Supplemental Digital Content is available in the text.
Keywords: female, intracranial aneurysm, lifestyle, subarachnoid hemorrhage
Background and Purpose:
The prevalence of unruptured intracranial aneurysms (UIAs) in the adult population is ≈3%. Rupture of an intracranial aneurysm can have devastating consequences, which emphasizes the importance of identification of potentially modifiable determinants for the presence and size of UIAs. Our aim was to study the association of a broad spectrum of potential determinants with the presence and size of UIAs in a general adult population.
Methods:
Between 2005 and 2015, 5841 participants from the population-based Rotterdam Study (mean age, 64.4 years, 45.0% male) underwent brain magnetic resonance imaging (1.5T). These scans were evaluated for the presence of incidental UIAs. We determined number and volume of the UIAs. Using logistic and linear regression models, we assessed the association of cardiovascular, lifestyle and emerging inflammatory and hormonal determinants with the presence and volume of UIAs.
Results:
In 134 (2.3%) participants, ≥1 UIAs were detected (149 UIAs in total), with a median volume of 61.1 mm3 (interquartile range, 33.2–134.0). In multivariable models, female sex (odds ratio, 1.92 [95% CI, 1.33–2.84]), hypertension (odds ratio, 1.73 [95% CI, 1.13–2.68]), and current smoking (odds ratio, 3.75 [95% CI, 2.27–6.33]) were associated with the presence of UIAs. We found no association of alcohol use, physical activity, or diet quality with UIA presence. Finally, we found white blood cell count to relate to larger aneurysm volume (difference in volume of 33.6 mm3 per 109/L increase in white blood cell [95% CI, 3.92–63.5]).
Conclusions:
In this population-based study, female sex, hypertension, and smoking, but no other lifestyle determinants, were associated with the presence of UIAs. White blood cell count is associated with size of UIAs. Preventive strategies should focus on treating hypertension and promoting cessation of smoking.
Unruptured intracranial aneurysms (UIAs) occur in ≈3% of the adult population,1–3 of whom ≈20% to 30% harbor multiple UIAs.4 Given the potentially devastating consequences of rupture of such aneurysms, development of preventive strategies for intracranial aneurysm formation is key. Yet, to develop such preventive strategies, detailed knowledge on cause is required. Over the last decades, several studies on risk factors for UIAs have been performed. However, these studies were generally performed in small, highly selected populations, including populations of patients with previous aneurysmal subarachnoid hemorrhage5–7 or patients with a genetically increased risk (eg, the Finnish population).5,8,9
Unfortunately, data on the cause of UIAs in unselected, community-dwelling individuals remain scarce. Therefore, it is important to assess the role of previously established major risk factors (eg, female sex, hypertension, and smoking6–8) in the general population, while also shedding light on additional potentially modifiable risk factors, including lifestyle and inflammation. Evidence shows that adhering to a healthy lifestyle, including, for example, a healthy diet and regular physical activity, decreases the risk of other cardiovascular diseases (CVDs), including stroke.10,11 Nonetheless, little is known about an association between lifestyle, which consists of largely modifiable risk factors, and the presence of UIAs. Improved knowledge about the determinants is paramount and may provide important handholds for future prevention of intracranial aneurysms.
Besides preventing aneurysm formation, another way of preventing aneurysm rupture is by limiting its growth. Growth and size, which can be seen as a surrogate marker for aneurysms grown in the past, both have been shown to be associated with risk of aneurysm rupture.12 As the causes of aneurysmal growth are incompletely understood, it is important to first investigate novel potential determinants for increased aneurysm size.
Given the above, the aim of this study was to investigate the association of a broad range of determinants with presence and size of UIAs in the general adult population, with a focus on the role of lifestyle.
Methods
Settings and Study Population
This cross-sectional study was embedded in the Rotterdam Study, a population-based, prospective study cohort in over 14 000 adults aged 45 or over, located in Ommoord, a neighborhood in Rotterdam, the Netherlands.13 Ninety-six percent of the participants is of white descent. Participants were invited to undergo a range of extensive examinations at study entry and subsequently every 3 to 4 years at a dedicated study center. These examinations ranged from elaborate questionnaires to medical imaging, such as magnetic resonance imaging (MRI).
Since 2005, brain MRI examination has been incorporated in the study protocol and provided to all participants without contraindications as part of the Rotterdam Scan Study, which was embedded in the original Rotterdam Study. Between 2005 and 2015, 5841 unique persons have undergone MRI of the brain, which comprises the population for the current study. Assessment of UIAs was not incorporated in the original study aim but was part of a review of all scans for potentially relevant incidental findings.3
Requests to access the data set from qualified researchers trained in human subject confidentiality protocols may be sent to the Department of Epidemiology, Erasmus MC University Medical Center at f.vanrooij@erasmusmc.nl. The Rotterdam Study has been approved by the Medical Ethics Committee of the Erasmus MC (registration number MEC 02.1015) and by the Dutch Ministry of Health, Welfare and Sport (Population Screening Act WBO, license number 1071272-159521-PG). The Rotterdam Study has been entered into the Netherlands National Trial Register (www.trialregister.nl) and into the World Health Organization International Clinical Trials Registry Platform (www.who.int/ictrp/network/primary/en/) under shared catalogue number NTR6831.13 All participants provided written informed consent to participate in the study and to have their information obtained from treating physicians.
Assessment of UIAs
A study-dedicated 1.5T MRI unit with an 8-channel head coil (General Electric Healthcare, Milwaukee) was used for brain MRI.14 The MRI protocol, as described elsewhere,13 consisted of 4 high-resolution axial sequences: a 3-dimensional T1-weighted sequence; a 2-dimensional proton-density-weighted (PDw) sequence; a 2-dimensional fluid-attenuated inversion recovery sequence; and a 3-dimensional T2-weighted gradient-recalled echo sequence. There was no administration of contrast material.
Per protocol, every brain MRI scan was rated within 2 weeks of acquisition for the presence of incidental findings by trained research physicians3 and assessed for intracranial aneurysms on the PDw sequence (see example in the Figure). All potential intracranial aneurysms reported by research physicians were reassessed by experienced neuroradiologists and were categorized properly for location and size. According to our management of incidental findings protocol,3 all persons with an aneurysm in the posterior circulation or an aneurysm >7 mm in the anterior circulation were directly referred for further clinical evaluation. After subsequent clinical diagnostic imaging (ie, computed tomography angiography or magnetic resonance angiography), these participants were all proven to actually harbor a UIA, indicating a low false-positive rate. None of the participants had a history of subarachnoid hemorrhage at baseline.
Figure.
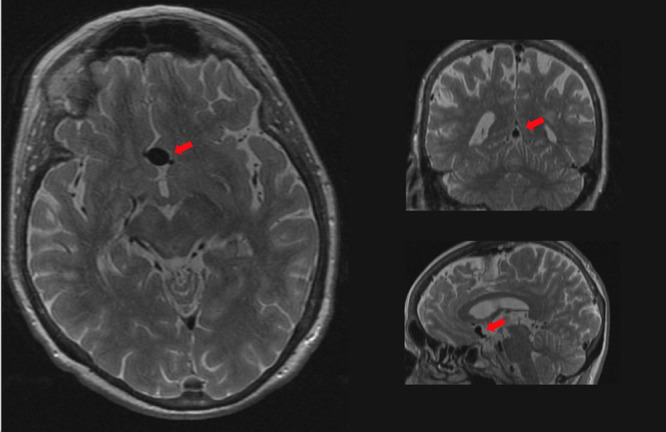
Unruptured intracranial aneurysm on brain MRI. Arrow indicates an aneurysm of the anterior communicating artery (diameter, 10 mm) on the proton-density-weighted image.
All intracranial aneurysms, regardless of referral, were manually segmented by a single rater on axial PDw slices using MeVisLab 2.6.1 (MeVis Medical Solutions AG). Manual segmentation was used for a 3-dimensional reconstruction, yielding maximum diameter and volume for each aneurysm. For 4 participants, it was not possible to estimate maximum diameter or volume because of motion artifacts on the PDw scan. A random subset (n=20) of scans was segmented separately by an independent rater to assess the interrater intraclass correlation coefficient of maximum diameter (intraclass correlation coefficient=0.71) and volume (intraclass correlation coefficient=0.87).
Assessment of Determinants
We divided the potential determinants into 3 categories: cardiovascular, lifestyle, and emerging determinants. Cardiovascular determinants were body mass index hypertension, smoking, hypercholesterolemia, diabetes mellitus, and history of CVD. Height (cm) and weight (kg) were measured under standardized conditions, and body mass index was calculated as weight/height2 (kg/m2). Blood pressure was measured twice using a random zero sphygmomanometer, and the average was taken. Hypertension was defined as a systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg, and/or use of blood pressure–lowering medication. Smoking status was acquired through home interviews and was categorized into never, former, and current smoking. Cholesterol and glucose levels were measured using fasting blood samples.15 Hypercholesterolemia was defined as total cholesterol concentration ≥6.2 mmol/L and/or use of lipid-lowering medication. Diabetes mellitus was defined as having fasting blood glucose concentrations >7.0 mmol/L and/or nonfasting blood glucose >11.1 mmol/L and/or use of glucose-lowering medication. CVD history was defined as having a history of stroke, myocardial infarction, or percutaneous coronary intervention.
Our assessment of lifestyle consisted of a diet adherence score, a physical activity score, and use of alcohol. Dietary intake was assessed with a validated self-administered food frequency questionnaire consisting of 389 items, based on which we evaluated the adherence (yes/no) of the participants to 14 items of the Dutch dietary guidelines. By adding the adhered items, a score between 0 and 14 was obtained, with a higher score reflecting better diet quality.16 Physical activity was assessed using the validated17,18 Longitudinal Ageing Study Amsterdam (LASA) physical activity questionnaire, which contains questions about frequency and duration of a range of physical activities (walking, cycling, gardening, sports, and housekeeping16). For each participant, these data were converted into metabolic equivalent of task-hours per week.19 Finally, alcohol consumption was measured through home interviews and was converted to a universal unit (g/day, where 10 g of alcohol equals 1 glass).
Assessment of emerging determinants consisted of inflammatory markers (CRP [C-reactive protein] and white blood cell [WBC] count) and a hormonal marker (estradiol). These biomarkers were all obtained through a fasting blood sample.15
Statistical Analysis
To assess the association of the determinants with the presence of UIAs, we used logistic regression models. In the first set of models (model 1), we investigated the determinants individually, while adjusting for sex and age. In the second set of models (model 2), we additionally adjusted for all cardiovascular determinants (body mass index, hypertension, smoking, hypercholesterolemia, diabetes mellitus, CVD history). As data on diet score, physical activity, alcohol intake, CRP, WBC count, and estradiol were only collected in specific subcohorts, we decided not to include these determinants in model 2 (see below). Associations of estradiol levels with UIAs were analyzed stratified by sex, given the fundamentally different distribution of estradiol levels between males and females.
To assess whether the potential determinants were associated with aneurysm volume, we used linear regression while adjusting for confounders using models 1 and 2. This analysis was limited to the 130 participants with aneurysms, in whom aneurysm volume could be calculated. We reported both models, as model 1 mainly serves a descriptive purpose, whereas model 2 primarily determines the independent relationship of the determinants with UIA presence and volume.
In the total cohort, we had missing values for body mass index (0.4%), hypertension (0.5%), systolic blood pressure and diastolic blood pressure (0.4%), blood pressure–lowering medication (0.6%), smoking status (0.6%), total cholesterol and non-HDL (high-density lipoprotein) cholesterol (1.7%), lipid-lowering medication (0.6%), fasting glucose (1.7%), diabetes mellitus (1.2%), alcohol intake (5.7%), and WBC count (0.01%). In the subgroup used for analysis of aneurysm volume, these values ranged from 0% to 3.7%. Missing values for these variables were imputed using the expectation-maximization algorithm based on the cardiovascular variables, WBC count, alcohol intake, and aneurysm presence. Some measurements were only performed in specific subcohorts of the Rotterdam Study, resulting in limited available data on such variables. Accordingly, diet score, physical activity, CRP, and estradiol were available in only 77.7%, 62.1%, 49.8%, and 49.6% of the participants, respectively. For these variables, we analyzed the data using complete-case analysis, while still adjusting for other variables in models 1 and 2. For data analysis, R version 3.4.1 was used (www.cran.r-project.org).
Results
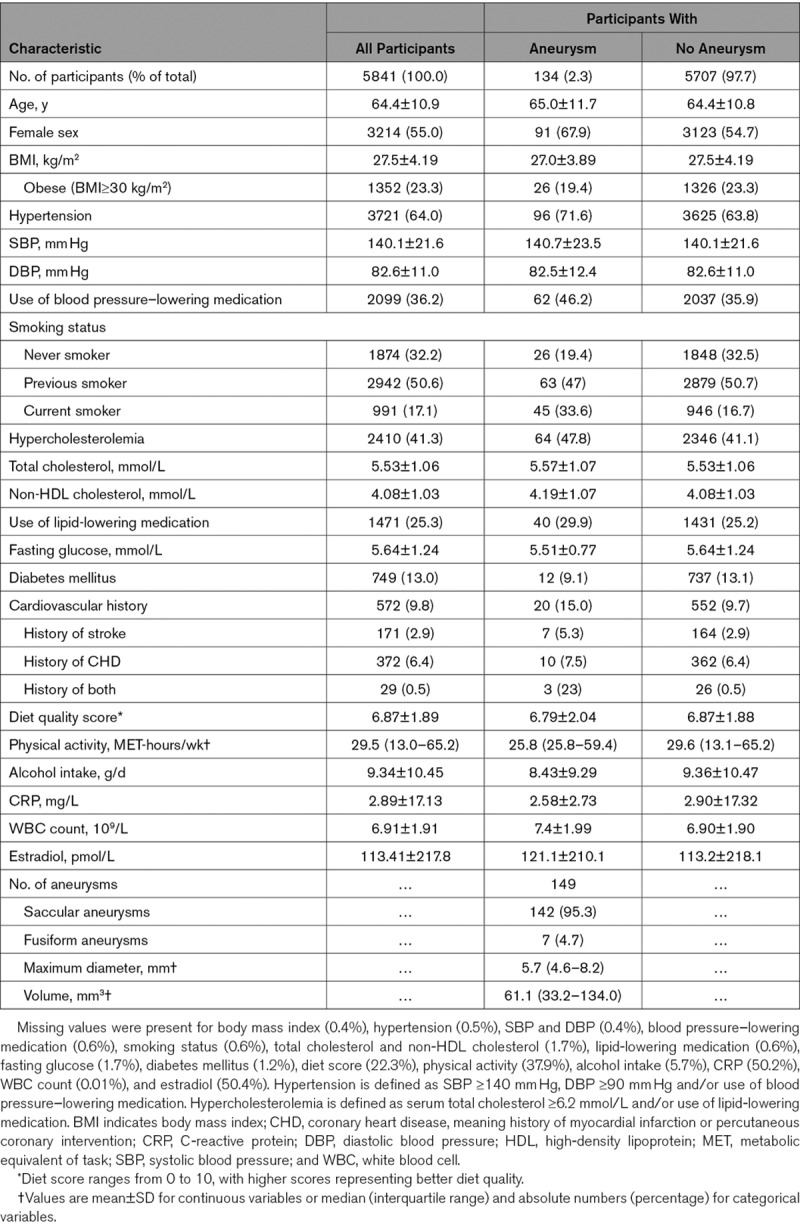
In nearly all cases, the MRI scan was acquired after the assessment of determinants, with a median time interval of 20 days (interquartile range, 12–56). The mean age at time of brain MRI was 64.4 years (SD=10.9 years), and 55.0% of participants were female (Table 1). The prevalence of UIAs was 2.3% (95% CI, 1.9–2.7), with a prevalence of 2.9% (95% CI, 2.3–3.4) in females and 1.7% (95% CI, 1.2–2.1) in males, respectively. Median maximum diameter of UIAs was 5.7 mm (interquartile range, 4.6–8.2) and median volume 61.1 mm3 (interquartile range, 33.2–134.0). Almost all aneurysms were saccular (95.3%). Most frequently affected artery was the anterior communicating artery (16.8%). Only 6 aneurysms (4.1%) were located in the posterior circulation.
Table 1.
Characteristics of the Study Population
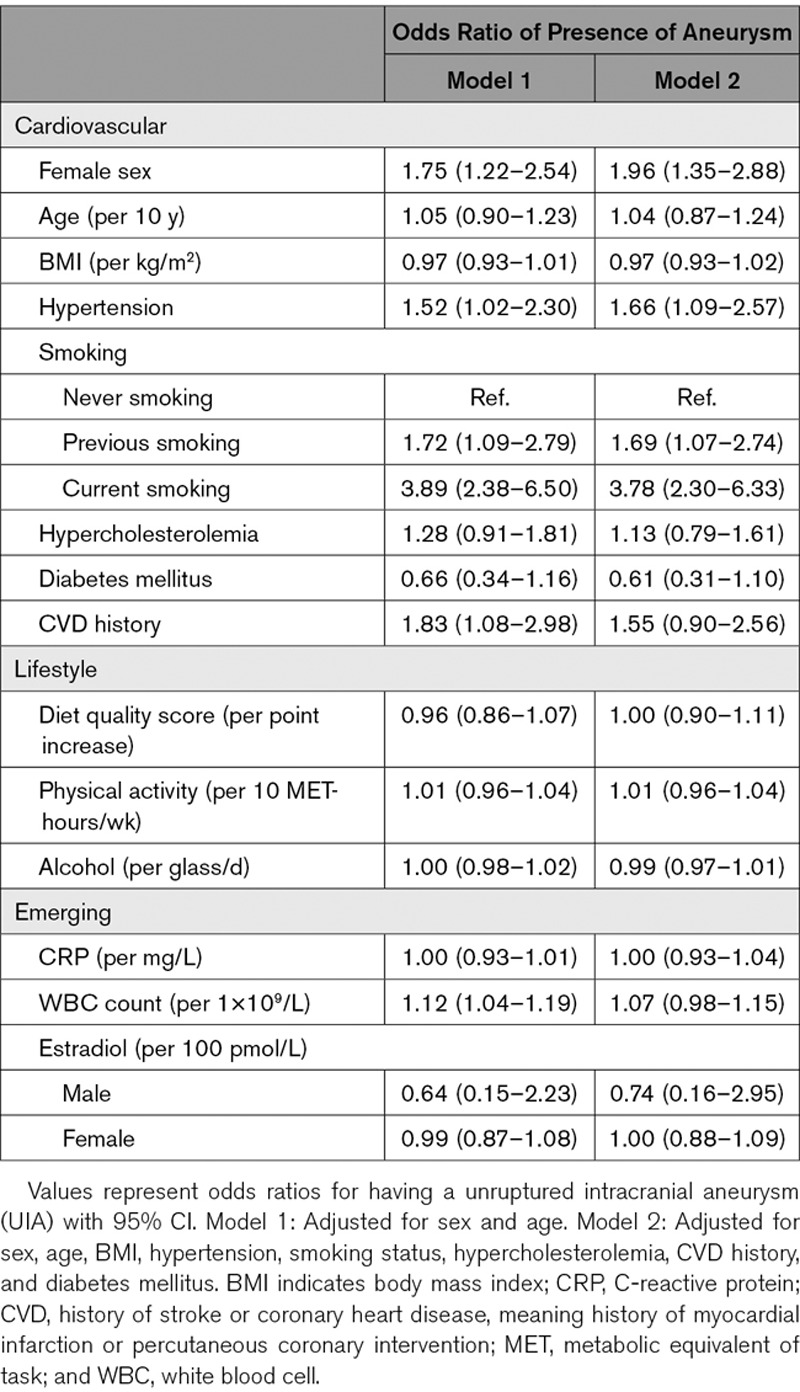
In age- and sex-adjusted analyses, we found female sex (odds ratio [OR], 1.75 [95% CI, 1.22–2.54]), hypertension (OR, 1.52 [95% CI, 1.02–2.30]), smoking (OR, 3.89 [95% CI, 2.38–6.50]), CVD history (OR, 1.91 [95% CI, 1.12–3.11]), and WBC count (OR, 1.11 per 109/L increase [95% CI, 1.04–1.19]) to be associated with the presence of UIAs (Table 2, model 1). In the fully adjusted analyses, female sex (OR, 1.92 [95% CI, 1.33–2.84]), hypertension (OR, 1.73 [95% CI, 1.13–2.68]), and smoking (OR, 3.75 [95% CI, 2.27–6.33]) remained associated with presence of UIAs (Table 2, model 2).
Table 2.
Determinants for the Presence of Unruptured Intracranial Aneurysms
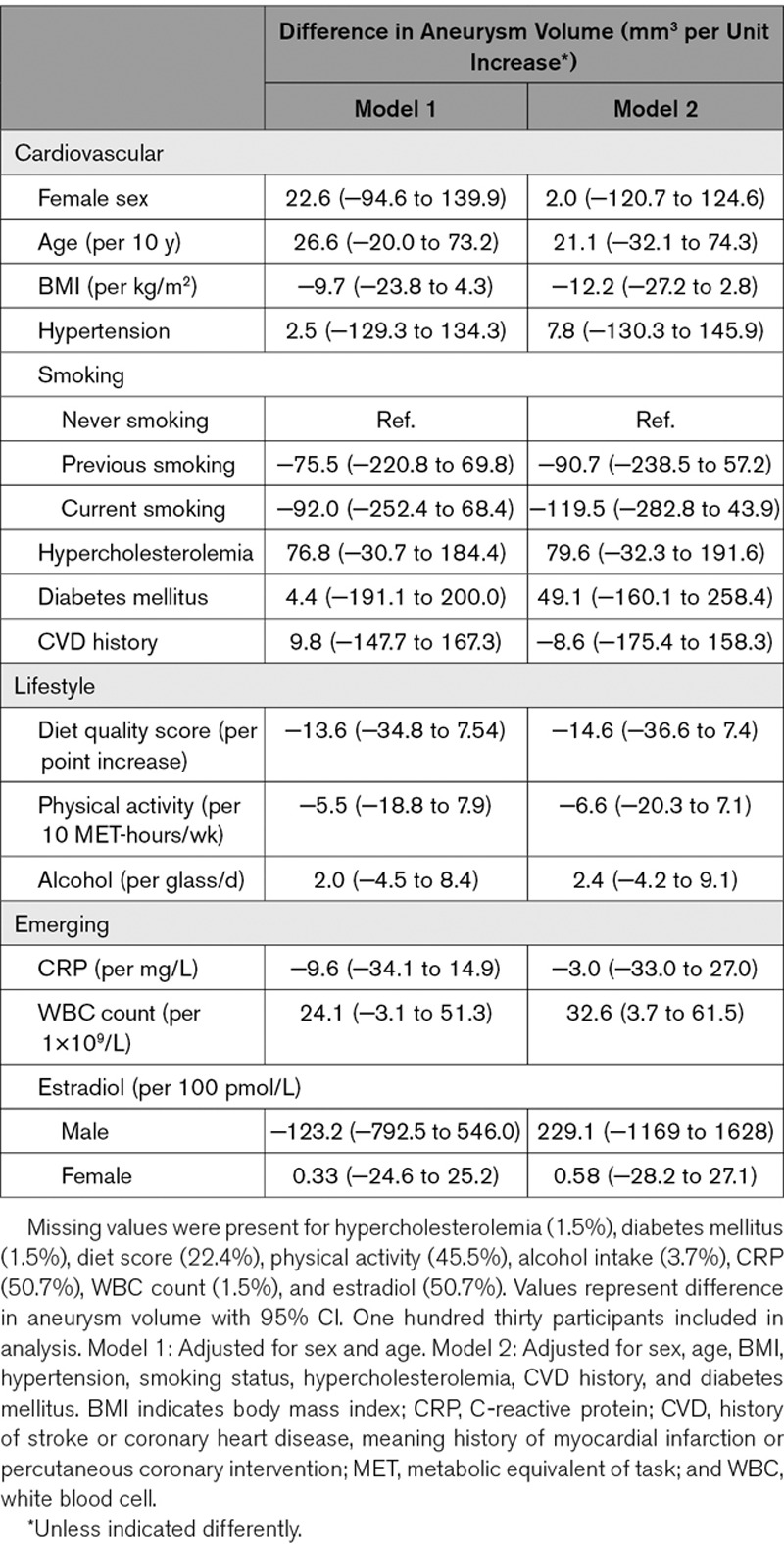
In age- and sex-adjusted analyses, we did not find any of the determinants to be associated with volume (Table 3) of UIAs. In fully adjusted analyses, only WBC count was found to relate to aneurysm volume (β=33.6 mm3 per 109/L increase [95% CI, 3.92–63.5]). After stratifying for sex (Tables I and II in the Data Supplement), we found that only in females, WBC count was associated with aneurysm volume (β=39.3 mm3 per 109/L increase [95% CI, 0.35–78.2)).
Table 3.
Determinants of Volume of Unruptured Intracranial Aneurysms
Discussion
In this study among middle-aged and elderly community-dwelling individuals, we found female sex, hypertension, and smoking to be associated with the presence of an UIA. Other lifestyle factors, such as alcohol use, diet, and physical activity, did not influence the presence of UIAs. We found WBC count to be associated with volume of UIAs in the general population.
The results of this study are largely in agreement with results from previous research. However, the effect of female sex appears to be less strong in the general population than a previous clinical study among patients with a history of subarachnoid hemorrhage indicated.8 Besides obvious differences in study population, which may explain this, another interesting explanation of this difference may also be that women are more prone to aneurysm rupture than men. Nevertheless, that would be in contrast with the largest meta-analysis investigating risk factors for aneurysm rupture,9 which did not find female sex as a risk factor for aneurysm rupture. Furthermore, our reported prevalence of posterior circulation aneurysms is lower than in previous studies.2 This can be explained by their tendency to be smaller, leading to lower detection and thus an underestimation in our study, as we did not use diagnostic computed tomography or magnetic resonance angiography. An alternative explanation for the discrepancy is the higher prevalence of such aneurysms in populations with comorbidities, which were overrepresented in previous studies, than in the general population. Finally, these aneurysms are more prone to rupture,9 which may have led to underrepresentation in the current study.
Hypertension is thought to exert its effect on formation of UIAs by increasing hemodynamic stress on cerebral vessel walls and subsequent remodeling of the vessel wall.20 As previous research suggests that hypertension is associated with increased risk of aneurysm growth,6 and thus potentially with aneurysm size, it is possible that hypertension causes aneurysm growth through similar mechanisms. Nonetheless, we did not find a relationship between aneurysm size and hypertension in the general population. This may be partly explained by the fact that a considerable number of participants with hypertension use antihypertensive medication, thereby attenuating the association. Conversely, it is possible that daily or long-term fluctuations in blood pressure influence aneurysm presence and size. Investigating such an association requires long-term and extensive blood pressure monitoring but may be helpful in dissecting the cause of UIAs.
Several studies5–8 have previously demonstrated the considerable, detrimental effects of smoking on aneurysm formation, although the exact pathophysiological pathways remain undefined. The findings of this study support these studies and point to smoking as one of the major known modifiable risk factors of aneurysm presence. Preventative measures of UIAs focusing on cessation of smoking may therefore prove to be highly successful. There was an incremental increase in risk of aneurysm presence across nonsmokers, previous smokers, and current smokers, which supports the hypothesis that cessation of smoking may at any given moment prove helpful in prevention of intracranial aneurysms.
Despite playing a considerable part in many CVDs, such as coronary heart disease and stroke,10,11 we found no role of other lifestyle factors, such as alcohol use, diet, and physical activity, in aneurysm presence. These findings may call into question the efficacy of potential interventions focused on improving diet and physical activity and limiting alcohol use to prevent formation of intracranial aneurysms. However, it is possible that such interventions decrease the risk of aneurysm formation through other pathways, such as lowering blood pressure.
Interestingly, our study found WBC count to be associated with presence of UIAs. However, after adjusting for cardiovascular risk factors, this effect did not remain statistically significant. Moreover, WBC count was associated with increased aneurysm volume. Nonetheless, as this association would not survive a multiple comparison correction, it should be approached with caution. There is a growing body of evidence implicating inflammation in the formation of UIAs. Prior research has shown an association between WBC count and incidence of subarachnoid hemorrhage.21 Our study suggests that this association may be driven by an increased risk of aneurysm presence. Moreover, previous imaging studies22–24 have demonstrated vessel wall changes related to inflammation in aneurysms. Circumferential enhancement along the aneurysm wall on MRI, likely a reflection of inflammation activity, was associated with aneurysm growth (and thus size), further supporting this pathway and highlighting a potential diagnostic or prognostic role for imaging studies in aneurysm development and growth.23,24 Finally, data from a case-control study suggest that anti-inflammatory therapies (eg, aspirin) may potentially limit aneurysm rupture,25 possibly in part through inhibition of aneurysm growth. A randomized controlled trial investigating the effects of aspirin and intensive blood pressure lowering on aneurysm rupture and growth is currently ongoing.26 The role of inflammation in aneurysm development remains controversial, and our findings should be regarded as hypothesis-generating rather than conclusive evidence.
Despite previous efforts, the pathophysiological mechanisms behind sex differences in aneurysm presence remain obscure. Hormones are a fundamental part of sex differences and may be the main underlying cause of sex differences in aneurysm prevalence. To shed light on this topic, we investigated the association of estradiol levels with UIAs. Nevertheless, we did not find an association between estradiol levels and aneurysm presence or volume. However, it must be pointed out that the majority of women in our study was (post-)menopausal and that we did not have data on their premenopausal estradiol levels. As prevalence of intracranial aneurysms quickly increases among women after menopause,1 it is possible that not so much the postmenopausal level of estrogens, but more the shift in estrogen levels, is a risk factor for aneurysm formation. Longitudinal data, especially from younger subjects, are necessary to further investigate this.
Sex, hypertension, and smoking have previously been shown to be risk factors for aneurysm growth.6,8 We therefore hypothesized that these determinants would also relate to aneurysm size in the general population. Nonetheless, besides WBC count, we did not find any of the determinants to be associated with aneurysm volume. A potential explanation is that certain determinants (eg, smoking and hypertension) cause rupture early after growth, thereby causing underrepresentation of these determinants among those with large aneurysms.27 However, as the vast majority of aneurysms were similar in volume, it is possible that we did not find any associations due to lack of statistical power.
The main strengths of this study are the large sample size and the population-based study design, which enabled us to assess the determinants in a truly unselected population. Knowledge on determinants in the general population is essential, as this forms the target population for interventions to prevent the development and rupture of UIAs. However, some limitations have to be considered. First, we used a proton-density T2-weighted sequence to evaluate presence of UIAs, possibly leading to underestimation of the prevalence of aneurysms and decreasing our power. The slice thickness of the PDw sequence was 1.6 mm, meaning that some aneurysms smaller than this might have been missed due to the limits in spatial resolution. However, although the found effects would be attenuated, they would remain valid. On the other hand, it is also possible that people who were judged to have an UIA did not actually harbor one. Finally, another potential limitation is the relatively homogeneous constitution of this geographically defined study population, consisting primarily of white, middle-class persons.28 Consequentially, our results may not be generalizable to other ethnic or socioeconomic groups.
Our results may have implications for clinical practice and future research. Although diet and physical activity show no association with the presence of UIAs, it is too early to rule out possible protective effects with respect to rupture risk, which was not studied here. Longitudinal data, with detailed registration of diet and physical activity, or intervention studies focused on promoting a healthy diet and increased physical activity are necessary in the future to further elucidate their potential role. For now, preventive strategies should be primarily focused on treating hypertension and promoting cessation of smoking.
Acknowledgments
We gratefully acknowledge the study participants of the Ommoord district and their general practitioners and pharmacists for their devotion in contributing to the Rotterdam Study. We also thank all staff who facilitated assessment of participants in the Rotterdam Study throughout the years.
Sources of Funding
The Rotterdam Study is supported by the Erasmus Medical Center and Erasmus University Rotterdam; the Netherlands Organization for Scientific Research (NWO); the Netherlands Organization for Health Research and Development (ZonMw); The Research Institute for Diseases in the Elderly (RIDE); the Ministry of Education, Culture and Science; the Ministry of Health, Welfare and Sports; the European Commission (DG XII); and the Municipality of Rotterdam.
The funding source had no role in study design, collection, analysis, interpretation of data, writing of the report, or decision to submit the article for publication.
Disclosures
None.
Supplementary Material
Footnotes
Drs Vernooij and Roozenbeek contributed equally.
For Sources of Funding and Disclosures, see page 2109.
The Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/STROKEAHA.120.029296.
References
- 1.Vlak MH, Algra A, Brandenburg R, Rinkel GJ. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: a systematic review and meta-analysis. Lancet Neurol. 2011;10:626–636. doi: 10.1016/S1474-4422(11)70109-0. doi: 10.1016/S1474-4422(11)70109-0. [DOI] [PubMed] [Google Scholar]
- 2.Rinkel GJ, Djibuti M, Algra A, van Gijn J. Prevalence and risk of rupture of intracranial aneurysms: a systematic review. Stroke. 1998;29:251–256. doi: 10.1161/01.str.29.1.251. doi: 10.1161/01.str.29.1.251. [DOI] [PubMed] [Google Scholar]
- 3.Vernooij MW, Ikram MA, Tanghe HL, Vincent AJ, Hofman A, Krestin GP, et al. Incidental findings on brain MRI in the general population. N Engl J Med. 2007;357:1821–1828. doi: 10.1056/NEJMoa070972. doi: 10.1056/NEJMoa070972. [DOI] [PubMed] [Google Scholar]
- 4.Weir B. Unruptured intracranial aneurysms: a review. J Neurosurg. 2002;96:3–42. doi: 10.3171/jns.2002.96.1.0003. doi: 10.3171/jns.2002.96.1.0003. [DOI] [PubMed] [Google Scholar]
- 5.Lindgren AE, Räisänen S, Björkman J, Tattari H, Huttunen J, Huttunen T, et al. De Novo Aneurysm Formation in Carriers of Saccular Intracranial Aneurysm Disease in Eastern Finland. Stroke. 2016;47:1213–1218. doi: 10.1161/STROKEAHA.115.012573. doi: 10.1161/STROKEAHA.115.012573. [DOI] [PubMed] [Google Scholar]
- 6.Wermer MJ, van der Schaaf IC, Velthuis BK, Algra A, Buskens E, Rinkel G ASTRA Study Group. Follow-up screening after subarachnoid haemorrhage: frequency and determinants of new aneurysms and enlargement of existing aneurysms. Brain. 2005;128:2421–2429. doi: 10.1093/brain/awh587. doi: 10.1093/brain/awh587. [DOI] [PubMed] [Google Scholar]
- 7.Vlak MH, Rinkel GJ, Greebe P, Algra A. Independent risk factors for intracranial aneurysms and their joint effect: a case-control study. Stroke. 2013;44:984–987. doi: 10.1161/STROKEAHA.111.000329. doi: 10.1161/STROKEAHA.111.000329. [DOI] [PubMed] [Google Scholar]
- 8.Juvela S, Poussa K, Porras M. Factors affecting formation and growth of intracranial aneurysms: a long-term follow-up study. Stroke. 2001;32:485–491. doi: 10.1161/01.str.32.2.485. doi: 10.1161/01.str.32.2.485. [DOI] [PubMed] [Google Scholar]
- 9.Greving JP, Wermer MJ, Brown RD, Jr, Morita A, Juvela S, Yonekura M, et al. Development of the PHASES score for prediction of risk of rupture of intracranial aneurysms: a pooled analysis of six prospective cohort studies. Lancet Neurol. 2014;13:59–66. doi: 10.1016/S1474-4422(13)70263-1. doi: 10.1016/S1474-4422(13)70263-1. [DOI] [PubMed] [Google Scholar]
- 10.Colpani V, Baena CP, Jaspers L, van Dijk GM, Farajzadegan Z, Dhana K, et al. Lifestyle factors, cardiovascular disease and all-cause mortality in middle-aged and elderly women: a systematic review and meta-analysis. Eur J Epidemiol. 2018;33:831–845. doi: 10.1007/s10654-018-0374-z. doi: 10.1007/s10654-018-0374-z. [DOI] [PubMed] [Google Scholar]
- 11.Larsson SC, Akesson A, Wolk A. Healthy diet and lifestyle and risk of stroke in a prospective cohort of women. Neurology. 2014;83:1699–1704. doi: 10.1212/WNL.0000000000000954. doi: 10.1212/WNL.0000000000000954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Villablanca JP, Duckwiler GR, Jahan R, Tateshima S, Martin NA, Frazee J, et al. Natural history of asymptomatic unruptured cerebral aneurysms evaluated at CT angiography: growth and rupture incidence and correlation with epidemiologic risk factors. Radiology. 2013;269:258–265. doi: 10.1148/radiol.13121188. doi: 10.1148/radiol.13121188. [DOI] [PubMed] [Google Scholar]
- 13.Ikram MA, Brusselle GGO, Murad SD, van Duijn CM, Franco OH, Goedegebure A, et al. The Rotterdam Study: 2018 update on objectives, design and main results. Eur J Epidemiol. 2017;32:807–850. doi: 10.1007/s10654-017-0321-4. doi: 10.1007/s10654-017-0321-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ikram MA, van der Lugt A, Niessen WJ, Koudstaal PJ, Krestin GP, Hofman A, et al. The Rotterdam Scan Study: design update 2016 and main findings. Eur J Epidemiol. 2015;30:1299–1315. doi: 10.1007/s10654-015-0105-7. doi: 10.1007/s10654-015-0105-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bos D, van der Rijk MJ, Geeraedts TE, Hofman A, Krestin GP, Witteman JC, et al. Intracranial carotid artery atherosclerosis: prevalence and risk factors in the general population. Stroke. 2012;43:1878–1884. doi: 10.1161/STROKEAHA.111.648667. doi: 10.1161/STROKEAHA.111.648667. [DOI] [PubMed] [Google Scholar]
- 16.Voortman T, Kiefte-de Jong JC, Ikram MA, Stricker BH, van Rooij FJA, Lahousse L, et al. Adherence to the 2015 Dutch dietary guidelines and risk of non-communicable diseases and mortality in the Rotterdam Study. Eur J Epidemiol. 2017;32:993–1005. doi: 10.1007/s10654-017-0295-2. doi: 10.1007/s10654-017-0295-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stel VS, Smit JH, Pluijm SM, Visser M, Deeg DJ, Lips P. Comparison of the LASA Physical Activity Questionnaire with a 7-day diary and pedometer. J Clin Epidemiol. 2004;57:252–258. doi: 10.1016/j.jclinepi.2003.07.008. doi: 10.1016/j.jclinepi.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 18.Caspersen CJ, Bloemberg BP, Saris WH, Merritt RK, Kromhout D. The prevalence of selected physical activities and their relation with coronary heart disease risk factors in elderly men: the Zutphen Study, 1985. Am J Epidemiol. 1991;133:1078–1092. doi: 10.1093/oxfordjournals.aje.a115821. doi: 10.1093/oxfordjournals.aje.a115821. [DOI] [PubMed] [Google Scholar]
- 19.Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DR, Jr, Tudor-Locke C, et al. 2011 Compendium of Physical Activities: a second update of codes and MET values. Med Sci Sports Exerc. 2011;43:1575–1581. doi: 10.1249/MSS.0b013e31821ece12. doi: 10.1249/MSS.0b013e31821ece12. [DOI] [PubMed] [Google Scholar]
- 20.Morimoto M, Miyamoto S, Mizoguchi A, Kume N, Kita T, Hashimoto N. Mouse model of cerebral aneurysm: experimental induction by renal hypertension and local hemodynamic changes. Stroke. 2002;33:1911–1915. doi: 10.1161/01.str.0000021000.19637.3d. doi: 10.1161/01.str.0000021000.19637.3d. [DOI] [PubMed] [Google Scholar]
- 21.Söderholm M, Zia E, Hedblad B, Engström G. Leukocyte count and incidence of subarachnoid haemorrhage: a prospective cohort study. BMC Neurol. 2014;14:71. doi: 10.1186/1471-2377-14-71. doi: 10.1186/1471-2377-14-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aoki T, Saito M, Koseki H, Tsuji K, Tsuji A, Murata K, et al. MR Macrophage Imaging Study Investigators. Macrophage Imaging of Cerebral Aneurysms with Ferumoxytol: an Exploratory Study in an Animal Model and in Patients. J Stroke Cerebrovasc Dis. 2017;26:2055–2064. doi: 10.1016/j.jstrokecerebrovasdis.2016.10.026. doi: 10.1016/j.jstrokecerebrovasdis.2016.10.026. [DOI] [PubMed] [Google Scholar]
- 23.Omodaka S, Endo H, Niizuma K, Fujimura M, Inoue T, Endo T, et al. Circumferential wall enhancement in evolving intracranial aneurysms on magnetic resonance vessel wall imaging. J Neurosurg. 2018;131:1262–8. doi: 10.3171/2018.5.JNS18322. [DOI] [PubMed] [Google Scholar]
- 24.Edjlali M, Guédon A, Ben Hassen W, Boulouis G, Benzakoun J, Rodriguez-Régent C, et al. Circumferential Thick Enhancement at Vessel Wall MRI Has High Specificity for Intracranial Aneurysm Instability. Radiology. 2018;289:181–187. doi: 10.1148/radiol.2018172879. doi: 10.1148/radiol.2018172879. [DOI] [PubMed] [Google Scholar]
- 25.Hasan DM, Mahaney KB, Brown RD, Jr, Meissner I, Piepgras DG, Huston J, et al. International Study of Unruptured Intracranial Aneurysms Investigators. Aspirin as a promising agent for decreasing incidence of cerebral aneurysm rupture. Stroke. 2011;42:3156–3162. doi: 10.1161/STROKEAHA.111.619411. doi: 10.1161/STROKEAHA.111.619411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vergouwen MD, Rinkel GJ, Algra A, Fiehler J, Steinmetz H, Vajkoczy P, et al. Prospective Randomized Open-label Trial to evaluate risk faCTor management in patients with Unruptured intracranial aneurysms: Study protocol. Int J Stroke. 2018;13:992–998. doi: 10.1177/1747493018790033. doi: 10.1177/1747493018790033. [DOI] [PubMed] [Google Scholar]
- 27.Etminan N, Beseoglu K, Steiger HJ, Hänggi D. The impact of hypertension and nicotine on the size of ruptured intracranial aneurysms. J Neurol Neurosurg Psychiatry. 2011;82:4–7. doi: 10.1136/jnnp.2009.199661. doi: 10.1136/jnnp.2009.199661. [DOI] [PubMed] [Google Scholar]
- 28.Rossum C, Grobbee D. Socioeconomic inequalities in cardiovascular disease in an ageing population: (Ph.D. thesis. Rotterdam, the Netherlands: Erasmus University Rotterdam; 1999. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


