkalinization, due to the reduction in pCO2 in the perfusate, followed by a prolonged acidification. A steady-state pHi was not reached for more than an hour after switching solutions, suggesting that it takes at least this long for the HCO3− in the retina to be washed out. The change in steady-state pHi produced by switching from HCO3−- to HEPES-buffered perfusate could not be determined accurately due to the slow drift in pHi in the preparation, although a net acidification beyond 7.25 was observed in all trials.
Effect of DIDS on Intracellular pH
If the K+-induced alkalinization is generated by the Na+/HCO3− cotransport system, it should be blocked by the stilbene 4,4′-diisothiocyanato-stilbene-2,2′-disulfonic acid (DIDS). This proved to be the case (Fig. 5).
Fig. 5.
DIDS sensitivity of K+-induced alkalinization in HCO3− perfusate. In astrocytes, the K+-induced alkalinization is blocked by addition of 0.5 mM DIDS. In Müller cells, the alkalinization is transformed into an acidification by DIDS. Addition of DIDS also produces an acid shift in steady-state pHi in both astrocytes and Müller cells.
In astrocytes, addition of 0.5 mM DIDS to HCO3− perfusate reduced the K+-induced alkalinization from 0.21 ± 0.05 (17) to 0.02 ± 0.04 (12) pH units (P < 0.001; measured at 12 min after addition of the high K+ perfusate). In Müller cells, the pHi response was actually transformed from a 0.09 ± 0.02 (13) pH unit alkalinization to a 0.07 ± 0.03 (8) pH unit acidification (P < 0.001). The K+-induced acidification in the presence of DIDS was consistently observed.
Addition of 0.5 mM DIDS to the HCO3− perfusate also produced a rapid change in steady-state pHi. In astrocytes, DIDS reduced steady-state pHi 0.08 pH units to 7.17 ± 0.05 (17) (P < 0.01). In Müller cells, steady-state pHi was reduced 0.11 pH units, to 7.14 ± 0.04 (13) (P < 0.001).
Effect of Na+-Free Perfusate on Intracellular pH
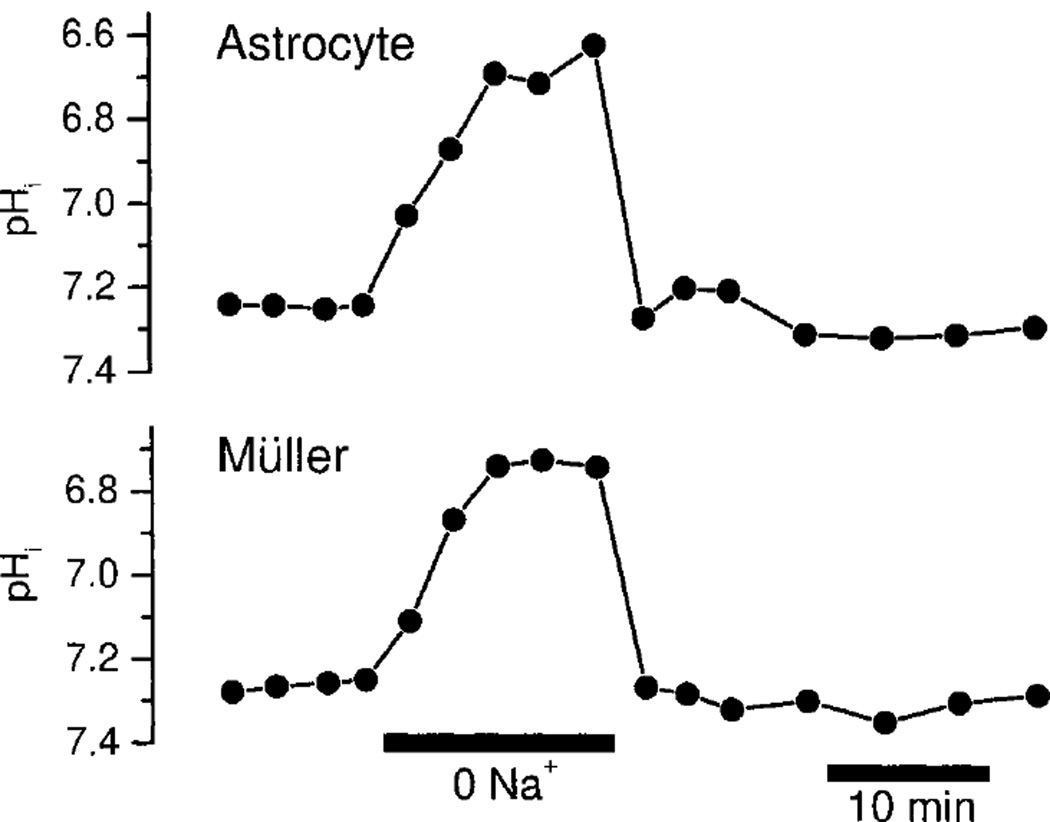
Na+/HCO3− cotransport activity should also be Na+-dependent. Indeed, removal of Na+ from the HCO3− perfusate generated a rapid acidification in both astrocytes and Müller cells (Fig. 6). The acidifications were large, averaging 0.47 ± 0.07 (3) pH units in astrocytes and 0.48 ± 0.06 (3) pH units in Müller cells, measured 12 min after removal of Na+. The acidifications are consistent with the presence of the Na+/HCO3− cotransporter, but may also be generated by the action of a Na+/H+ exchange system. Due to the rapid acidification produced by Na+ removal, it was not practical to measure K+-induced alkalinization in Na+-free perfusate.
Fig. 6.
Sodium dependence of glial cell pHi. Substitution of nominally Na+-free perfusate for control HCO3− perfusate evokes a large acidification in both astrocytes and Müller cells.
DISCUSSION
Na+/HCO3− Cotransport
The results presented in this article indicate the presence of a Na+/HCO3− cotransport system in both astrocytes and Müller cells in the intact rat retina. Raising extracellular [K+], which in turn depolarizes cells, induced an intracellular alkalinization in both types of glial cells. This alkalinization is generated, at least in part, by the Na+/HCO3− cotransporter, as indicated by its HCO3−-dependence and DIDS sensitivity.
The K+-induced alkalinization was only partially HCO3−-dependent. The HCO3−-independent component of the alkalinization could be generated by other acid/base transport systems or pumps (Pappas and Ransom, 1993). However, in other glial cell preparations, including salamander Müller cells (Newman, 1996), this component of the alkalinization is not generated by transmembrane acid/base flux and is most likely due to K+-induced changes in the metabolic state of the cell (Orkand et al., 1973; Salem et al., 1975).
Even in the absence of HCO3− in the perfusate, it is likely that millimolar concentrations of HCO3− remain within the tissue. These levels are generated by the aerobic production of CO2, which is converted to HCO3−. Thus, a component of the K+-induced alkalinization observed in HEPES-buffered perfusate could be generated by HCO3−-dependent processes. It should also be noted that the total buffering capacity of glial cells is considerably reduced when HEPES-buffered perfusate is substituted for HCO3−-buffered perfusate (Chesler, 1990). Thus, observed alkalinizations in HEPES-buffered perfusate are generated by smaller acid/base fluxes than they are in HCO3−-buffered perfusate.
The K+-induced alkalinization was effectively reduced by DIDS, a stilbene which blocks Na+/HCO3− cotransport in some glial systems (Newman, 1991; O’Connor et al., 1994; Shrode and Putnam, 1994; Brune et al., 1994). Interestingly, the stilbenes DIDS and 4,4′-dinitrostilbene-2,2′-disulphonic acid (DNDS) are ineffective in blocking the cotransporter in gliotic brain astrocytes (Grinchtchenko and Chesler, 1994a; Grichtchenko and Chesler, 1994b) and in cultured oligodendrocytes (Kettenmann and Schlue, 1988). In Müller cells, addition of DIDS unmasked a K+-induced acidification. The nature of this acidification is not known. It is not present in astrocytes of the rat retina, nor in dissociated salamander Müller cells (Newman, 1996).
The K+-induced alkalinization was more rapid in time course and greater in magnitude in astrocytes than it was in Müller cells. This may indicate that cotransporter properties differ in the two types of glial cells. Alternately, these differences may reflect differences in transporter density or cell surface-to-volume ratios. The slow rise time of the alkalinization in Müller cells may also be due to the opposing K+-induced acidification present in these cells.
The results suggest that, during steady-state conditions, the Na+/HCO3− cotransporter generates a HCO3− influx into astrocytes and Müller cells in the rat retina. When cotransporter activity is reduced by HEPES substitution, the cells acidify, indicating an interruption of HCO3− influx. A similar acidification occurs when cotransporter activity is blocked by DIDS. It should be noted that the results are also consistent with HCO3− influx being generated by HCO3−-dependent and DIDS-sensitive transport processes other than the Na+/HCO3− cotransporter. An influx of HCO3− through the cotransporter during steady-state conditions has been suggested in previous experiments (O’Connor et al., 1994; Newman, 1996).
Function of Na+/HCO3− Cotransport
Retinal activation by light stimulation results in an extracellular alkalinization generated by increased synaptic activity, as well as by a reduction in the metabolic activity of photoreceptors (Borgula et al., 1989; Yamamoto et al., 1992). This change in pHo can have profound effects on subsequent neuronal activity. Voltage-gated ion channels (Barnes et al., 1993; Harsanyi and Mangel, 1993; Tombaugh and Somjen, 1996), as well as neurotransmitter receptors (Traynelis and Cull-Candy, 1990; Tang et al., 1990; Vyklicky et al., 1990), are sensitive to pHo, and small variations in pHo can lead to modulation of synaptic efficacy. In the salamander retina, for instance, an alkalinization of 0.05 pH units results in a 24% increase in synaptic transmission between photoreceptors and bipolar cells (Barnes et al., 1993).
The Na+/HCO3− cotransporter of rat astrocytes and Müller cells may function to counter this alkalinization, thus helping to regulate retinal pHo (Ransom, 1992). In several glial cell preparations, including dissociated salamander Müller cells (Newman, 1996) and hippocampal slices (Grichtchenko and Chesler, 1994a), activation of the cotransport system results in acid efflux and an acidification of extracellular space. The acidification may counterbalance the alkalinization generated directly by neuronal activity. This mechanism of glial regulation of pHo remains to be confirmed experimentally.
ACKNOWLEDGMENTS
The author thanks Paul Ceelen for his excellent technical assistance and Janice I. Gepner and Kathleen R. Zahs for their helpful comments on the manuscript.
Grant sponsor: National Institutes of Health; Grant number: EY04077.
REFERENCES
- Astion ML, Orkand RK. Electrogenic Na+/HCO3− cotransport in neuroglia. Glia. 1988;1:355–357. doi: 10.1002/glia.440010508. [DOI] [PubMed] [Google Scholar]
- Balestrino M, Somjen GG. Concentration of carbon dioxide, interstitial pH and synaptic transmission in hippocampal formation of the rat. J Physiol. 1988;396:247–266. doi: 10.1113/jphysiol.1988.sp016961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes S, Merchant V, Mahmud F. Modulation of transmission gain by protons at the photoreceptor output synapse. Proc Natl Acad Sci USA. 1993;90:10081–10085. doi: 10.1073/pnas.90.21.10081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgula GA, Karwoski CJ, Steinberg RH. Light-evoked changes in extracellular pH in frog retina. Vision Res. 1989;29:1069–1077. doi: 10.1016/0042-6989(89)90054-0. [DOI] [PubMed] [Google Scholar]
- Boussouf A, Lambert RC, Gaillard S. Voltage-dependent Na+-HCO3− cotransporter and Na+/H+ exchanger are involved in intracellular pH regulation of cultured mature rat cerebellar oligodendrocytes. Glia. 1997;19:74–84. doi: 10.1002/(sici)1098-1136(199701)19:1<74::aid-glia8>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Boyarsky G, Ransom B, Schlue W-R, Davis MBE, Boron WF. Intracellular pH regulation in single cultured astrocytes from rat forebrain. Glia. 1993;8:241–248. doi: 10.1002/glia.440080404. [DOI] [PubMed] [Google Scholar]
- Brookes N, Turner RJ. K+-induced alkalinization in mouse cerebral astrocytes mediated by reversal of electrogenic Na+-HCO3− cotransport. Am J Physiol. 1994;267:C1633–C1640. doi: 10.1152/ajpcell.1994.267.6.C1633. [DOI] [PubMed] [Google Scholar]
- Brune T, Fetzer S, Backus KH, Deitmer JW. Evidence for electrogenic sodium-bicarbonate cotransport in cultured rat cerebellar astrocytes. Pflugers Arch. 1994;429:64–71. doi: 10.1007/BF02584031. [DOI] [PubMed] [Google Scholar]
- Chaillet JR, Boron WF. Intracellular calibration of a pH-sensitive dye in salamander proximal tubules. J Gen Physiol. 1985;86:765–794. doi: 10.1085/jgp.86.6.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JCT, Chesler M. Modulation of extracellular pH by glutamate and GABA in rat hippocampal slices. J Neurophysiol. 1992;67:29–36. doi: 10.1152/jn.1992.67.1.29. [DOI] [PubMed] [Google Scholar]
- Chesler M. The regulation and modulation of pH in the nervous system. Prog Neurobiol. 1990;34:401–427. doi: 10.1016/0301-0082(90)90034-e. [DOI] [PubMed] [Google Scholar]
- Chesler M, Kaila K. Modulation of pH by neuronal activity. TINS. 1992;15:396–402. doi: 10.1016/0166-2236(92)90191-a. [DOI] [PubMed] [Google Scholar]
- Chesler M, Kraig RP. Intracellular pH transients of mammalian astrocytes. J Neurosci. 1989;9:2011–2019. doi: 10.1523/JNEUROSCI.09-06-02011.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitmer JW, Schlue WR. An inwardly directed electrogenic sodium-bicarbonate co-transport in leech glial cells. J Physiol. 1989;411:179–194. doi: 10.1113/jphysiol.1989.sp017567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfried JA, Chesler M. Endogenous H+ modulation of NMDA receptor-mediated EPSCs revealed by carbonic anhydrase inhibition in rat hippocampus. J Physiol. 1994;478.3:373–378. doi: 10.1113/jphysiol.1994.sp020258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grichtchenko II, Chesler M. Depolarization-induced acid secretion in gliotic hippocampal slices. Neuroscience. 1994a;62:1057–1070. doi: 10.1016/0306-4522(94)90343-3. [DOI] [PubMed] [Google Scholar]
- Grichtchenko II, Chesler M. Depolarization-induced alkalinization of astrocytes in gliotic hippocampal slices. Neuroscience. 1994b;62:1071–1078. doi: 10.1016/0306-4522(94)90344-1. [DOI] [PubMed] [Google Scholar]
- Harsanyi K, Mangel SC. Modulation of cone to horizontal cell transmission by calcium and pH in the fish retina. Visual Neurosci. 1993;10:81–91. doi: 10.1017/s0952523800003242. [DOI] [PubMed] [Google Scholar]
- Kettenmann H, Schlue WR. Intracellular pH regulation in cultured mouse oligodendrocytes. J Physiol. 1988;406:147–162. doi: 10.1113/jphysiol.1988.sp017373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettenmann H, Ransom BR, Schlue WR. Intracellular pH shifts capable of uncoupling cultured oligodendrocytes are seen only in low HCO3− solution. Glia. 1990;3:110–117. doi: 10.1002/glia.440030204. [DOI] [PubMed] [Google Scholar]
- Liu J, Diwu Z, Klaubert DH. Fluorescent molecular probes III. 2′,7′-bis-(3-carboxypropyl)-5-(and 6)-carboxyfluorescein (BCPCF): a new polar dual-excitation and dual emission pH indicator with a PKA of 7.0. Bioorg Med Chem. 1997;7:3069–3072. [Google Scholar]
- Newman EA. Distribution of potassium conductance in mammalian Müller (glial) cells: a comparative study. J Neurosci. 1987;7:2423–2432. [PMC free article] [PubMed] [Google Scholar]
- Newman EA. Electrogenic sodium-bicarbonate cotransport in retinal Müller cells of the spiny dogfish (Squalus acanthias) Bulletin (Mt.Desert Island Biol Lab) 1990;29:102–103. [Google Scholar]
- Newman EA. Sodium-bicarbonate cotransport in retinal Müller (glial) cells of the salamander. J Neurosci. 1991;11:3972–3983. doi: 10.1523/JNEUROSCI.11-12-03972.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman EA. A physiological measure of carbonic anhydrase in Müller cells. Glia. 1994;11:291–299. doi: 10.1002/glia.440110402. [DOI] [PubMed] [Google Scholar]
- Newman EA. Acid efflux from retinal glial cells generated by sodium-bicarbonate cotransport. J Neurosci. 1996;16:159–168. doi: 10.1523/JNEUROSCI.16-01-00159.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman EA, Zahs KR. Modulation of neuronal activity by glial cells in the retina. J Neurosci. 1998;18:4022–4028. doi: 10.1523/JNEUROSCI.18-11-04022.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor ER, Sontheimer H, Ransom BR. Rat hippocampal astrocytes exhibit electrogenic sodium-bicarbonate cotransport. J Neurophysiol. 1994;72:2580–2589. doi: 10.1152/jn.1994.72.6.2580. [DOI] [PubMed] [Google Scholar]
- Orkand PM, Bracho H, Orkand RK. Glial metabolism: alteration by potassium levels comparable to those during neural activity. Brain Res. 1973;55:467–471. doi: 10.1016/0006-8993(73)90315-6. [DOI] [PubMed] [Google Scholar]
- Pappas CA, Ransom BR. A depolarization-stimulated, bafilomycin-inhibitable H+-pump in hippocampal astrocytes. Glia. 1993;9:280–291. doi: 10.1002/glia.440090406. [DOI] [PubMed] [Google Scholar]
- Ransom BR. Glial modulation of neural excitability mediated by extracellular pH: a hypothesis. Prog Brain Res. 1992;94:37–46. doi: 10.1016/s0079-6123(08)61737-9. [DOI] [PubMed] [Google Scholar]
- Salem RD, Hammerschlag R, Bracho H, Orkand RK. Influence of potassium ions on accumulation and metabolism of [14C]glucose by glial cells. Brain Res. 1975;86:499–503. doi: 10.1016/0006-8993(75)90903-8. [DOI] [PubMed] [Google Scholar]
- Shrode LD, Putnam RW. Intracellular pH regulation in primary rat astrocytes and C6 glioma cells. Glia. 1994;12:196–210. doi: 10.1002/glia.440120305. [DOI] [PubMed] [Google Scholar]
- Spray DC, Bennett MVL. Physiology and pharmacology of gap junctions. Annu Rev Physiol. 1985;47:281–303. doi: 10.1146/annurev.ph.47.030185.001433. [DOI] [PubMed] [Google Scholar]
- Taira T, Smirnov S, Voipio J, Kaila K. Intrinsic proton modulation of excitatory transmission in rat hippocampal slices. Neuroreport. 1993;4:93–96. doi: 10.1097/00001756-199301000-00024. [DOI] [PubMed] [Google Scholar]
- Tang C-M, Dichter M, Morad M. Modulation of the N-methyl-D-aspartate channel by extracellular H+ Proc Natl Acad Sci USA. 1990;87:6445–6449. doi: 10.1073/pnas.87.16.6445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombaugh GC, Somjen GG. Effects of extracellular pH on voltage-gated Na+, K+, and Ca2+ currents in isolated rat CA1 neurons. J Physiol. 1996;493:719–732. doi: 10.1113/jphysiol.1996.sp021417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong C-K, Chesler M. Activity-evoked extracellular pH shifts in slices of rat dorsal lateral geniculate nucleus. Brain Res. 1998;815:373–381. doi: 10.1016/s0006-8993(98)01059-2. [DOI] [PubMed] [Google Scholar]
- Traynelis SF, Cull-Candy SG. Proton inhibition of N-methyl-D-aspartate receptors in cerebellar neurons. Nature. 1990;345:347–350. doi: 10.1038/345347a0. [DOI] [PubMed] [Google Scholar]
- Vyklicky L, Vlachova V, Krusek J. The effect of external pH changes on responses to excitatory amino acids in mouse. J Physiol. 1990;430:497–517. doi: 10.1113/jphysiol.1990.sp018304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto F, Borgula GA, Steinberg RH. Effects of light and darkness on pH outside rod photoreceptors in the cat retina. Exp Eye Res. 1992;54:685–697. doi: 10.1016/0014-4835(92)90023-l. [DOI] [PubMed] [Google Scholar]