Supplemental Digital Content is available in the text.
Keywords: anticoagulant, apixaban, atrial fibrillation, mortality, safety
Abstract
Background and Purpose:
The effects of direct oral anticoagulants in nonvalvular atrial fibrillation should be assessed in actual conditions of use. France has near-universal healthcare coverage with a unified healthcare information system, allowing large population-based analyses. NAXOS (Evaluation of Apixaban in Stroke and Systemic Embolism Prevention in Patients With Nonvalvular Atrial Fibrillation) aimed to compare the safety, effectiveness, and mortality of apixaban with vitamin K antagonists (VKAs), rivaroxaban, and dabigatran, in oral anticoagulant-naive patients with nonvalvular atrial fibrillation.
Methods:
This was an observational study using French National Health System claims data and including all adults with nonvalvular atrial fibrillation who initiated oral anticoagulant between 2014 and 2016. Outcomes of interest were major bleeding events leading to hospitalization (safety), stroke and systemic thromboembolic events (effectiveness), and all-cause mortality. Four approaches were used for comparative analyses: matching on propensity score (PS; 1:n); as a sensitivity analysis, matching on high-dimensional PS; adjustment on PS; and adjustment on known confounders. For each outcome, cumulative incidence rates accounting for competing risks of death were estimated.
Results:
Overall, 321 501 patients were analyzed, of whom 35.0%, 27.2%, 31.1%, and 6.6% initiated VKAs, apixaban, rivaroxaban, and dabigatran, respectively. Apixaban was associated with a lower PS–matched risk of major bleeding compared with VKAs (hazard ratio [HR], 0.43 [95% CI, 0.40–0.46]) and rivaroxaban (HR, 0.67 [95% CI, 0.63–0.72]), but not dabigatran (HR, 0.93 [95% CI, 0.81–1.08]). Apixaban was associated with a lower risk of stroke and systemic thromboembolic event compared with VKAs (HR, 0.60 [95% CI, 0.56–0.65]), but not rivaroxaban (HR, 1.05 [95% CI, 0.97–1.15]) or dabigatran (HR, 0.93 [95% CI, 0.78–1.11]). All-cause mortality was lower with apixaban than with VKAs, but not lower than with rivaroxaban or dabigatran.
Conclusions:
Apixaban was associated with superior safety, effectiveness, and lower mortality than VKAs; with superior safety than rivaroxaban and similar safety to dabigatran; and with similar effectiveness when compared with rivaroxaban or dabigatran. These observational data suggest potentially important differences in outcomes between direct oral anticoagulants, which should be explored in randomized trials.
Although vitamin K antagonists (VKAs) are highly effective in the prevention and treatment of thromboembolic events, they have limitations related to drug and food interactions. VKAs also have a narrow therapeutic margin, requiring frequent monitoring, with a particular concern for an increased risk of intracranial bleeding.1 Direct oral anticoagulants (DOACs) represent an alternative to VKAs and inhibit coagulation by directly and specifically binding to the active site of either thrombin (dabigatran) or factor Xa (rivaroxaban and apixaban). Due to a large therapeutic index, DOACs can be given in fixed doses without routine coagulation monitoring and have limited drug and food interactions.2 Randomized trials have generally established superior safety and at least similar efficacy of DOACs with VKAs in patients with nonvalvular atrial fibrillation (NVAF).3
ARISTOTLE (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation), a randomized, double-blind trial, compared apixaban with warfarin in patients with NVAF and ≥1 additional risk factor for thromboembolism.4 In that trial, apixaban reduced stroke/systemic embolism, bleeding, and mortality compared with warfarin.4 On the basis of ARISTOTLE, French regulators (Health Authority and Transparency Committee) approved apixaban for the prevention of stroke and systemic embolism in patients with NVAF and ≥1 risk factor(s), but required a postmarketing effectiveness study. To satisfy this request, the NAXOS study (Evaluation of Apixaban in Stroke and Systemic Embolism Prevention in Patients With Nonvalvular Atrial Fibrillation) was designed to describe the real-world use of apixaban and other oral anticoagulants (OACs) available in France (VKAs, dabigatran, and rivaroxaban). NAXOS aimed to evaluate the risks of major bleeding (safety), stroke and systemic thromboembolic events (STEs; effectiveness), and all-cause mortality comparing apixaban with other OACs. In the absence of randomized, controlled head-to-head comparisons between DOACs, high-quality observational data can provide useful information on the comparative effectiveness of DOACs. Because France has near-universal healthcare coverage and a unified national healthcare data system (covering >90% of the total French population of ≈66 million individuals), NAXOS provided a good opportunity for a large population-based analysis of the comparative effectiveness of the OACs used in France for NVAF, focused on apixaban.
Methods
Because of their sensitive nature, the data are available only to investigators habilitated by the French National Health System. Patient consent was not required for this study (not applicable in anonymized claims database).
Study Design and Data Source
This historical, population-based cohort study used French National Health System claims data (Système National des Données de Santé [SNDS]), which contains anonymous individual information on sociodemographic characteristics, all nonhospital reimbursed healthcare expenditures (without the corresponding medical indication or results), and all hospital discharge summaries.
The SNDS does not provide direct information on clinical history, clinical or paraclinical examination (tobacco smoking, blood pressure level, body mass index, etc), biologic results or any information on drug dispensed during a hospital stay (except very costly medication), and any data on cause of death; however, the outcome events of interest for this study (stroke, major bleeding, death) are captured by SNDS. This claims database currently covers >90% of the country’s population.5
The study population consisted of all patients aged ≥18 years covered by the SNDS, with ≥1 reimbursement for OAC treatments (VKAs, apixaban, rivaroxaban, or dabigatran) between January 2014 and December 2016, and newly initiating one of the study OAC treatments, that is, without the use of the same OAC in the 24 months before the index date (ie, date of the first dispensation).6 Patients with atrial fibrillation diagnosed in the 24 months before inclusion were identified using a validated algorithm previously used by Bouillon et al7 in the NACORA study. A second algorithm was developed to identify NVAF (based on the European Society of Cardiology definition).8
Eligible patients were allocated to 4 distinct subcohorts based on whether they received VKAs, apixaban, rivaroxaban, or dabigatran during the study period.
Patients with several OAC treatments, multiple doses or multiple prescribers at the index date, and patients possibly treated for indications other than stroke prevention in NVAF were excluded.
Selected patients were followed up during their exposure to the studied anticoagulant treatment. Hence, each patient was studied from the index date until switch to another anticoagulant treatment, treatment discontinuation, patient’s last health record (last care recorded in the database prior a 6-month period without any reimbursed care, which may include emigration and admissions to geriatric homes), death, or end of the study period (ie, December 31, 2016), whichever occurred earliest. Patients were censored at the first occurrence of one of these events. Details/definitions of switching treatment, discontinuation, and drug coverage can be found in the Supplemental Materials in the Data Supplement.
Outcomes
The outcomes of interest were safety, defined as the risk of major bleeding events leading to hospitalization and identified through main hospital discharge diagnoses; effectiveness, defined as the risk of stroke and STE and identified through main hospital discharge diagnoses; and all-cause mortality, identified using the date of death recorded in the SNDS (Table I in the Data Supplement). Safety was also investigated considering 3 specific bleeding sites. For patients with >1 bleeding site at their date of first major bleeding event, the clinically most severe event was considered according to the following order of priority: intracranial bleeding, gastrointestinal bleeding, and other bleeding. The proxies of outcomes have been adapted from Friberg et al9 and have been validated by Bouillon et al7 on the SNDS.
Statistical Methods
For each cohort of OAC-naive patients, sociodemographic characteristics and comorbidities were described using descriptive statistics.
The main analysis, comparing apixaban versus each of the other OACs, was performed using propensity score (PS) matching. Three PS were estimated for each comparison between apixaban and other OACs, using a logistic regression model that included the following variables: sociodemographic characteristics, specialty of the prescriber who initiated the OAC treatment, comorbid conditions, CHA2DS2-VASc score, modified HAS-BLED score, Charlson score, and drugs dispensed within 3 months before index date. In case of collinearity, collinear variables were removed from the PS. Several checks were performed to ensure a good balance of PS and of covariates between apixaban and comparison groups: first, the treatment group PS distribution was analyzed graphically. Then, the balance of covariates across treatment and comparison groups was checked using standardized difference.10 Apixaban patients were matched with those treated with other anticoagulants using sequential pairwise nearest neighbor 1:n (n variable and n≤3) matching without replacement, using the logit of PS and specified caliper of width 0.2 of SD of the logit of PS.11 The quality of the matching was checked with absolute weighted standardized differences on the demographics and clinical covariates (standardized differences <0.1 indicating good balance between treatment groups).11
After PS matching, the risk for each outcome was compared between apixaban and each of the other OACs using a Cox proportional hazard model with robust variance estimator to account for matching. To account for the competing risk of mortality (as the mortality was >10% in the VKAs cohort), Fine and Gray models were used to compare safety and effectiveness outcomes between apixaban and VKAs. Proportionality assumption was checked for exposure by including the interaction between a time function and exposure. If the proportionality assumption was violated, then 2 models were computed: the first without considering the nonproportionality that computed an average hazard ratio (HR) for each exposure over the period, and the second with the inclusion of the interaction between time function (log) and exposure to identify potential change of the HR over time.
To support the robustness of the main analysis, 3 additional analyses were performed (see Supplemental Materials in the Data Supplement).
All statistical analyses were performed using SAS (SAS Institute, NC), version 9.4.
Sensitivity Analysis
Sensitivity analyses were performed using modified definitions of the outcomes: for the safety outcome, addition of associated diagnoses of hospital stays for major bleeding events and addition of transfusion (through medical procedures codes); and for the effectiveness outcome, exclusion of diagnoses of hemorrhagic stroke. The comparisons between apixaban and each of the other OACs were performed using the same method as for the main analysis.
Ethics
This study was approved by the French Institute for Health Data (Institut National des Données de Santé, approval No. 136 from September 8, 2015). It was conducted using anonymized data, approved by the National Informatics and Liberty Committee (Commission Nationale Informatique et Libertés, approval No. 1877931 from March 17, 2016), and registered at URL: https://www.clinicaltrials.gov; Unique identifier: NCT02640222.
Results
Identification of the Study Population
Among the 1 951 218 patients included in the French National Health System database who had been dispensed ≥1 course of VKA, apixaban, rivaroxaban, or dabigatran between 2014 and 2016, 1 040 864 patients (53.3%) initiated one of the study OAC treatments. Almost all patients (99.8%) were aged ≥18 years at OAC initiation, 438 280 patients had atrial fibrillation and 93.9% (411 700 patients) had NVAF. After excluding patients without valid dosage or prescriber information, 411 077 patients were included in the study population.
Of those included, 321 501 OAC-naive patients with NVAF were identified: 112 628 (35.0%) initiated VKAs (69.8% used fluindione, 27.3% used warfarin, and 2.9% used acenocoumarol), whereas 87 565 (27.2%), 100 063 (31.1%), and 21 245 patients (6.6%) initiated apixaban, rivaroxaban, and dabigatran, respectively (Figure 1).
Figure 1.
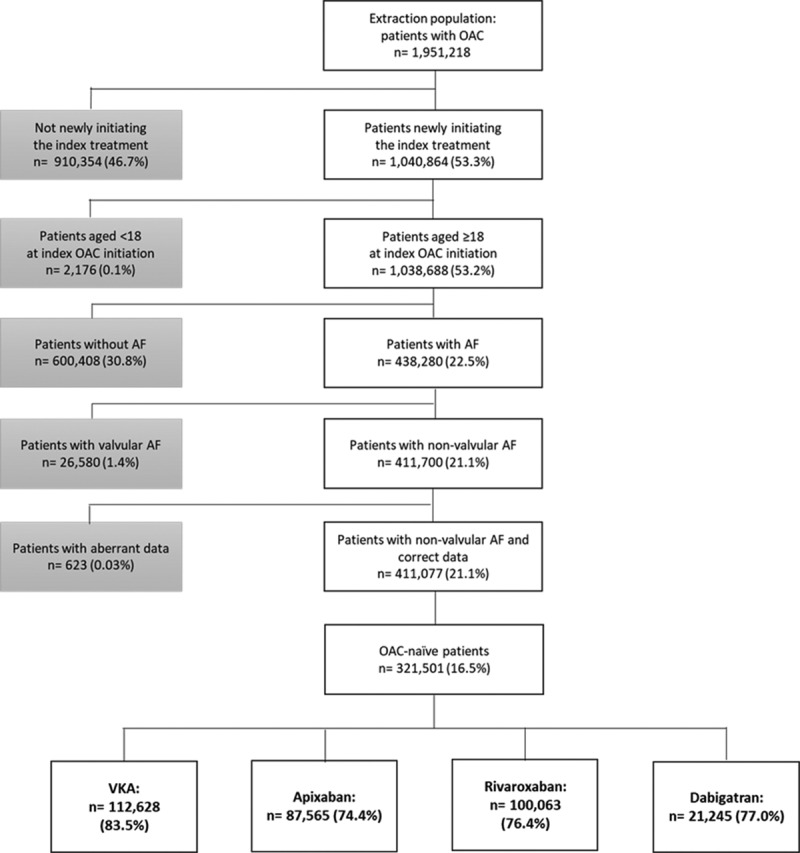
Selection of the study population. The percentages of patients who initiated vitamin K antagonists (VKAs), apixaban, rivaroxaban, and dabigatran were reported based on the number of oral anticoagulant (OAC)-naive patients (n=321 501). AF indicates atrial fibrillation.
Patient Characteristics
Prematched data showed notable differences in patient characteristics between the cohorts initiating VKA, apixaban, rivaroxaban, and dabigatran (Table). Patients in the VKA cohorts were older, had a higher risk of stroke, and more comorbid conditions than patients receiving DOACs. Among DOAC patients, those initiating apixaban were older, had a higher risk of stroke, and more frequently had comorbidities than those in the rivaroxaban and dabigatran cohorts.
Table.
Prematched Baseline Demographic and Clinical Characteristics of Patients in the 24 mo Before the Index Date by OAC Treatment in OAC-Naive Cohorts
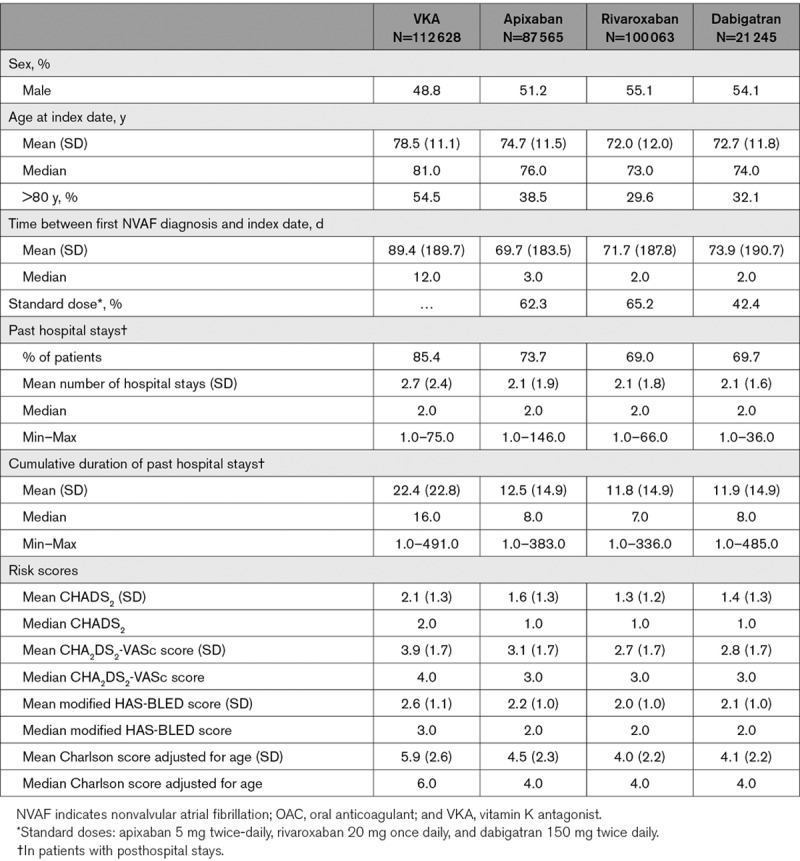
The mean follow-up duration was 316 days (median=218 days) for anticoagulant-naive patients treated with VKAs. For those receiving apixaban, rivaroxaban, and dabigatran, the mean follow-up duration was 286 (median=213), 318 (median=205), and 329 days (median=186), respectively.
Among apixaban-treated patients, 68 208 could be matched to 107 558 VKA patients, 81 759 could be matched to 100 050 rivaroxaban patients, and 21 245 could be matched to 21 245 dabigatran patients (Table II in the Data Supplement).
After propensity score matching, the absolute weighted standardized differences of all confounding factors were <10% (Figure I in the Data Supplement). The confounding factors after weighting for each of the matched cohorts are given in Table III in the Data Supplement.
Outcomes During Follow-Up
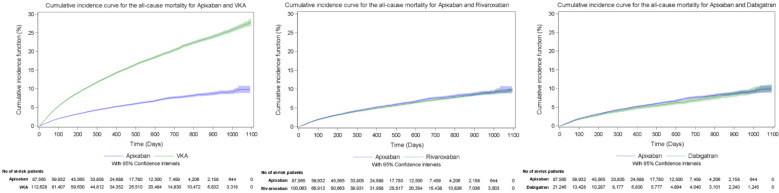
The cumulative incidence of major bleeding during the follow-up period, taking into account the competing risk of death in the VKA cohort, was 4.00% for apixaban and 9.54% for VKA, 4.24% for apixaban and 6.38% for rivaroxaban, and 3.80% for apixaban and 4.31% for dabigatran (Figure 2). The cumulative incidence of stroke or STE during the follow-up period, taking into account competing risks in the VKA cohort, was 3.16% for apixaban and 6.13% for VKA, 2.93% for apixaban and 3.22% for rivaroxaban, and 2.56% for apixaban and 3.28% for dabigatran (Figure 3). The cumulative incidence of all-cause mortality during the follow-up period was 11.10% for apixaban and 26.55% for VKA, 9.18% for apixaban and 9.66% for rivaroxaban, and 8.36% for apixaban and 10.05% for dabigatran (Figure 4).
Figure 2.
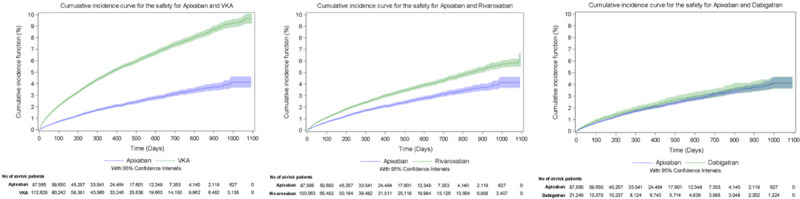
Cumulative incidence curves for the evaluation of major bleeding events leading to hospitalization (safety) during the overall follow-up period. VKA indicates vitamin K antagonist.
Figure 3.
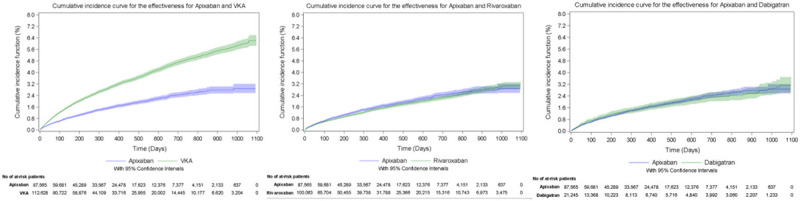
Cumulative incidence curves for the evaluation of risk of stroke and systemic thromboembolic events (effectiveness) outcomes during the overall follow-up period. VKA indicates vitamin K antagonist.
Figure 4.
Cumulative incidence curves for the evaluation of all-cause mortality outcome during the overall follow-up period. VKA indicates vitamin K antagonist.
Comparison of Outcomes
After propensity score matching, patients initiating apixaban were at a lower risk of major bleeding (all major bleeding HR, 0.43 [95% CI, 0.40–0.46]; gastrointestinal bleeding HR, 0.44 [95% CI, 0.40–0.50]; intracranial bleeding HR, 0.42 [95% CI, 0.37–0.48]; and other bleeding HR, 0.43 [95% CI, 0.39–0.47]) and stroke or STE (HR, 0.60 [95% CI, 0.56–0.65]) and had a lower risk of all-cause mortality (HR, 0.44 [95% CI, 0.42–0.45]) than matched patients initiating VKAs (Figure 5). Patients treated with apixaban had a lower risk of major bleeding than those treated with rivaroxaban (HR, 0.67 [95% CI, 0.63–0.72]), although the risk of stroke and STE (HR, 1.05 [95% CI, 0.97–1.15) and the risk of all-cause mortality (HR, 0.97 [95% CI, 0.93–1.02]) were comparable (Figure 5). Patients initiating apixaban were also associated with lower risks of gastrointestinal bleeding (HR, 0.63 [95% CI, 0.56–0.70]) and other bleeding (HR, 0.64 [95% CI, 0.57–0.71]), but a comparable risk of intracranial bleeding (HR, 0.87 [95% CI, 0.75–1.01]) than those initiating rivaroxaban. Compared with patients treated with dabigatran, patients treated with apixaban had comparable risks of major bleeding (HR, 0.93 [95% CI, 0.81–1.08]), of stroke and STE (HR, 0.93 [95% CI, 0.78–1.11]), and of all-cause mortality (HR, 0.94 [95% CI, 0.85–1.04]) (Figure 5). Patients initiating apixaban had also a lower risk of gastrointestinal bleeding (HR, 0.60 [95% CI, 0.48–0.76]), a comparable risk of other bleeding (HR, 1.13 [95% CI, 0.90–1.44]), and a higher risk of intracranial bleeding (HR, 1.72 [95% CI, 1.20–2.48]) than those initiating dabigatran. In case of violation of the proportionality assumption, the direction of the association was not modified after inclusion of the interaction between time function and exposure.
Figure 5.
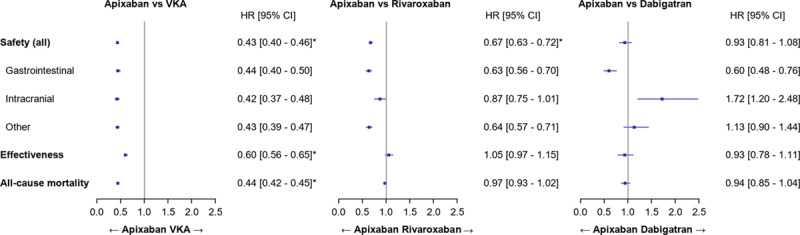
Forest plots of the results of the main analysis (propensity score matched). HR indicates hazard ratio; and VKA, vitamin K antagonist.
The findings of the additional analyses using adjustment on confounding factors, adjustment on PS, and matching on high-dimensional propensity score were mostly consistent with those of the main analysis, as detailed in Figure II in the Data Supplement. Sensitivity analyses using modified outcome definitions (safety and effectiveness) were also overall consistent with the main analysis (Figure III in the Data Supplement).
Discussion
With a total cohort of 321 501 OAC-naive patients with NVAF, NAXOS is among the largest observational studies to assess the use and effects of OACs, and it offers a nationwide population-based perspective of the use of anticoagulants in everyday life. In this study, the choice of anticoagulant clearly differed according to the patient profile, with patients who initiated DOACs having a lower cardiovascular risk profile than patients initiating VKAs. Among patients prescribed DOACs, those receiving apixaban had a higher cardiovascular risk, higher CHA2DS2-VASc score, and more comorbid conditions (higher Charlson score) than those receiving other agents. After PS matching, apixaban was associated with superior safety and effectiveness compared with VKAs. Apixaban was also associated with superior safety compared with rivaroxaban, although effectiveness of the 3 DOACs was comparable. The risk of all-cause mortality was lower with apixaban versus VKAs, although it was comparable between the 3 DOACs.
To support the robustness of the main analysis and to include the full population (no attrition bias), we performed 2 additional analysis, using adjustment for confounding factors, and using adjustment for PS. Moreover, to try to improve the classical PS, we performed a third additional analysis, using matching on high-dimensional propensity score. In addition, to support the validity of the definitions used to identify outcomes, sensitivity analyses were conducted using modified definitions of these outcomes. Overall, these additional sensitivity analyses produced results consistent with the main analysis.
Identification of patients with NVAF and associated risk scores from claims databases is complex.12 The algorithm used in NAXOS relied on the criteria previously used in the ARISTOTLE trial4 and in the NACORA study (Nouveaux Anticoagulants Oraux et Risques Associés).7 In terms of sociodemographic and clinical data, patients included in the NAXOS study were comparable to those from 3 previous large French observational studies,7,13,14 in which patients treated with VKAs were older than patients treated with DOACs. The age and comorbidities of patients included in the NAXOS study were similar to those of patients from the PAROS study (Apixaban in Patients With Atrial Fibrillation in a Real Life Setting: Cross-Sectional Study in France), a recent French cross-sectional survey of OAC prescriptions in patients with NVAF.15
The safety, effectiveness, and all-cause mortality observations pertaining to apixaban and VKAs in NAXOS are consistent with the results of the ARISTOTLE trial.4 The mortality of VKA-treated patients, however, was higher in NAXOS (13.39%) (data not shown) than in ARISTOTLE (3.94%), likely due to the older age of patients in the NAXOS cohort and due to the stringent selection process used to identify eligibility in randomized trials. As a rule, patients enrolled in observational studies in routine clinical practice tend to be at substantially higher risk of adverse outcomes than patients in clinical trials.16–18
The relative risk of major bleeding events leading to hospitalization, the risk of stroke and STEs, and the risk of all-cause mortality with apixaban compared with VKAs were lower in NAXOS than in ARISTOTLE (HR, 0.43 versus 0.69, 0.60 versus 0.79, and 0.44 versus 0.89, respectively). These relative risks may reflect poorer international normalized ratio (INR) control among patients treated with VKAs in routine clinical practice, as opposed to the highly controlled environment of a randomized clinical trial and is consistent with data suggesting that the majority of patients treated with VKAs in France have inadequate INR control,19,20 compared with higher control rates in clinical trials that implement careful INR monitoring.4 However, this assumption remains speculative, as INR results are not available in the French claims database. Another difference between NAXOS and randomized trials is that fluindione is the most frequently used VKA in France, whereas warfarin was preferentially used in randomized controlled trials.20
The results of the NAXOS study are in line with those of previous observational studies, both in terms of safety21,22 and effectiveness.22,23 In the study conducted by Vinogradova et al24 in the United Kingdom, apixaban was associated with a lower risk of bleeding compared with VKAs, but no difference was observed in the incidence of ischemic stroke and mortality. In a US study, apixaban, but not dabigatran or rivaroxaban, was associated with a lower risk of stroke/thromboembolism compared with warfarin; all 3 DOACs were associated with a reduced risk of intracranial bleeding, and both apixaban and dabigatran were associated with a decreased risk of major bleeding.22 In a further US study that relied on claims data, both apixaban and dabigatran were associated with a reduced risk of major bleeding compared with warfarin, whereas rivaroxaban had a similar risk.21 In a Danish claims data study,25 all 3 DOACs were associated with a reduced risk of intracranial bleeding compared with VKAs, with no difference in stroke or thromboembolic events. In our study, dabigatran was associated with significantly increased risks of gastrointestinal and other bleedings, but significantly reduced risk of intracranial bleeding, compared with apixaban. These findings were consistent with those of the study from Graham et al.26 In a US study from Noseworthy et al,27 apixaban, rivaroxaban, and dabigatran appeared to have similar effectiveness, although apixaban had a lower bleeding risk and rivaroxaban had an elevated bleeding risk; among DOAC users in another large US-based analysis, the benefit-harm profiles for apixaban and dabigatran were more favorable than rivaroxaban.26
Although observational analyses have provided somewhat inconsistent results with respect to mortality, safety, and effectiveness differences,24,28–31 NAXOS, as one of the largest, includes the vast majority of the French population and suggests potentially clinically relevant differences between OACs, with possible public health impact.
In the ARISTOPHANES study (Anticoagulants for Reduction in Stroke: Observational Pooled Analysis on Health Outcomes and Experience of Patients), the incidence of stroke/systemic embolism with lower and standard doses of apixaban and rivaroxaban was similar to that with warfarin, although it was higher with dabigatran than with warfarin.23 The risk of major bleeding was lower with apixaban and dabigatran than with warfarin at both low and standard doses and higher with rivaroxaban at both doses. At both low and standard doses, the risk of stroke/systemic embolism was lower with apixaban than with dabigatran or rivaroxaban, whereas the risk was higher with dabigatran than with rivaroxaban at low doses. Whatever the dose, the risk of major bleeding was lower with apixaban and dabigatran than with rivaroxaban, in agreement with the NAXOS findings.23
The strengths of NAXOS are that it used a nationwide claims database (SNDS) covering both primary and secondary care, providing a broad range of data, including sociodemographic information, major medical history, and comprehensive healthcare reimbursement records, and comprises >90% of the French population. It is one of the largest nationwide observational cohorts of patients initiating OACs for the treatment of NVAF to date, providing high statistical power. Results were consistent across sensitivity analyses. Most importantly, it was population based and relied on a single payer (French National Health System) database, thereby minimizing the potential for selection bias. Finally, although it is not possible to exclude residual bias favoring apixaban in the comparisons, the fact that, among patients treated with DOACs, those receiving apixaban had higher CHA2DS2-VASc and Charlson scores than patients receiving the other DOACs suggests that residual bias by indication is an unlikely explanation for the superior safety of apixaban and that the findings are conservative estimates. The magnitude of some differences between DOACs suggests that head-to-head randomized clinical trials appear warranted. From an exploratory calculation conducted by authors based on the proportion of events, around 10 000 patients per treatment arm would be needed to evaluate outcomes across DOACs in a hypothetical head-to-head trial (assuming an 80% power).
Like all observational studies, NAXOS has limitations: patients with NVAF were identified by an algorithm that may have excluded patients with less severe disease, such as those with uncomplicated paroxysmal atrial fibrillation. The SNDS database does not collect diagnoses other than discharge diagnoses; therefore, some cardiovascular risk factors had to be identified from proxies based on treatments and hospital diagnoses (the drawbacks of using DRG and inability to fully ascertain the nature/cause of some outcome events are well known), and information was missing for major confounders (clearance of creatinine, hemoglobin, smoking status, weight, diet, and alcohol consumption), as is often the case for claims data, or even for medical records when data are not regularly updated. Thus, the CHADS2, CHA2DS2-VASc, modified HAS-BLED, and Charlson scores adjusted for age were assessed through algorithms based on proxies. It would have been of interest to separate the etiological subtypes of ischemic stroke and intracerebral hemorrhage, as we aware that the exact etiology is important for determining a causal relationship to anticoagulants’ use. However, in the database, we could not fully ascertain the nature/cause of these outcome events (for instance, in some elderly patients, no radiological investigation was performed, when the overall health status was poor).
Although significant efforts were made to minimize confounding, residual confounding may be present, as in all observational analyses. Nonetheless, the consistency of our findings is reassuring, suggesting robust evidence. Large numbers of models were produced, opening a possibility of correction for multiple testing. However, the majority of our findings were highly statistically significant, and the application of corrections for multiple testing would not have affected the critical findings, even if a threshold of P=0.001 and Bonferroni corrections have been used. Finally, edoxaban is not reimbursed in France for treatment of NVAF and therefore could not be included in the present analysis.
Conclusions
In NAXOS, apixaban-treated patients had a lower risk of major bleeding events leading to hospitalization, lower risk of stroke and STEs, and lower risk of all-cause mortality compared with patients receiving VKAs. NAXOS also suggests comparable effectiveness for all 3 DOACs (apixaban, rivaroxaban, and dabigatran), with superior safety of apixaban versus rivaroxaban, and no difference in mortality between agents. Given the lack of randomized comparisons between DOACs, these observations are of interest to patients, clinicians, regulators, and payers.
Acknowledgments
All authors critically reviewed the manuscript. Drs Van Ganse and Steg provided supervision, conceived and designed the study, interpreted the data, and drafted the manuscript. Drs Danchin, Mahé, Hanon, and Falissard were members of the Scientific Committee. F. Jacoud and M. Nolin performed the statistical analysis. F. Dalon, Dr Lefevre, Dr Cotte, Dr Gollety, and Dr Belhassen contributed to study design and interpretation of data. Dr Van Ganse is the guarantor of the study. We thank Georgii Filatov of Springer Nature, who conducted an English language edit of this manuscript.
Sources of Funding
This work was supported by Bristol-Myers Squibb/Pfizer. The study was designed, conducted and written by the investigators and the academic group at PELyon. PELyon performed statistical analyses independently from the sponsor.
Disclosures
Dr Van Ganse reports personal fees from PELyon, outside the submitted work. Dr Danchin reports personal fees and nonfinancial support from BMS, personal fees and nonfinancial support from Bayer, personal fees and nonfinancial support from Pfizer during the conduct of the study, personal fees and nonfinancial support from Amgen, personal fees and nonfinancial support from AstraZeneca, personal fees from Boehringer Ingelheim, personal fees from Intercept, personal fees from MSD, personal fees from Servier, personal fees from Sanofi, and personal fees from Novo Nordisk outside the submitted work. Dr Mahé reports grants and personal fees from BMS/Pfizer, during the conduct of the study; personal fees from Daiichi Sankyo, BMS, Pfizer, and Bayer; and grants and personal fees from Leo Pharma, outside the submitted work. Dr Hanon reports personal fees from Pfizer, personal fees from BMS, grants and personal fees from Bayer, personal fees from Boehringer, personal fees from Boston scientific, personal fees from Leo Pharma, and personal fees from Aspen during the conduct of the study, personal fees from Servier, personal fees from AstraZeneca, personal fees from Bouchara Recordati, personal fees from Vifor, personal fees from Novartis, and personal fees from HAC Pharma outside the submitted work. F. Jacoud, M. Nolin, F. Dalon, and Dr Belhassen are employees of PELyon. Drs Lefevre, Cotte, and Gollety are employees of Bristol-Myers Squibb. Dr Falissard reports personal fees and nonfinancial support from BMS-Pfizer, during the conduct of the study; and personal fees from Eli Lilly, BMS, Servier, Sanofi, GSK, HRA, Roche, Boehringer Ingelheim, Bayer, Almirall, Allergan, Stallergenes, Genzyme, Pierre Fabre, AstraZeneca, Novartis, Janssen, Astellas, Biotronik, Daiichi Sankyo, Gilead, MSD, Lundbeck, Actelion, UCB, Otsuka, Grunenthal, and ViiV, outside the submitted work. Dr Steg reports personal fees from BMS/Pfizer during the conduct of the study; grants and personal fees from Amarin; personal fees from Amgen; grants and personal fees from Bayer; personal fees from Boehringer Ingelheim; personal fees from AstraZeneca; personal fees from Idorsia; personal fees from Novartis; personal fees from Pfizer; grants, personal fees, and nonfinancial support from Sanofi/Regeneron; and grants and personal fees from Servier outside the submitted work; and he is an inventor on a patent on alirocumab use after acute coronary syndrome to reduce cardiovascular risk (all royalties assigned to Sanofi).
Supplementary Material
Footnotes
This manuscript was sent to Michael Brainin, MD, Dr (hon), for review by expert referees, editorial decision, and final disposition.
Presented in part at the European Society of Cardiology Congress, Paris, France, August 31 to September 4, 2019.
For Sources of Funding and Disclosures, see page 2074.
The Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/STROKEAHA.120.028825.
References
- 1.Tendera M, Syzdół M, Parma Z. ARISTOTLE RE-LYs on the ROCKET. What’s new in stroke prevention in patients with atrial fibrillation? Cardiol J. 2012;19:4–10. doi: 10.5603/cj.2012.0002. doi: 10.5603/cj.2012.0002. [DOI] [PubMed] [Google Scholar]
- 2.Desai J, Kolb JM, Weitz JI, Aisenberg J. Gastrointestinal bleeding with the new oral anticoagulants–defining the issues and the management strategies. Thromb Haemost. 2013;110:205–212. doi: 10.1160/TH13-02-0150. doi: 10.1160/TH13-02-0150. [DOI] [PubMed] [Google Scholar]
- 3.Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, Camm AJ, Weitz JI, Lewis BS, Parkhomenko A, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955–962. doi: 10.1016/S0140-6736(13)62343-0. doi: 10.1016/S0140-6736(13)62343-0. [DOI] [PubMed] [Google Scholar]
- 4.Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al-Khalidi HR, Ansell J, Atar D, Avezum A, et al. ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. doi: 10.1056/NEJMoa1107039. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 5.Tuppin P, Rudant J, Constantinou P, Gastaldi-Ménager C, Rachas A, de Roquefeuil L, Maura G, Caillol H, Tajahmady A, Coste J, et al. Value of a national administrative database to guide public decisions: From the système national d’information interrégimes de l’Assurance Maladie (SNIIRAM) to the système national des données de santé (SNDS) in France. Rev Epidemiol Sante Publique. 2017;65(suppl 4):S149–S167. doi: 10.1016/j.respe.2017.05.004. doi: 10.1016/j.respe.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 6.Picard F, Van Ganse E, Ducrocq G, Danchin N, Falissard B, Hanon O, Belhassen M, Ginoux M, Lefevre C, Cotte F-E, et al. EvaluatioN of ApiXaban in strOke and systemic embolism prevention in patients with non-valvular atrial fibrillation in clinical practice Setting in France, rationale and design of the NAXOS: SNIIRAM study. Clin Cardiol. 2019;42:851–859. doi: 10.1002/clc.23231. doi: 10.1002/clc.23231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouillon K, Bertrand M, Maura G, Blotière PO, Ricordeau P, Zureik M. Risk of bleeding and arterial thromboembolism in patients with non-valvular atrial fibrillation either maintained on a vitamin K antagonist or switched to a non-vitamin K-antagonist oral anticoagulant: a retrospective, matched-cohort study. Lancet Haematol. 2015;2:e150–e159. doi: 10.1016/S2352-3026(15)00027-7. doi: 10.1016/S2352-3026(15)00027-7. [DOI] [PubMed] [Google Scholar]
- 8.Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH, Hindricks G, Kirchhof P ESC Committee for Practice Guidelines (CPG) 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012;33:2719–2747. doi: 10.1093/eurheartj/ehs253. doi: 10.1093/eurheartj/ehs253. [DOI] [PubMed] [Google Scholar]
- 9.Friberg L, Rosenqvist M, Lip GY. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182 678 patients with atrial fibrillation: the Swedish Atrial Fibrillation cohort study. Eur Heart J. 2012;33:1500–1510. doi: 10.1093/eurheartj/ehr488. doi: 10.1093/eurheartj/ehr488. [DOI] [PubMed] [Google Scholar]
- 10.Garrido MM, Kelley AS, Paris J, Roza K, Meier DE, Morrison RS, Aldridge M. Methods for constructing and assessing propensity scores. Health Serv Res. 2014;49:1701–1720. doi: 10.1111/1475-6773.12182. doi: 10.1111/1475-6773.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46:399–424. doi: 10.1080/00273171.2011.568786. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Billionnet C, Alla F, Bérigaud É, Pariente A, Maura G. Identifying atrial fibrillation in outpatients initiating oral anticoagulants based on medico-administrative data: results from the French national healthcare databases. Pharmacoepidemiol Drug Saf. 2017;26:535–543. doi: 10.1002/pds.4192. doi: 10.1002/pds.4192. [DOI] [PubMed] [Google Scholar]
- 13.Moore N, Blin P, Dureau C, Cottin Y, Mismetti P, Lassalle R, Abouelfath A, Benichou J, Droz C. Effectiveness and safety of direct oral anticoagulants compared with vitamin-k antagonists: initial results from a cohort study in the nationwide french claims and hospitalization database (sniiram). J Am Coll Cardiol. 2017;69:307. [Google Scholar]
- 14.Blin P, de Pouvourville G, Cottin Y, Dureau-Pournin C, Abouelfath A, Lassalle R, Bénichou J, Droz-Perroteau C, Mismetti P, Moore N. Comparative effectiveness and medical cost of dabigatran versus vitamin k antagonists from engel 2: a french nationwide cohort of 100,000 patients with non-valvular atrial fibrillation. Value Health. 2017;20:A608–A609. [Google Scholar]
- 15.Falissard B, Picard F, Mahe I, Hanon O, Touzé E, Danchin N, Lamy F-X, Ricci L, Steg P. Apixaban for prevention of stroke and systemic embolism in patients with non-valvular atrial fibrillation in France: the PAROS cross-sectional study of routine clinical practice. Arch Cardiovasc Dis. 2019;112:400–409. doi: 10.1016/j.acvd.2019.02.003. doi: 10.1016/j.acvd.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 16.Booth CM, Tannock IF. Randomised controlled trials and population-based observational research: partners in the evolution of medical evidence. Br J Cancer. 2014;110:551–555. doi: 10.1038/bjc.2013.725. doi: 10.1038/bjc.2013.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steg PG, López-Sendón J, Lopez de Sa E, Goodman SG, Gore JM, Anderson FA, Jr, Himbert D, Allegrone J, Van de Werf F GRACE Investigators. External validity of clinical trials in acute myocardial infarction. Arch Intern Med. 2007;167:68–73. doi: 10.1001/archinte.167.1.68. doi: 10.1001/archinte.167.1.68. [DOI] [PubMed] [Google Scholar]
- 18.Rothwell PM. External validity of randomised controlled trials: “to whom do the results of this trial apply?”. Lancet. 2005;365:82–93. doi: 10.1016/S0140-6736(04)17670-8. doi: 10.1016/S0140-6736(04)17670-8. [DOI] [PubMed] [Google Scholar]
- 19.Mahé I, Bal dit Sollier C, Duru G, Lamarque H, Bergmann JF, Drouet L. [Use and monitoring of vitamin K antagonists in everyday medical practice. French results of the international ISAM study of patients with nonvalvular atrial fibrillation]. Presse Med. 2006;35(12 pt 1):1797–1803. doi: 10.1016/s0755-4982(06)74904-1. doi: 10.1016/s0755-4982(06)74904-1. [DOI] [PubMed] [Google Scholar]
- 20.Cotté FE, Benhaddi H, Duprat-Lomon I, Doble A, Marchant N, Letierce A, Huguet M. Vitamin K antagonist treatment in patients with atrial fibrillation and time in therapeutic range in four European countries. Clin Ther. 2014;36:1160–1168. doi: 10.1016/j.clinthera.2014.07.016. doi: 10.1016/j.clinthera.2014.07.016. [DOI] [PubMed] [Google Scholar]
- 21.Lip GY, Keshishian A, Kamble S, Pan X, Mardekian J, Horblyuk R, Hamilton M. Real-world comparison of major bleeding risk among non-valvular atrial fibrillation patients initiated on apixaban, dabigatran, rivaroxaban, or warfarin. A propensity score matched analysis. Thromb Haemost. 2016;116:975–986. doi: 10.1160/TH16-05-0403. doi: 10.1160/TH16-05-0403. [DOI] [PubMed] [Google Scholar]
- 22.Yao X, Abraham NS, Sangaralingham LR, Bellolio MF, McBane RD, Shah ND, Noseworthy P. Effectiveness and safety of dabigatran, rivaroxaban, and apixaban versus warfarin in nonvalvular atrial fibrillation. J Am Heart Assoc. 2016;5:e003725. doi: 10.1161/JAHA.116.003725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lip GYH, Keshishian A, Li X, Hamilton M, Masseria C, Gupta K, Luo X, Mardekian J, Friend K, Nadkarni A, et al. Effectiveness and safety of oral anticoagulants among nonvalvular atrial fibrillation patients. Stroke. 2018;49:2933–2944. doi: 10.1161/STROKEAHA.118.020232. doi: 10.1161/STROKEAHA.118.020232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vinogradova Y, Coupland C, Hill T, Hippisley-Cox J. Risks and benefits of direct oral anticoagulants versus warfarin in a real world setting: cohort study in primary care. BMJ. 2018;362:k2505. doi: 10.1136/bmj.k2505. doi: 10.1136/bmj.k2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Staerk L, Fosbøl EL, Lip GYH, Lamberts M, Bonde AN, Torp-Pedersen C, Ozenne B, Gerds TA, Gislason GH, Olesen JB, et al. Ischaemic and haemorrhagic stroke associated with non-vitamin K antagonist oral anticoagulants and warfarin use in patients with atrial fibrillation: a nationwide cohort study. Eur Heart J. 2017;38:907–915. doi: 10.1093/eurheartj/ehw496. doi: 10.1093/eurheartj/ehw496. [DOI] [PubMed] [Google Scholar]
- 26.Graham DJ, Baro E, Zhang R, Liao J, Wernecke M, Reichman ME, Hu M, Illoh O, Wei Y, Goulding MR, et al. Comparative stroke, bleeding, and mortality risks in older medicare patients treated with oral anticoagulants for nonvalvular atrial fibrillation. Am J Med. 2019;132:596 e511–604 e511. doi: 10.1016/j.amjmed.2018.12.023. [DOI] [PubMed] [Google Scholar]
- 27.Noseworthy PA, Yao X, Abraham NS, Sangaralingham LR, McBane RD, Shah ND. Direct comparison of dabigatran, rivaroxaban, and apixaban for effectiveness and safety in nonvalvular atrial fibrillation. Chest. 2016;150:1302–1312. doi: 10.1016/j.chest.2016.07.013. doi: 10.1016/j.chest.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 28.Larsen TB, Skjøth F, Nielsen PB, Kjældgaard JN, Lip GY. Comparative effectiveness and safety of non-vitamin K antagonist oral anticoagulants and warfarin in patients with atrial fibrillation: propensity weighted nationwide cohort study. BMJ. 2016;353:i3189. doi: 10.1136/bmj.i3189. doi: 10.1136/bmj.i3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nielsen PB, Skjøth F, Søgaard M, Kjældgaard JN, Lip GY, Larsen TB. Effectiveness and safety of reduced dose non-vitamin K antagonist oral anticoagulants and warfarin in patients with atrial fibrillation: propensity weighted nationwide cohort study. BMJ. 2017;356:j510. doi: 10.1136/bmj.j510. doi: 10.1136/bmj.j510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Staerk L, Gerds TA, Lip GYH, Ozenne B, Bonde AN, Lamberts M, Fosbøl EL, Torp-Pedersen C, Gislason GH, Olesen JB, et al. Standard and reduced doses of dabigatran, rivaroxaban and apixaban for stroke prevention in atrial fibrillation: a nationwide cohort study. J Intern Med. 2018;283:45–55. doi: 10.1111/joim.12683. doi: 10.1111/joim.12683. [DOI] [PubMed] [Google Scholar]
- 31.Graham DJ, Reichman ME, Wernecke M, Hsueh YH, Izem R, Southworth MR, Wei Y, Liao J, Goulding MR, Mott K, et al. Stroke, bleeding, and mortality risks in elderly Medicare beneficiaries treated with dabigatran or rivaroxaban for nonvalvular atrial fibrillation. JAMA Intern Med. 2016;176:1662–1671. doi: 10.1001/jamainternmed.2016.5954. doi: 10.1001/jamainternmed.2016.5954. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.