Abstract
Background:
Transcranial magnetic stimulation (TMS) can be paired with functional magnetic resonance imaging (fMRI) in concurrent TMS-fMRI experiments. These multimodal experiments enable causal probing of network architecture in the human brain which can complement alternative network mapping approaches. Critically, merely introducing the TMS coil into the scanner environment can sometimes produce substantial magnetic field inhomogeneities and spatial distortions which limit the utility of concurrent TMS-fMRI.
Method and results:
We assessed the efficacy of point spread function corrected echo planar imaging (PSF-EPI) in correcting for the field inhomogeneities associated with a TMS coil at 3 T. In phantom and brain scans, we quantitatively compared the coil-induced distortion artifacts measured in EPI scans with and without PSF correction. We found that the application of PSF corrections to the EPI data significantly improved signal-to-noise and reduced distortions. In phantom scans with the PSF-EPI sequence, we also characterized the temporal profile of dynamic artifacts associated with TMS delivery and found that image quality remained high as long as the TMS pulse preceded the RF excitation pulses by at least 50 ms. Lastly, we validated the PSF-EPI sequence in human brain scans involving TMS and motor behavior as well as resting state fMRI scans.
Conclusions:
Our collective results demonstrate the potential benefits of PSF-EPI for concurrent TMS-fMRI when coil-related artifacts are a concern. The ability to collect high quality resting state fMRI data in the same session as the concurrent TMS-fMRI experiment offers a unique opportunity to interrogate network architecture in the human brain.
Keywords: Point spread function, Transcranial magnetic stimulation, Functional magnetic resonance imaging, Echo planar imaging, Metal-induced distortion, Networks
1. Introduction
Transcranial magnetic stimulation (TMS) is a non-invasive approach for manipulating human brain activity (Hallett, 2007). Because TMS-induced activity in a targeted region can lead to activity changes in remote but connected regions, combining TMS with functional magnetic resonance imaging (fMRI) enables the characterization of functional and distributed cortical networks through the causal manipulation of human brain activity (Baudewig et al., 2000; Bestmann et al., 2004; Bestmann and Feredoes, 2013; Blankenburg et al., 2010; Hanakawa et al., 2009; Leitão et al., 2017, 2015; Peters et al., 2012; Ruff et al., 2007, 2006; Shastri et al., 1999). A major advantage of concurrent TMS-fMRI over other network mapping approaches like resting state fMRI and diffusion tensor imaging is the ability to characterize state-dependent changes in network architecture (Bestmann et al., 2005; Blankenburg et al., 2008; Feredoes et al., 2011; Leitão et al., 2015, 2012; Rahnev et al., 2016; Ruff et al., 2006; Sack et al., 2007, 2006).
Historically, a major challenge with concurrent TMS-fMRI is magnetic field inhomogeneity, generated by the introduction of the TMS coil into the MRI environment, which produces spatial distortions and signal loss in the acquired images. Importantly, the presence and magnitude of coil-related artifacts may differ dramatically depending on the particular hardware used in the experiments. When coil-related artifacts are present, the spatial distortions in the images depend on the orientation of image slices with respect to the orientation of the TMS coil (Baudewig et al., 2000; Bestmann et al., 2003a): Substantially greater geometric distortions and ghost artifacts are present when the frequency-encoding gradient is perpendicular to the TMS coil plane as compared to when the frequency-encoding direction and slice orientation are parallel to the TMS coil. Accordingly, the parallel alignment scheme has been used as a common strategy to reduce coil-related artifacts in concurrent TMS-fMRI experiments (Bestmann et al., 2003a; Bungert et al., 2012; de Lara et al., 2017; Moisa et al., 2009; Navarro de Lara et al., 2015; Weiskopf et al., 2009). However, this strategy has limitations. First, there is no automated and reliable method for optimally aligning functional prescriptions with the TMS coil even with the use of vitamin capsules (Bestmann et al., 2006; Sack et al., 2007) or water-filled tubes (Bestmann et al., 2003a; de Weijer et al., 2014; Leitão et al., 2012) to visualize the TMS coil. Moreover, though using a parallel alignment can improve signal quality over the brain generally, this strategy may actually cause a greater signal reduction in specific brain regions. For instance, a consequence of aligning the slice prescriptions to the TMS coil plane can be an exacerbation of the signal loss that is already prominent in areas such as the orbitofrontal cortices (Moisa et al., 2009; Weiskopf et al., 2006). Lastly, susceptibility artifacts may persist even after achieving parallel alignment between the frequencyencoding direction and the TMS coil.
When using MRI-compatible TMS systems that produce artifacts, alternative hardware-based strategies have also been employed to treat the static artifacts and signal loss associated with the introduction of the TMS coil to the scanner environment. A relay-diode combination can be effective in reducing image artifacts caused by leakage currents (Weiskopf et al., 2009). Susceptibility artifacts can also be reduced through passive shimming (Bungert et al., 2012). Furthermore, customized radiofrequency coil arrays may be used to improve MR sensitivity (de Lara et al., 2017; Navarro de Lara et al., 2015). These strategies require specialized hardware which may not be generally available.
While much of the efforts to improve data quality in concurrent TMS-fMRI experiments have focused on procedural strategies or hardware innovations, there have been limited efforts to address TMS-induced static field artifacts using specialized pulse sequences, though such sequences have been developed as general solutions to correct for susceptibility artifacts. Point spread function (PSF) corrected echo planar imaging (PSF-EPI) has been used to minimize susceptibility artifacts and distortion, which is a critical problem particularly with ultrahigh field imaging (Chung et al., 2011; In et al., 2015, 2017b; In and Speck, 2012; In et al., 2016; Loureiro et al., 2017; Oh et al., 2012; Robson et al., 1997; Zaitsev et al., 2004; Zeng and Constable, 2002). In brief, PSF mapping is based on the combination of EPI phase-encoding gradients (which are vulnerable to distortions) with spin-warp phaseencoding gradients (which are geometrically accurate). PSF mapping provides a summary of the EPI distortions – caused by B0 field inhomogeneity, susceptibility, chemical shift, or eddy currents – which can then be corrected. In addition to its use in correcting for image stretching and compression at ultra-high fields, PSF-EPI has also been used in fMRI scans with deep brain stimulation devices to minimize the metal-induced distortion near the implants (In et al., 2017a).
Here, we tested the utility of PSF-EPI for concurrent TMS-fMRI performed at 3 T. In phantom and brain scans, we measured and compared the signal distortion and loss associated with the mere introduction of a TMS coil into the scanner environment in EPI data evaluated with and without PSF-correction. In addition to characterizing the static artifacts related to the presence of the TMS coil, we also characterized the acute artifacts associated with delivery of TMS pulses before and during image acquisition. We validated the use of PSF-EPI for concurrent TMS-fMRI in an in vivo experiment by quantifying fMRI BOLD signal changes associated with TMS application and the performance of a motor task. Lastly, we acquired resting state fMRI datasets using the PSF-EPI sequence and characterized functional connectivity patterns with the TMS coil positioned over different regions. These collective experiments enabled us to establish the utility of the PSF-EPI sequence for concurrent TMS-fMRI experiments in which coil-related artifacts impact image quality. Our finding that high quality resting state fMRI data can be acquired along with concurrent TMS-fMRI data using the same experimental setup establishes the feasibility of relating these complementary approaches for characterizing network architecture in the human brain.
2. Materials and methods
2.1. General overview
We performed 5 separate experiments. Experiment 1 was aimed at characterizing the static spatial artifacts in phantom scans generated by the introduction of a TMS coil into the scanner in the absence TMS coil discharging in EPI scans with and without PSF-correction. Experiment 2 was aimed at characterizing the same static artifacts in EPI human brain scans with and without PSF-correction. Experiment 3 was aimed at characterizing the acute artifacts in images acquired with PSF-EPI associated with delivery of a single TMS pulse at different intensities and times relative to image acquisition at multiple coil orientations. Experiment 4 was aimed at validating PSF-EPI for concurrent TMS-fMRI by characterizing BOLD signal changes associated with the performance of a cued motor task and TMS application over sensorimotor cortex. Experiment 5 was aimed at characterizing resting state functional connectivity (RSFC) using the PSF-EPI sequence in the presence of the TMS coil to test whether consistent connectivity patterns could be attained with the coil positioned over different brain regions.
2.2. Magnetic resonance imaging
Functional and structural imaging data were acquired on a Siemens MAGNETOM Trio 3 T scanner (Siemens Medical Solutions, Erlangen, Germany) using the posterior elements (6 channels) of the 12-channel head matrix coil (part #08622644) and the flexible 6-channel body matrix coil (part #08622651). Note that this particular combination and configuration of coil arrays may be differentially vulnerable to TMS-related artifacts compared to alternative rigid or flexible head coils and depending on the specific TMS system used. This issue is beyond the scope of our current study, as we focused on characterizing the potential benefits of PSF-correction of EPI data for a particular setup; however, the topic of imaging coil designs for concurrent TMS-fMRI has been extensively considered previously (Navarro de Lara et al., 2015). In all experiments, we first performed a localizer scan (1.1 × 1×7 mm3, TR/TE =8.6/4 ms, field of view (FOV) = 250 mm, flip angle =20°, 1 slice, readout bandwidth =320 Hz/pixel). We then acquired functional data using a PSF-EPI sequence (Loureiro et al., 2017) (3-mm isotropic voxels, TR/TE =3000/30 ms, FOV = 210 mm, flip angle =84°, 46 slices, readout bandwidth =1374 Hz/pixel, PAT mode: GRAPPA, parallel imaging acceleration factor= 2). Critically, because uncorrected EPI data are retained, we can directly evaluate how PSF-correction impacts image quality metrics defined for the same EPI data with and without correction. This ensures that differences observed between the uncorrected and corrected images cannot be attributed to subtle differences in image reconstruction which could conceivably result in substantial signal differences. We additionally acquired two separate 3D gradient echo (GRE) images (2.4-mm isotropic voxels, TR =488 ms, TE =4.92 and 7.38 ms, FOV = 230 mm, flip angle = 40°, 45 slices, readout bandwidth =301Hz/pixel) and used these data to generate field maps using AFNI (Cox, 1996; Cox and Jesmanowicz, 1999) and FSL (Jenkinson et al., 2012). The field maps (Fig. 1) reveal clear susceptibility distortion in the B0 field in the vicinity of the TMS coil. We also acquired T1-weighted structural images using a 3D fast low angle shot (FLASH) sequence (1.6 × 1.6 × 3 mm3, TR/TE = 6.62/1.85 ms, FOV =210 mm, flip angle = 15°, 48 slices, readout bandwidth =130Hz/pixel, parallel imaging acceleration factor =2). Matching the prescriptions of the 3D FLASH scans to the functional scans enabled a direct comparison between the EPI data that are vulnerable to field inhomogeneities to T1-weighted images that are not. In sessions involving brain scans, we also acquired structural images using a magnetization prepared rapid gradient echo (MPRAGE) sequence (1-mm isotropic voxel resolution, TR/TE = 2600/3.02 ms, FOV = 256 mm, flip angle =8°, 176 slices, readout bandwidth =130 Hz/pixel, parallel imaging acceleration factor = 2). These structural data were used for estimating the TMS target site, data visualization, and co-registration with the functional data from the concurrent TMS-fMRI and resting state fMRI experiments.
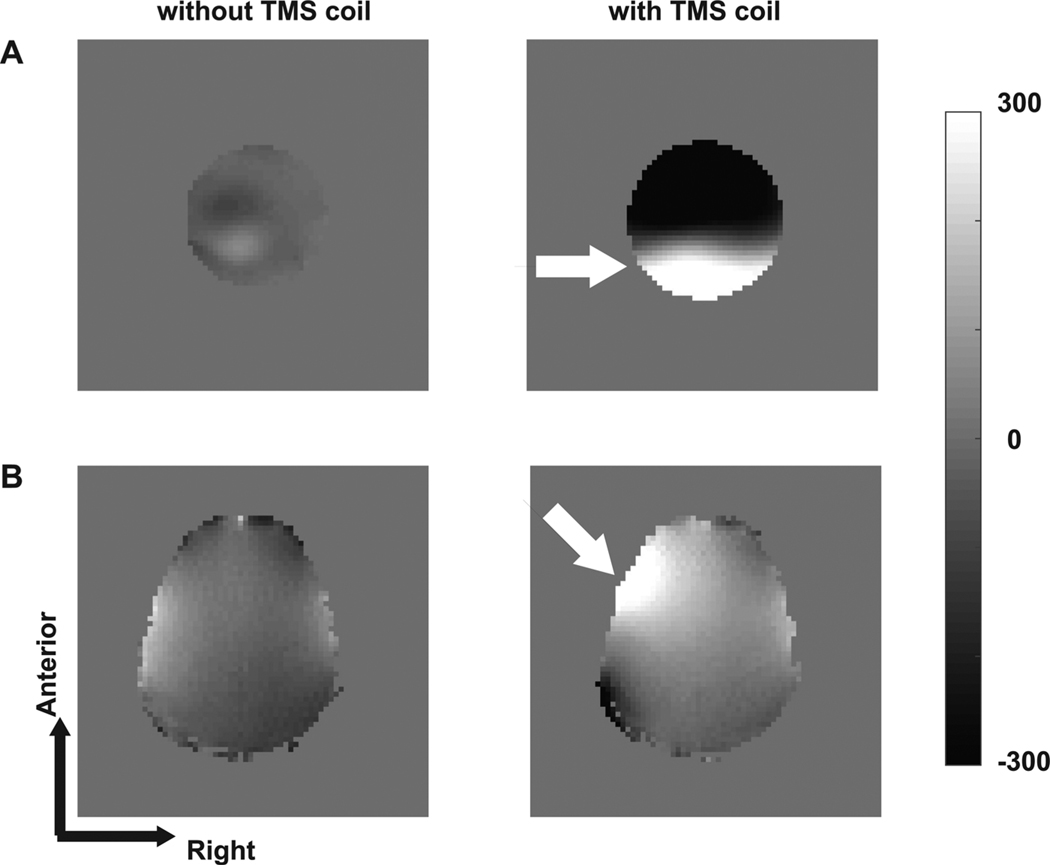
Fig. 1.
(A) Field map of a spherical phantom without and with the TMS coil. (B) Field map of brain without and with the TMS coil. The field maps were calculated in units of rad/s. The TMS coil (white arrow) was positioned superior to the phantom with its plane aligned to the transverse imaging plane and over the left sensorimotor cortex.
2.3. Transcranial magnetic stimulation
A MRI-compatible figure-of-eight coil (Magstim Co. UK; inner diameter: 42 mm; outer diameter: 80 mm; number of turns: 10; cable length: 5.75 m to the filter box) was connected to 2 Magstim Rapid2 stimulator units located outside the MRI suite via a 2-to-1 filter adaptor (Magstim Co. UK). This system was used to deliver biphasic TMS pulses. A filter box and ferrite sleeves served to attenuate radio frequency (RF) noise. The filter box (Magstim Co. UK) contains a shielded inductive filter to help prevent spurious RF interference from entering the MRI room via the stimulating coil cable. Such RF interference can come from the Magstim unit or from the antenna effect of the long cable. In addition the filter box contains a relay that can be used to short any direct current coming from the Magstim equipment itself. A custom-built platform and coil holder secured the TMS coil to the scanner bed. This platform also supported the body matrix coil that provided anterior coverage of the phantom and brain.
The TMS coil was labeled by four microcentrifuge tubes (0.5 ml) filled with 1 part Gadolinium (Gd; 1:300) and 1 part 0.9% sodium chloride solution. These Gd-filled tubes were highly visible markers that defined the position and orientation of the TMS coil in the localizer scans and in the T1-weighted scans. In Experiment 4, we localized the TMS coil and its position and orientation with respect to the brain in the T1-weighted images using a pipeline, analogous to our previously described method (Yau et al., 2013), implemented in AFNI and custom Matlab scripts (R2017a. The MathWorks, Natick, MA, USA). This procedure enables the identification of the cortical region falling immediately beneath the center of the TMS coil (Yau et al., 2013).
TMS timing was controlled by Psychtoolbox-3 (Kleiner et al., 2007) in MATLAB (2011b, MathWorks) running on a MacBook Pro (model A1278; OS X 10.9.5, 2.5 GHz Core i5, 4 GB of RAM). TMS was triggered by TTL pulses sent via a DAQ device (model USB-1208FS, Measurement Computing Corporation).
2.4. Experiment 1. Static artifact characterization in phantom scans
In phantom scans (agarose gel 2% phantom; 48-mm circumference) acquired using PSF-EPI, we quantified signal loss and image distortion artifacts induced by the TMS coil in the absence of discharging in data with and without PSF-correction. We scanned the phantom with and without the TMS coil. Each scan comprised 100 volumes. When present, the TMS coil was positioned superior to the phantom with its plane aligned to the transverse imaging plane. To establish the dependence of the static artifacts on the relative orientation of the image slices and the TMS coil, in separate scans we prescribed functional slices that were either parallel or oblique to the TMS coil surface as guided by the Gd-filled markers (Fig. 2A). Functional data were acquired in transverse planes with the phase encoding gradients in a right-left direction. For the parallel prescription, the orientation of the image slices was aligned parallel to the TMS coil surface. For the oblique prescription, the slices were tilted approximately 15° from the parallel prescription in both the sagittal and coronal planes.
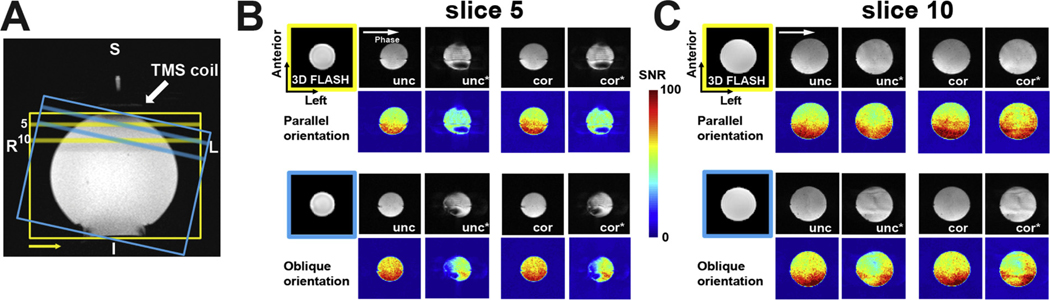
Fig. 2.
(A) Phantom measurement setup. EPI data acquired with either parallel (yellow) or oblique (blue) prescriptions with respect to the TMS coil surface. Slice prescriptions are guided by Gadolinium-filled markers labeling the TMS coil which are visible in a set of three-plane localizer scans. The phase encoding direction was a right-left direction (marked with the yellow arrow). The 5th and 10th slices in the image stacks are indicated by the bars. (B) Comparisons of the uncorrected EPI (unc) and PSF-corrected EPI (cor) data and tSNR maps calculated for the 5th slice. The asterisks indicate data acquired in the presence of the TMS coil. Data acquired in the parallel orientation (top 2 rows) and oblique orientation (bottom 2 rows) reveal the dependence of image quality on acquisition orientation. Structural images acquired using the 3D FLASH sequence are undistorted by the TMS coil. (C) Comparisons of the 10th slice. Conventions as in B. R = right, L =left, S = superior, I = inferior, Phase =phase-encoding direction.
2.5. Experiment 2. Static artifact characterization in brain scans
In brain scans acquired using PSF-EPI, we quantified signal loss and image distortion artifacts induced by the TMS coil in the absence of TMS discharging in data with and without PSF-correction. Images were only acquired with the TMS coil positioned over left sensorimotor cortex (Talairach: −51, −5, 42) (Fig. 4A). In this experiment, we did not manipulate the slice prescriptions with respect to the TMS coil: Slice acquisition orientation was maintained in the transverse plane. Each dataset comprised 100 volumes.
Fig. 4.
Comparison of raw images acquired in the presence of the TMS coil. (A) Left: Volume rendering depicting the position of the TMS coil with respect to the scalp. Right: Slice prescriptions used for PSF-EPI scans. (B) PSF-corrected (Cor) and uncorrected (Unc) EPI slices in the presence of the TMS coil (white arrow). The difference images reveal distortion and ghosting artifacts in slices in close proximity to the TMS coil. L = left, R = right, A =anterior, P =posterior.
2.6. Experiment 3. Characterization of acute artifacts associated with TMS discharge
Eddy currents produced by TMS discharge interfere with subsequent image acquisition by causing signal loss and distortion in specific spatial-temporal patterns (Shastri et al., 1999). A common strategy for avoiding these acute artifacts is to introduce a temporal gap between TMS discharge and subsequent RF-excitation and image acquisition (Bestmann et al., 2003b; Ruff et al., 2006). In Experiment 3, we wished to characterize the acute artifact induced by a single TMS pulse in functional data acquired using PSF-EPI to establish the temporal delay required after the TMS pulse to avoid image perturbation. Critically, although the PSF-EPI sequence we used had a nominal repetition time of 3000 ms, a single volume was acquired in 2750 ms thereby leaving a 250-ms gap between consecutive volume acquisitions during which TMS could be delivered. In separate scans on a phantom, we manipulated the time of a single TMS discharge during and after this 250-ms interval and quantified its impact on image acquisition. TMS was delivered −125, −100, −75, −50, 0, and +50 ms with respect to RF excitation and image acquisition. To characterize how acute discharge-related artifacts scaled with TMS intensity, we repeated the experiment at 2 TMS intensities, expressed as a percentage of maximum stimulator output (70% and 100%). Thus, we performed 12 scans in this experiment. Each scan began with an initial 4 TMS-free volumes. Subsequently, a TMS-containing “cycle” began: A volume was acquired without TMS, a single TMS pulse was delivered at the designated time, and 3 additional volumes were acquired. This cycle repeated 10 times over the scan yielding a total of 44 volumes per TMS condition. We tested each TMS condition with the coil positioned superior or lateral to the phantom (i.e., with the TMS coil pointing in a direction aligned with or perpendicular to the B0 field, respectively). The slices were acquired parallel to the plane of the TMS coil in each scan.
2.7. Experiment 4. Concurrent TMS-fMRI with PSF-EPI
We performed a set of concurrent TMS-fMRI scans to validate the use of the PSF-EPI sequence for measuring BOLD signal changes related to acute TMS and the performance of a motor behavior. A right-handed male (20 years old) participated in the experiment after giving the written informed consent. All procedures were approved by the Institutional Review Board for human subject research at the Baylor College of Medicine. The participant reported no history of neurological disorders or impaired sensorimotor function. Head movement was limited by foam padding and the subject wore earplugs and noise-at-tenuating earmuffs to minimize noise from the scanner and the click sounds associated with TMS discharge. The TMS coil was positioned over the left sensorimotor cortex (x = −51, y = −5, z =42), and slice acquisition orientation was maintained in the transverse plane.
The primary goals of this experiment were to verify that robust BOLD signal changes could be measured in the vicinity of the TMS coil and in regions remote from the coil rather than to characterize activation patterns for the purpose of inferring network architecture. We used a block design paradigm to characterize BOLD signal changes associated with TMS and bimanual finger tapping (Fig. 8A). In separate scans, blocks consisted of either low intensity TMS (TMSLow; 40% maximum stimulator output), high intensity TMS (TMSHigh; 50% maximum stimulator output), or a motor task performed concurrently with low intensity TMS (FT +TMSLow) (Bestmann et al., 2005; Yau et al., 2013). Note that these TMS intensities were both below the threshold required to produce visible muscle activation at rest, but subthreshold TMS can result in BOLD signal changes distributed over cortical and subcortical sensorimotor networks (Bestmann et al., 2005; Yau et al., 2013). The motor task involved bimanual finger-tapping: The participant touched his thumbs to each finger in sequence continuously over the duration of the block (9 s). Each block comprised 3 volumes and blocks were separated by 7 volumes during which the participant rested without receiving TMS. During each block, the subject received 3 bursts of TMS with each burst (3 pulses, 16 Hz, inter-pulse interval: 62.5 ms) triggered during the 250-ms gaps separating volume acquisitions. Each burst began at the onset of the inter-volume gap thereby providing 125 ms for the eddy currents to dissipate before acquisition of the subsequent volume. Each scan comprised 10 blocks (104 volumes, total scan time: 5:2 min). This scan began with 4 TMS-free volumes.
Fig. 8.
Concurrent TMS-fMRI scans. (A) In separate block-design scans, a subject received low-and high-intensity TMS bursts (40% and 50% maximum stimulator output, respectively; Materials and methods). In a separate scan, the subject also performed bimanual finger tapping when cued by low intensity TMS. TMS bursts (triplet; 16 Hz) were applied during a 250-ms gap separating consecutive volume acquisitions. (B) Activation pattern associated with bimanual finger tapping and low intensity TMS. BOLD signal time series from the left and right precentral gyri reveal robust signal changes associated with finger tapping. The temporal response profiles and response magnitudes in the left and right hemispheres were comparable, indicating that the TMS coil (positioned over the left hemisphere) did not substantially attenuate signal locally. (C) Activation patterns associated with low-and high-intensity TMS alone. The temporal response profiles were highly similar for the finger-tapping and TMS conditions. Error bars indicate SEM.
2.8. Experiment 5. Resting state fMRI with PSF-EPI
We performed a series of resting state fMRI scans using the PSF-EPI sequence in the presence of the TMS coil. The goal of this experiment was to verify that clear resting state correlation patterns were attainable in the presence of the TMS coil with the PSF-EPI sequence and that these patterns were invariant to the positioning of the TMS coil. In each of 3 sessions (inter-session interval: 6 days), we acquired fifteen continuous minutes of resting state fMRI data (300 volumes) in the one participant who also participated in Experiment 4. The TMS coil was positioned over different scalp locations in each session. In session 1, the TMS coil was over the left middle frontal gyrus (x = −44, y =3, z =54). In session 2, the coil was positioned over the right superior frontal gyrus (x =39, y = 26, z =49). In session 3, the coil was positioned over the left inferior parietal lobule (x = −49, y = −32, z =50). Slice acquisition orientation was maintained in the transverse plane irrespective of TMS coil position. The subject underwent each resting state fMRI experiment with eyes closed. No TMS pulses were triggered during these scans.
2.9. Data analysis
All data analyses were performed using AFNI and Matlab (R2018a).
2.9.1. Static artifacts in phantom data
We computed 2 metrics to compare the impact of TMS coil presence and slice prescription on the EPI phantom data with and without PSF-correction. As a measure of signal strength, we generated temporal SNR (tSNR) maps for each sequence under each condition. For each voxel, we computed:
where is the mean signal of the fMRI time series and is the standard deviation of the signal. To focus our analysis on the voxels contained within the phantom, we defined an analysis mask using the T1-weighted volume acquired with the 3D FLASH sequence: The mask, which served as a ground truth for the spatial extent of the phantom, comprised all voxels that exceeded an intensity threshold defined as 0.1 of the maximum signal in the T1-weighted volume. The mask was applied identically to the uncorrected and PSF-corrected datasets which we compared slice-wise. To compare the tSNR values computed for each sequence, we conducted a 3-way repeated-measures ANOVA with correction status, TMS coil presence, and prescription orientation as factors. We performed post-hoc comparisons, with Bonferroni-corrections, based on significant main and interaction effects.
Because the presence of the TMS coil causes spatial distortions as well as signal drop, we compared the T1-weighted 3D FLASH volume, which is not susceptible to the presence of the TMS coil, to the mean volumes acquired with PSF-EPI, with and without correction, to characterize the relative image distortions related to the TMS coil presence and slice prescriptions in the EPI data. Using the 3D FLASH volume as a reference, we computed structure similarity (SSIM) indices for each EPI sequence (Wang et al., 2004). SSIM is a reference metric that quantifies the quality of a comparison image with respect to a reference image as a weighted combination of comparisons between the images’ luminance, contrast, and structure. Because SSIM depends on luminance, we first normalized the reference (R) and comparison (C) volumes by each volume’s maximum intensity. For each slice in the EPI volumes, we then computed:
where μR is the average normalized intensity in the reference slice, μC is the average normalized intensity in the comparison slice, is the variance of the reference slice, is the variance of the comparison slice, σRC is the covariance of the reference and comparison slices, and K1 and K2 are stabilizing parameters fixed at 0.01 and 0.03, respectively. SSIM values range from 0 to 1 with larger values of SSIM indicating greater similarity between the reference and comparison data. To compare the SSIM values computed for each sequence, we conducted a 3-way repeated-measures ANOVA with correction status, TMS coil presence, and prescription orientation as factors. We performed post-hoc comparisons, with Bonferroni-corrections, based on significant main and interaction effects.
2.9.2. Static artifacts in brain data
To compare the impact of the TMS coil on resting EPI brain data with and without PSF-correction, we calculated and compared tSNR and SSIM using analogous analyses as those described for the phantom data. The tSNR analyses only comprised voxels contained within the skull as defined in the 3D FLASH data (threshold: greater than 0.1 of the maximum intensity). We visualized tSNR brain maps using previously described methods (Taylor et al., 2018). In the SSIM analyses, we first performed skull-stripping on only the 3D FLASH data (using the 3dSkullStrip function in AFNI) and then compared this reference volume to the EPI volumes without thresholding. Removing the skull from the 3D FLASH data better enabled us to account for spatial distortions in the EPI data that pushed the signals from the brain outside of the skull. We performed a paired-sample t-test to contrast tSNR and SSIM values achieved with PSF-corrected and uncorrected EPI data acquired only in the presence of the TMS coil and with a single prescription.
2.9.3. Acute artifacts in phantom data
To quantify acute artifacts in the PSF-EPI data related to TMS discharge, we compared volumes acquired during or subsequent to TMS delivery to a reference volume acquired before any TMS discharges. We used the 4th volume in the scan as the reference. Because magnetic fields produced by TMS decay over space and time, we computed the root mean square error (RMSE) between corresponding slices in the reference and comparison volumes for each TMS pulse time, as done previously (de Lara et al., 2017). Note that we excluded data from a single slice measurement from the analysis because its signal intensity differed from the mean of the other 9 slices acquired under identical conditions by ~33 times the standard deviation across the 9 slices. To test if TMS coinciding with RF-excitation results in altered signal intensities in subsequent acquisitions, as shown previously (Bestmann et al., 2003a), we also assessed potential signal changes in the 2nd and 3rd volumes acquired after TMS delivery.
2.9.4. Concurrent TMS-fMRI data
Data analysis for the concurrent TMS-fMRI experiment was performed in volume space using AFNI. As we have done previously (Pérez-Bellido et al., 2017), we performed data preprocessing including slice timing correction, motion correction, despiking, and coregistration with the structural data. The functional data were spatially smoothed by convolution with a 4-mm FWHM isotropic Gaussian kernel after spatial normalization into Talairach space. We analyzed the data by fitting a general linear model that included 3 regressors of interest corresponding to the TMSLow, TMSHigh, and FT + TMSLow blocks convolved with gamma-variate functions. Head motion parameters and drift parameters (linear, quadratic, cubic, and quartic) were included as nuisance regressors. To localize regions whose signals changed in association with finger-tapping and low-intensity TMS, statistical results for the FT + TMSLow >baseline contrast were thresholded at a false discovery rate (FDR) corrected q <0.05 over the whole brain. To localize regions within the sensorimotor system (established from the FT + TMSLow responses) whose signals changed in association with TMS, we contrasted TMSLow and TMSHigh against baseline (FDR q <0.05, masked by FT + TMSLow).
2.9.5. Resting state fMRI data
Data analysis for the resting state fMRI experiment was performed in surface space using AFNI. A surface model of the brain was created using FreeSurfer (Dale et al., 1999). We performed standard data preprocessing (slice timing correction, motion correction, and despiking) before projecting the data into surface space. A bandpass filter (0.01 0.1 Hz) was applied to reduce the effect of low-and high-frequency physiological noise (Bright and Murphy, 2015). The data were spatially smoothed by convolution with a 4-mm FWHM isotropic Gaussian kernel in surface space. For each session separately, the data in each hemisphere were sorted into 180 discrete parcels (Glasser et al., 2016). We calculated the mean time series in each parcel and computed the correlation between the mean time series in each pair of parcels to generate a resting state functional connectivity (RSFC) matrix for each session.
As a first step in determining the overall similarity of the RSFC patterns across sessions, we calculated the pairwise correlations between the upper triangle of the RSFC matrices generated for the sessions (Gordon et al., 2017). To determine whether the TMS coil influenced RSFC patterns specifically in regions near the coil, we computed the RSFC profile for the parcel identified as each session’s TMS target site (T) and compared these RSFC profiles across sessions (S). For instance, we identified the parcel corresponding to the session 1 TMS target (i.e., the left middle frontal gyrus) and computed the RSFC strength between this parcel and all parcels across the two hemispheres for the session 1 data to define a vector of 360 correlation values, . We then calculated the RSFC profiles for the same parcel using the session 2 and session 3 data to define and . If the TMS coil distorted the resting state data locally near the coil, we might expect that the RSFC profiles for the target parcel T would differ across sessions in which the coil was moved. To quantify RSFC profile similarity for the same parcel across sessions, we calculated the pairwise correlations between and averaged the correlation values for a single similarity metric, . We repeated this procedure for the parcels identified as targets for sessions 2 and 3 to calculate and , respectively. We then compared these RSFC profile similarity metrics to the “null” distribution of similarity metrics for all other parcels (N = 357) that were not associated with the TMS coil positions. We reasoned that would differ from the null distribution if the TMS coil substantially influenced RSFC profiles for parcels centered beneath the coil.
We separately tested whether the coil impacted RSFC magnitude because the RSFC profile similarity metric only describes the consistency of the relative patterns of RSFC. Conceivably, RSFC strength between parcel pairs could be impacted specifically for parcels near the TMS coil. To test this possibility, for each TMS target parcel (T), we computed the summed Euclidean distances between each session’s RSFC profile () and the average RSFC profile over the sessions for a single error metric, , that summarizes the connectivity strength differences for each parcel over the sessions. We then calculated analogous error metrics for all other parcels not associated with the TMS coil positions for a “null” distribution of errors. We reasoned that would differ from the null distribution if the TMS coil substantially altered RSFC strengths for parcels centered beneath the coil.
3. Results
3.1. Static artifacts in phantom scans
Fig. 2A shows the slice prescriptions for the PSF-EPI phantom scans. Separate scans were conducted with the acquisitions arranged in parallel or oblique orientations with respect to the plane of the TMS coil. Visual inspection of slices acquired 15 mm (Fig. 2B; slice 5) and 30 mm (Fig. 2C; slice 10) from the TMS coil reveal spatial distortions and signal loss that qualitatively varied according to whether PSF-correction was applied, TMS coil presence, and acquisition orientation, with more substantial artifacts observed in the slice closer to the TMS coil. The raw images and tSNR maps reveal slight distortions and signal loss in the corrected and uncorrected EPI data even in the absence of the TMS coil. These patterns are exacerbated by introducing the TMS coil into the scanner. Notably, the PSF-corrected EPI data contained considerably less distortion and less signal dropout relative to the uncorrected EPI data in both parallel and oblique acquisition orientations. We performed statistical analyses on image quality metrics calculated for the corrected and uncorrected PSF-EPI phantom scans to characterize the effects of the TMS coil and acquisition orientation quantitatively.
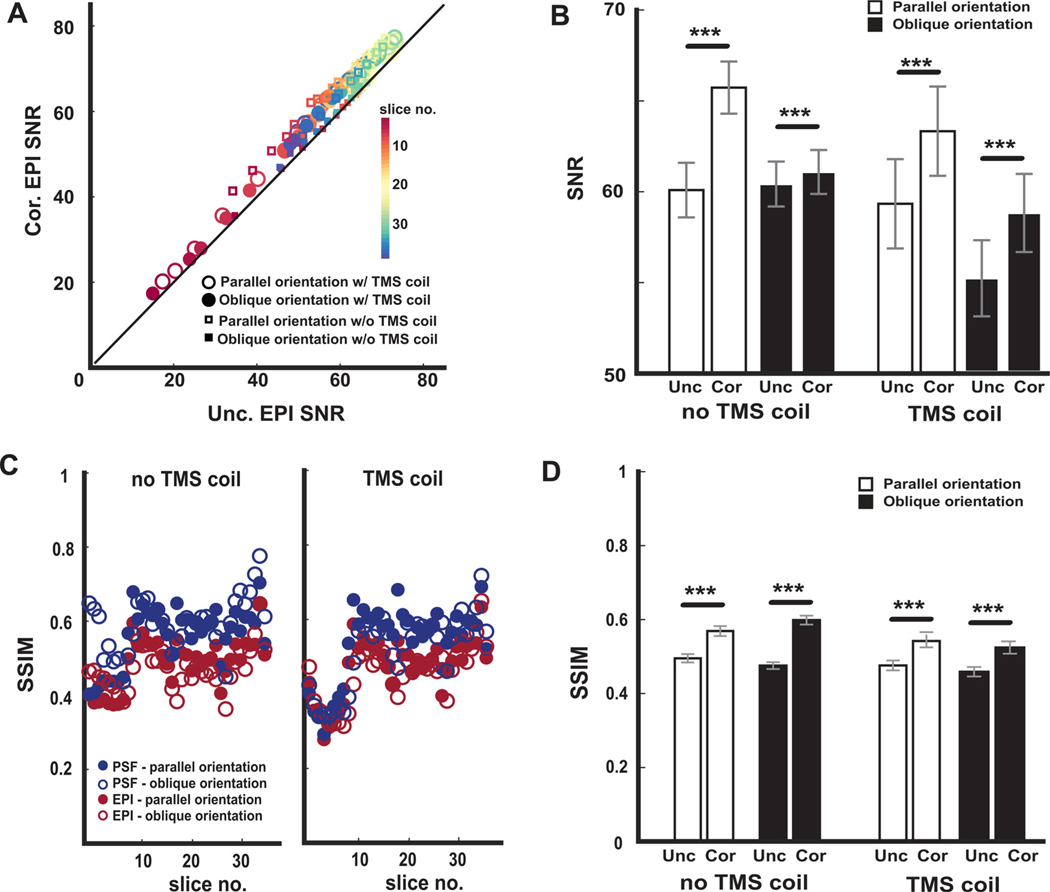
To compare TMS coil effects on signal strength, we calculated tSNR in each condition. Over all conditions, tSNR values were greater with PSF-corrected EPI data compared to uncorrected EPI data in nearly all image slices (Fig. 3A). A rmANOVA conducted on the slice-averaged tSNR values (Fig. 3B) revealed significant main effects of TMS coil presence (F1,36 = 5.02, p =0.031), correction status (F1,36 =1271.8, p =1.1e-29), and acquisition orientation (F1,36 =39.1, p= 3.2e-7). These significant main effects reveal that the presence of the TMS coil reduced tSNR, the PSF-correction produced images with higher tSNR, and higher tSNR values were observed when the acquisitions were oriented parallel to the TMS coil. Significant interaction effects were observed between the TMS coil and correction status (F1,36 = 8.7, p =0.0055), the TMS coil and acquisition orientation (F1,36 = 21.9, p =3.9e-5), and the correction status and acquisition orientation (F1,36 = 272.3, p =2.3e-18). Furthermore, the TMS coil X correction status X acquisition orientation interaction also achieved statistical significance (F1,36 =229.3, p =3.4e-17). The significant interaction effects indicate that the influences of coil presence, correction status, and acquisition orientation on tSNR were not independent. Because of the significant interaction effects, we directly compared the tSNR values associated with corrected and uncorrected images in each condition (Fig. 3B) and found that tSNR values were significantly higher with PSF-correction in all conditions (p < 6.3e-11 in all 4 paired t-tests).
Fig. 3.
Quantitative comparisons between PSF-corrected and uncorrected EPI data. (A) Markers indicate the average tSNR values for each slice of the uncorrected (Unc) and PSF-corrected EPI (Cor) data acquired in the phantom scans calculated for parallel and oblique acquisitions, with and without the TMS coil. Points above the unity line indicate higher tSNR with the PSF-correction. (B) Average tSNR values with PSF-corrected and uncorrected EPI data over the slices calculated for parallel and oblique acquisitions, with and without the TMS coil. (C) Markers indicate structural similarity (SSIM) values calculated between the 3D FLASH images and the PSF-corrected and uncorrected images. (D) Average SSIM values with PSF-corrected and uncorrected EPI data over the slices calculated for parallel and oblique acquisitions, with and without the TMS coil. Error bars indicate SEM. *** p < 0.001.
To compare TMS coil effects on image distortion, we calculated SSIM between the 3D FLASH phantom volume and the PSF-corrected and uncorrected EPI volumes under each condition (coil presence and acquisition orientation). Larger SSIM values reflect greater similarity with the 3D FLASH data – these data are not susceptible to spatial distortions and signal loss induced by the TMS coil presence so they serve as a ground truth. Over all conditions (Fig. 3C), SSIM values were greater with PSF-corrected data compared to uncorrected EPI data in nearly all image slices. A rmANOVA conducted on the slice-averaged SSIM values (Fig. 3D) revealed significant main effects of TMS coil presence (F1,36 = 28.8, p =2.8e-6) and correction status (F1,36 = 422.25, p= 1.8e-21), but not of acquisition orientation (F1,36 = 0.2, p=0.64). These significant main effects confirm that the presence of the TMS coil induces distortions in the EPI data and that PSF-correction produced images more similar to the 3D FLASH images. Significant 2-way interactions were observed between the TMS coil and correction status (F1,36 =40.8, p =2.1e-7), the TMS coil and acquisition orientation (F1,36 =16.5, p= 0.0002) and the correction status and acquisition orientation (F1,36 =40.82, p =2.1e-7). Furthermore, the TMS coil X correction status X acquisition orientation interaction also achieved statistical significance (F1,36 =44.3, p =9.4e-8). These significant interaction patterns indicate the importance of acquisition orientation and imply that the choice of slice prescriptions influences the magnitude of coil-related artifacts as well as the benefits of PSFbased correction. Because of the significant 3-way interactions, we compared SSIM values achieved with corrected and uncorrected EPI data in each condition (Fig. 3D) and found that SSIM values were significantly higher with PSF-correction in all conditions (p < 1.3e-12 in all 4 paired t-tests).
3.2. Static artifacts in brain scans
We compared how the placement of a TMS coil over left sensorimotor cortex impacted EPI data with and without PSF-correction (Fig. 4A). Faint image distortions and ghosting artifacts were observed in visual inspections of slices near the TMS coil. Importantly, the difference image calculated by comparing the PSF-corrected and uncorrected EPI data (Fig. 4B) revealed slight signal variations outside of the brain volume and more substantial signal differences on the perimeter of the brain as well as within the volume. Whole-brain tSNR maps (Fig. 5) also clearly reveal differences between corrected and uncorrected EPI data highlighted by substantially more signal loss in brain regions under and near the TMS coil in the uncorrected EPI data.
Fig. 5.
tSNR maps computed for PSF-corrected and uncorrected EPI. (A) tSNR maps for uncorrected and corrected data in top, left, and right views. The black arrow indicates the TMS coil location (see Fig. 4). (B) The difference maps (corrected minus uncorrected) reveal elevated tSNR with the corrected images in regions near the TMS coil and in remote regions that may have been more vulnerable to signal distortion and variability.
Quantitative comparisons between the corrected and uncorrected EPI sequences are shown in Fig. 6. Mean tSNR values of the PSF-corrected data exceeded those of the uncorrected data in all slices (Fig. 6A). A paired t-test conducted on the slice-averaged tSNR values (Fig. 6B) revealed that the tSNR values estimated from PSF-corrected EPI data were significantly higher than tSNR estimated from uncorrected EPI data (p =3.3e-30). These results indicate that PSF-correction enhances tSNR in brain data acquired in the presence of the TMS coil, consistent with the phantom scan results.
Fig. 6.
Quantitative comparisons between the PSF-corrected and uncorrected EPI data. (A) Markers indicate the average tSNR values for each EPI slice computed with and without PSF-correction. (B) Comparison between the average tSNR values of the PSF-corrected and uncorrected data averaged over slices. (C) Structural similarity (SSIM) values between the 3D FLASH images and the EPI data with (Cor) and without PSF-correction (Unc). (D) Comparison between the SSIM values achieved with the PSF-corrected and uncorrected data averaged over all slices. Error bars indicate SEM. *** p < 0.001.
Quantitative comparisons between the SSIM computed for correct and uncorrected EPI data similarly indicated the benefit of PSF-correction. With each slice, SSIM tended to be higher for corrected compared to uncorrected EPI data (Fig. 6C). A paired t-test (Fig. 6D) revealed that SSIM was significantly higher with PSF-corrected EPI data compared to uncorrected EPI data (p =1.4e-20).
3.3. Acute artifacts in phantom scans
To characterize the temporal profile of TMS pulse effects on image acquisition using PSF-EPI, we delivered a single TMS pulse at different times preceding and during volume acquisition (Fig. 7A). To quantify TMS effects as a function of delay times (i.e., the interval between the pulse and image acquisition), we calculated the RMSE between a volume acquired before TMS delivery and volumes acquired during or immediately following TMS on a slice-wise basis (Materials and methods). Because interactions between the scanner and TMS coil can depend on the orientation of the coil with respect to the B0 field (Yau et al., 2014), we characterized acute TMS effects on PSF-EPI data with the coil aligned with or perpendicular to the B0 field (Fig. 7B). RMSE profiles with TMS intensity set at 70% and 100% maximum stimulator output. For both coil positions, signal perturbations indexed by RMSE were observed mostly in slices close to the TMS coil which were acquired earlier in the slice sequence (Fig. 7C). In general, TMS applied during acquisition (0ms and 50 ms with respect to acquisition onset) induced the largest image perturbations. When the TMS coil was aligned to the B0 field, TMS preceding acquisition also perturbed images and errors were observed when image acquisition followed TMS pulse delivery by less than 75 ms (Fig. 7C). In this coil position, increasing TMS intensity from 70% to 100% maximum output resulted in larger errors at the longer delays and disrupted signal quality in more slices (Fig. 7C,D). When the TMS coil was oriented perpendicular to the B0 field, TMS pulses preceding image acquisition had little effect on RMSE and comparable distortions were observed with the 70% and 100% TMS intensities. The fact that the spatiotemporal RMSE profiles differed with the different TMS coil orientations (Fig. 7C,D) confirms that interactions between the scanner and TMS vary according to the relative orientation of the coil with respect to the B0 field. These differences may also reflect, in part, the slice prescriptions used under the two coil orientations: Subtle differences in tSNR could conceivably result in apparent differences in vulnerability to the eddy current produced by TMS. Critically, volumes acquired >3 s after TMS delivery did not contain substantial errors even when the TMS pulse potentially coincided with RF excitation and image acquisition (Fig. 7D).
Fig. 7.
Characterization of acute TMS artifacts. (A) Diagram indicates potential times a single TMS pulse was delivered with respect to image acquisition (Materials and methods). (B) The TMS coil (white bar) was placed superior to the phantom with its plane was aligned to the transverse imaging plane (Two left images in coronal and sagittal view) and approximately left lateral to the phantom (Two right images in coronal and axial view). In these configurations, the field generated by the coil was either aligned with or perpendicular to the B0 field, respectively. R =right, L = left, S =superior, I =inferior. (C) The root mean square error (RMSE) values calculated for each slice indicate the differences between reference images acquired prior to TMS and images acquired with TMS delivered at different times with respect to image acquisition. For each coil position (superior =left column; lateral = right column), separate RMSE profiles are indicated for TMS intensity set at 70% (upper panels) and 100% (lower panels) maximum stimulator output. (D) RMSE for the 4th slice (~12 mm from the TMS coil) with the stimulator output 70% (dashed line) and 100% (solid line) as a function of TMS delivery time. Red, yellow, and green traces indicate 1st, 2nd, and 3rd volume during or after TMS delivery, respectively. RMSE temporal profiles differed slightly depending on whether the TMS was positioned superior (left) or lateral (right) to the phantom. In both positions, RMSE increased in volumes acquired during or immediately after TMS delivery (red), but returned to baseline levels in volumes separated from the TMS pulse by intervals exceeding 3 s (yellow and green). Error bars indicate SEM.
3.4. Concurrent TMS-fMRI experiment
In one participant, we characterized BOLD signal changes associated with TMS and performance of a finger tapping task to validate the use of PSF-EPI (Fig. 8A). The goal of this experiment was simply to verify that BOLD signal changes could be measured in brain regions in the vicinity of the TMS coil and regions remote from the TMS coil. In separate block design scans, bursts of low-or high-intensity TMS were delivered between volume acquisitions during each block. Subjects also performed bimanual finger tapping, cued by low-intensity TMS, during one scan. The TMS coil was positioned over left sensorimotor cortex (Materials and methods). Bimanual finger tapping paired with TMSLow was associated with robust BOLD signal changes in a distributed sensorimotor network including the bilateral precentral gyrus, bilateral postcentral gyrus, bilateral cerebellum, left inferior parietal and right middle temporal gyrus (Fig. 8B). In order to compare BOLD signals in corresponding sensorimotor regions near to and remote from the TMS coil, we identified the peak activations associated with bimanual finger tapping in the left (x = −40, y = −19, z = 56) and right precentral gyri (x =44, y = −22, z =59) and defined two spherical regions-of-interest (ROI; 5-mm radius) for extracting response time series and activation estimates (Fig. 8B). The response equivalence between the two sensorimotor ROIs indicates that the PSF-EPI sequence successfully preserved BOLD signal changes associated with finger tapping in brain regions in the vicinity of the TMS coil. TMS alone was also associated with BOLD signal changes in the distributed sensorimotor network identified with finger tapping (Fig. 8C), with qualitatively greater BOLD signal changes in the high-intensity TMS condition compared to the low-intensity condition. Although BOLD signal changes related to TMS were weaker than those associated with finger tapping, the three conditions were marked by highly similar temporal response profiles.
3.5. Resting state functional connectivity
To test whether the PSF-EPI sequence could be used to acquire resting state fMRI data in the presence of the TMS coil, we conducted resting state fMRI scans with one subject over 3 sessions. Because the TMS coil was positioned over different scalp locations in each session (Materials and methods), we could characterize the consistency of RSFC patterns across the sessions and test whether the positioning of the TMS coil (Fig. 9A) specifically affected resting state networks comprising regions underneath the coil. We found that RSFC patterns over the whole brain (Fig. 9B), sorted according to an existing parcellation scheme (Glasser et al., 2016), were highly similar over sessions (mean r =0.78; range: 0.75–0.80). These correlations are comparable to published estimates of across-session RSFC consistency comparing 15min portions of resting state data acquired with conventional EPI at 3 T (Gordon et al., 2017; Laumann et al., 2015; Xu et al., 2016). We evaluated the across-session similarity of RSFC patterns for the parcels identified as the TMS targets (Materials and methods) and found that these values (target 1: 0.86, target 2: 0.84, target 3: 0.81) fell well within the distribution of average similarity values for all parcels not associated with TMS coil position (Fig. 9C; mean of null distribution: 0.77). Similarly, we evaluated the summed differences in RSFC strength (i.e., Euclidean distances, see Materials and methods) for the TMS target regions (target 1: 0.18, target 2: 0.18, target 3: 0.20) and found that these values also fell well within the null distribution defined by parcels not associated with TMS coil position. Together these, results indicate that the PSF-EPI sequence can produce high quality RSFC data in the presence of the TMS coil.
Fig. 9.

Resting state functional connectivity measured in the presence of the TMS coil. (A) Resting state fMRI data was acquired from a single subject in 3 sessions (15 min of data per session). The TMS coil was positioned over different scalp locations in each session. (B) Resting state functional connectivity (RSFC) matrices (Materials and methods) are depicted for 360 parcels spanning both hemispheres. The similarity of the RSFC patterns are indicated by the black arrows and associated correlations. The yellow, blue, and red arrows indicate the parcels associated with the TMS coil locations in sessions 1, 2, and 3, respectively. (C) The arrows indicate the average similarity in the RSFC patterns for the TMS-associated parcels (see B for reference). The grey bars indicate the null distribution of RSFC similarity values for all parcels not associated with the TMS coil positions. (D) The arrows indicate the total differences in the RSFC magnitudes for the TMS associated parcels (see B for reference). The grey bars indicate the null distribution for all parcels not associated with the TMS coil positions.
4. Discussion
We sought to demonstrate the feasibility of using PSF-corrected EPI to reduce metal-induced susceptibility artifacts and signal dropouts in concurrent TMS-fMRI experiments. In phantom and brain scans, we found that the PSF-corrected EPI data were marked by less spatial distortion, ghosting artifacts, and signal dropout compared to uncorrected EPI data. We then characterized the temporal profile of acute artifacts related to TMS discharge and established that 100 ms were required between the TMS pulse and subsequent image acquisition to avoid substantial signal loss and image perturbations. We combined TMS with performance of a bimanual motor task to confirm that robust BOLD signal changes could be measured using PSF-EPI in brain regions near and remote from the TMS coil. Lastly, we demonstrated that high quality resting state fMRI data could be acquired using the PSF-EPI sequence even in the presence of the TMS coil.
Our data confirm that the introduction of a TMS coil to the scanner environment can result in substantial image artifacts and signal loss in EPI data. This suggests that components of the TMS coil resulted in inhomogeneity of the static magnetic field and the magnetic field variations led to geometric distortion, signal loss, or signal pile-up in the EPI data. Note that we only tested a single TMS system and a particular combination of receiver coils, so our results may not generalize to setups comprising other TMS equipment or coil arrangements. That said, in our setup, we found in phantom and brain scans that spatial distortions and signal loss were significantly reduced in EPI data following PSF-correction in each of our tested conditions, though some artifacts remained. This benefit implies that PSF mapping using the combination of spin-warp phase-encoding gradients and EPI phase-encoding gradients provided a sufficient distortion map that could be used to correct for distortions in our EPI data. While the reduction in spatial distortions is sensible and consistent with the use of PSF-EPI in other experimental contexts (In et al., 2017a), the improved tSNR in PSFcorrected compared to uncorrected EPI is puzzling. One possibility is that the interpolation step in PSF-correction results in image filtering leading to an apparent increase in tSNR. In this case, our observed tSNR improvement with PSF-EPI may actually reflect a difference in image resolution. Importantly, the results of our concurrent TMS-fMRI and resting state fMRI experiments validate the use of PSF-EPI despite any potential resolution loss; however, future experiments are needed to explicitly evaluate whether interpolation in PSF-correction results in image filtering and meaningful changes in effective resolution.
Consistent with previous reports (Bestmann et al., 2003a), we found that signal loss and distortions with EPI depended on the orientation of the slice prescriptions with respect to the plane of the TMS coil: Greater artifacts are present when the frequency encoding direction is oblique rather than parallel to the plane of the TMS coil. Accordingly, previous studies using conventional EPI have attempted to reduce the coil-related static artifacts by prescribing the image acquisitions with respect to the TMS coil arrangement (Bestmann et al., 2003a; Bungert et al., 2012; de Lara et al., 2017; Moisa et al., 2009; Navarro de Lara et al., 2015; Weiskopf et al., 2009). Although we found that the PSF-EPI sequence, like conventional EPI sequences, was sensitive to the slice acquisition orientation with respect to the TMS coil in phantom scans, the PSF-correction yielded higher quality data in all orientations according to tSNR and SSIM estimates compared to uncorrected EPI data. Consistent with these phantom scan results, we observed that PSF-correction yielded substantially better signal and less distortion compared to uncorrected EPI data even as we used only transverse prescriptions in our brain scans. We also used the transverse prescription in the concurrent TMS-fMRI and resting state fMRI scans, and we observed robust BOLD signal changes and consistent RSFC patterns despite the fact that we did not tailor the image prescriptions to the TMS coil orientation and placement. Thus, in concurrent TMS-fMRI experiments where coilrelated artifacts are present and concerning, the use of PSF-EPI is advantageous over uncorrected EPI based on image quality metrics as well as ease and flexibility of use.
In the phantom and brain scans, we observed substantial ghost artifacts along the phase encoding direction which are presumably caused by systematic inconsistencies between odd and even k-space lines in the acquired data (Hu and Le, 1996). These ghost artifacts have also been reported in previous concurrent TMS-fMRI experiments (Bestmann et al., 2003a; Bungert et al., 2012). Earlier studies using conventional EPI dealt with this artifact by oversampling in the phase encoding direction, which shifted the ghost artifacts caused by the TMS coil (Blankenburg et al., 2008; Feredoes et al., 2011; Ruff et al., 2007). This strategy, though, can result in a subsequent decrease in tSNR and an increase in phase errors (Hu and Glover, 2006). Using PSF-EPI, we observed qualitatively less ghosting, consistent with earlier studies that reported reductions in ghost artifacts through the use of PSF-EPI sequences (Chung et al., 2011; Zaitsev et al., 2004).
We characterized the acute artifacts associated with discharging a single TMS pulse in order to identify the optimal delay between TMS and subsequent image acquisition. Depending on the hardware setup, the introduction of this delay can be important for allowing the eddy currents associated with TMS discharge to clear without impacting subsequent RF excitation and image acquisition. Because interactions between TMS and the scanner can depend on the relative orientation of the TMS coil with respect to the B0 field, we characterized the acute artifacts associated with a TMS pulse with the coil oriented parallel or perpendicular to the B0 field. When the TMS coil was aligned with the B0 field, we found that image quality remained high with delay times exceeding 50 ms. This timing is consistent with a recent report that delays on the order of 50 ms are sufficient to avoid distortions in the images with the 100% TMS output intensity (de Lara et al., 2017). Notably, we observed a different spatiotemporal perturbation pattern when the TMS coil was perpendicular to the B0 field (Fig. 7). In this orientation, pulse-related distortions were only observed when image acquisition occurred during or immediately following the TMS pulse (i.e., TMS pulse time at 0 and +50 ms relative to image acquisition). The different disruption patterns we observed confirm the sensitivity of TMS effects in the scanner environment to the relative orientation of the static field and highlight the variability in acute TMS influences on image quality even when using the same hardware. Importantly, while we found that image quality was minimally affected by TMS at delays exceeding 50 ms, some studies have recommended using longer delays on the order of 100 ms (Bestmann et al., 2003a; Shastri et al., 1999). This variation across studies is likely due to the use of different TMS stimulators and coils, different scanners and imaging hardware, and different EPI sequences. Indeed, TMS-related eddy currents may minimally affect image quality with some stimulator and coil designs. Moreover, some acute temporal artifacts related to TMS conceivably may be a consequence of imprecise coordination between the scanner and TMS system so better synchronization (e.g., timing control via a dedicated microprocessor) may also reduce TMS-related artifacts. Because acute TMS artifacts may differ dramatically across setups, researchers should carefully determine whether acute artifacts are present in their experiment and introduce a delay between TMS pulsing and image acquisition as needed.
We examined the feasibility of the PSF-EPI sequence in concurrent TMS-fMRI experiments by characterizing TMS and motor responses. Our goal in this experiment was simply to confirm that systematic BOLD signal changes could be measured over the brain rather than to characterize activation patterns and infer network organization. Using PSF-EPI, we observed robust BOLD signal changes associated with bimanual finger-tapping in bilateral regions comprising a well-established distributed sensorimotor network. Importantly, we confirmed the location of the TMS over coil left motor cortex using our marker processing procedure and found that finger-tapping BOLD responses in sensorimotor regions beneath and near the coil were just as robust as responses in homologous regions in the hemisphere contralateral to the coil. This response equivalence is important for indicating that the BOLD signal in the vicinity of the coil was largely preserved with PSFEPI, particularly because there has been some debate regarding the ability to measure significant BOLD signal changes in brain regions beneath the TMS coil. Indeed, many studies have failed to measure significant BOLD signal changes in motor cortex when delivering subthreshold TMS that successfully evokes significant activations in regions remote from the coil (Baudewig et al., 2001; Bestmann et al., 2004; Bohning et al., 1998; Kemna and Gembris, 2003). We also observed BOLD signal changes associated with TMS even in the absence of motor performance in distributed brain regions. These activation patterns, found in sensorimotor cortex, parietal operculum, and auditory regions, are largely consistent with the results of previous TMS-fMRI studies targeting on motor cortex (Bestmann et al., 2004; de Lara et al., 2017; Moisa et al., 2009; Shitara et al., 2011; Yau et al., 2013). Importantly, the responses to TMS were characterized by robust and systematic temporal response profiles that closely resemble the response time courses related to motor performance. Although the results from our feasibility experiment are insufficient for addressing specific questions related to network interrogation, the results confirm that PSF-EPI can be used to characterize BOLD signal changes in concurrent TMS-fMRI scans in distributed regions near to and remote from the TMS coil.
We demonstrated the feasibility of using PSF-EPI for acquiring resting state fMRI data in the presence of TMS coil. We confirmed that RSFC patterns were robust and highly correlated across sessions when comparing 15 min of data per session, consistent with previous reports (Gordon et al., 2017; Laumann et al., 2015; Xu et al., 2016). We additionally confirmed that RSFC patterns for brain regions under the TMS coil were reliable and robust. These results highlight the potential for combining concurrent TMS-fMRI and resting state fMRI in the same session; the combined use of these complementary approaches may reveal novel insights regarding the intrinsic architecture of the human brain (Fox et al., 2014; Hawco et al., 2018) and understanding how remote TMS effects relate to RSFC may inform clinical interventions utilizing targeted brain stimulation (Fox et al., 2012, 2013).
Although we have emphasized the potential benefit of PSF-EPI for efforts to characterize functional brain networks using concurrent TMS-fMRI, it is important to recognize the limitations of TMS and fMRI, their combined use, and the inferences one can draw about brain networks using concurrent TMS-fMRI. First, while TMS is a powerful tool that can induce electrical activity in neural populations, there are clear limits to the spatial precision with which TMS engages neural circuits. Given a figure-of-eight TMS coil configuration, the spatial resolution of TMS is likely less than 1 cm2 on the brain’s surface. This is relatively precise at a macro-level scale (i.e., brain regions or cortical areas), but this is relatively imprecise at the scale of individual cells or populations of neurons. This imprecision is not merely a consequence of the spatial focality of the magnetic fields produced by the TMS coil, but it also reflects the spatial relationship between the magnetic fields and the arrangement and morphology of the targeted neural structures (Lefaucheur et al., 2014; Rossi et al., 2009). Accordingly, the fact that TMS effects are non-selective with respect to specific neural populations in a small brain volume is a well-recognized limitation, but TMS is sufficiently precise to engage in different brain regions or networks that are topographically organized at an appropriate scale. In the temporal domain, TMS interacts with local neural activity at a millisecond scale and TMS effects can propagate to remote regions rapidly in tens to hundreds of milliseconds (Reis et al., 2008). In contrast, the hemodynamic signals measured with fMRI, which are interpreted as an indirect measure of neural activity (Logothetis and Wandell, 2004), are far more sluggish. Indeed, with TMS-related BOLD signal changes peaking multiple seconds after pulse delivery (Fig. 8), it would be inappropriate to draw inferences about when a particular brain region is active relative to TMS application or the temporal relationships between activated regions using fMRI data alone. However, given that concurrent TMS-fMRI can produce systematic and reproducible distributed activation patterns that are consistent with anatomical connectivity profiles, it may be more reasonable to interpret the spatial activation patterns associated with TMS application as reflecting network architecture on a macro (whole brain) level, matching that scale of alternative network characterization methods like resting state fMRI.
In summary, we have characterized the static artifacts induced by the introduction of a TMS coil into the scanner environment. PSF-corrected and uncorrected EPI data showed some degree of image distortion and signal loss relative to T1-weighted scans. Critically, the former suffered significantly less compared to the latter from the mere presence of our TMS coil, indicating that the characterization of the EPI distortions using point spread function mapping (In et al., 2017a, 2015; In and Speck, 2012; Zaitsev et al., 2004) and the subsequent adjustment of the EPI sufficiently corrected for the substantial magnetic field inhomogeneities induced by our TMS coil. Because PSF-EPI corrects for image distortion in a manner that is relatively invariant to changes in the prescription of the acquired volumes, its use enables flexible TMS coil positioning without the need to tailor prescriptions according to the specific coil placement and orientation. We additionally found that PSF-EPI yields functional data with minimal acute artifacts due to TMS discharge when the TMS pulse precedes RF excitation and image acquisition with a sufficient temporal gap. Based on the characterization of the static and acute artifacts, we conducted a concurrent TMS-fMRI scan and found robust BOLD signal changes in distributed brain regions. Our collective results indicate that concurrent TMS-fMRI experiments could benefit from the correction of EPI distortions using point spread function mapping. Importantly, some MRI-compatible TMS systems may not induce comparable spatial distortions and signal loss as we observed, so our current results may not generalize to all concurrent TMS-fMRI setups. Future experiments are required to establish the potential benefits for using PSF-EPI with TMS systems from different manufacturers (e.g., MagVenture MRi-B91). Nevertheless, in the event that the TMS system does introduce problematic spatial artifacts and signal loss, PSF-EPI may be helpful particularly when specialized hardware solutions for treating susceptibility effects are unavailable. PSF-EPI may also be a general solution for multimodal stimulation and imaging studies (e.g., EEG-fMRI (Abreu et al., 2018; Liu et al., 2006), tDCS-fMRI (Callan et al., 2016), and focused ultrasound-fMRI (Legon et al., 2014) that require the introduction of metallic hardware into the scanner environment. Moreover, PSF-EPI may hold great promise for measuring functional connectivity from the combination of concurrent TMS-fMRI and resting state fMRI (Fox et al., 2012).
Acknowledgements
This research was supported by R01NS097462 (JMY), The Dana Foundation (JMY), and K25HL131997 (JHK). We thank Gisela Hagberg for providing the PSF-EPI sequence. We thank Meghan Robinson for assisting with the resting state analysis and field map characterization. We thank Yau Lab members for thoughtful discussions. This work was performed in BCM’s Core for Advanced MRI (CAMRI).
Footnotes
Declaration of Competing Interest
None.
References
- Abreu R, Leal A, Figueiredo P, 2018. EEG-informed fMRI: a review of data analysis methods. Front. Hum. Neurosci 12, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudewig J, Paulus W, Frahm J, 2000. Artifacts caused by transcranial magnetic stimulation coils and EEG electrodes in T2*-weighted echo-planar imaging. Magn. Reson. Imaging 18, 479–484. [DOI] [PubMed] [Google Scholar]
- Baudewig J, Siebner HR, Bestmann S, Tergau F, Tings T, Paulus W, Frahm J, 2001. Functional MRI of cortical activations induced by transcranial magnetic stimulation (TMS). Neuroreport 12, 3543–3548. [DOI] [PubMed] [Google Scholar]
- Bestmann S, Baudewig J, Frahm J, 2003a. On the synchronization of transcranial magnetic stimulation and functional echo‐planar imaging. J. Magn. Reson. Imaging 17, 309–316. [DOI] [PubMed] [Google Scholar]
- Bestmann S, Baudewig J, Siebner HR, Rothwell JC, Frahm J, 2005. BOLD MRI responses to repetitive TMS over human dorsal premotor cortex. Neuroimage 28, 22–29. [DOI] [PubMed] [Google Scholar]
- Bestmann S, Baudewig J, Siebner HR, Rothwell JC, Frahm J, 2004. Functional MRI of the immediate impact of transcranial magnetic stimulation on cortical and subcortical motor circuits. Eur. J. Neurosci 19, 1950–1962. [DOI] [PubMed] [Google Scholar]
- Bestmann S, Baudewig J, Siebner HR, Rothwell JC, Frahm J, 2003b. Subthreshold high-frequency TMS of human primary motor cortex modulates interconnected frontal motor areas as detected by interleaved fMRI-TMS. Neuroimage 20, 1685–1696. [DOI] [PubMed] [Google Scholar]
- Bestmann S, Feredoes E, 2013. Combined neurostimulation and neuroimaging in cognitive neuroscience: past, present, and future. Ann. N. Y. Acad. Sci 1296, 11–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestmann S, Oliviero A, Voss M, Dechent P, Lopez-Dolado E, Driver J, Baudewig J, 2006. Cortical correlates of TMS-induced phantom hand movements revealed with concurrent TMS-fMRI. Neuropsychologia 44, 2959–2971. [DOI] [PubMed] [Google Scholar]
- Blankenburg F, Ruff CC, Bestmann S, Bjoertomt O, Eshel N, Josephs O, Weiskopf N, Driver J, 2008. Interhemispheric effect of parietal TMS on somatosensory response confirmed directly with concurrent TMS–fMRI. J. Neurosci 28, 13202–13208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenburg F, Ruff CC, Bestmann S, Bjoertomt O, Josephs O, Deichmann R, Driver J, 2010. Studying the role of human parietal cortex in visuospatial attention with concurrent TMS–fMRI. Cereb. Cortex 20, 2702–2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohning DE, Shastri A, Nahas Z, Lorberbaum JP, Andersen SW, Dannels WR, Haxthausen E-U, Vincent DJ, George MS, 1998. Echoplanar BOLD fMRI of brain activation induced by concurrent transcranial magnetic stimulation. Invest. Radiol 33, 336–340. [DOI] [PubMed] [Google Scholar]
- Bright MG, Murphy K, 2015. Is fMRI “noise” really noise? Resting state nuisance regressors remove variance with network structure. Neuroimage 114, 158–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bungert A, Chambers CD, Phillips M, Evans CJ, 2012. Reducing image artefacts in concurrent TMS/fMRI by passive shimming. Neuroimage 59, 2167–2174. [DOI] [PubMed] [Google Scholar]
- Callan DE, Falcone B, Wada A, Parasuraman R, 2016. Simultaneous tDCS-fMRI identifies resting state networks correlated with visual search enhancement. Front. Hum. Neurosci 10, 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung J-Y, In M-H, Oh S-H, Zaitsev M, Speck O, Cho Z-H, 2011. An improved PSF mapping method for EPI distortion correction in human brain at ultra high field (7T). Magn. Reson. Mater. Phys. Biol. Med 24, 179–190. [DOI] [PubMed] [Google Scholar]
- Cox RW, 1996. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res 29, 162–173. [DOI] [PubMed] [Google Scholar]
- Cox RW, Jesmanowicz A, 1999. Real‐time 3D image registration for functional MRI. Magn. Reson. Med 42, 1014–1018. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI, 1999. Cortical surface-based analysis: I. Segmentation and surface reconstruction. Neuroimage 9, 179–194. [DOI] [PubMed] [Google Scholar]
- de Lara LIN, Tik M, Woletz M, Frass-Kriegl R, Moser E, Laistler E, Windischberger C, 2017. High-sensitivity TMS/fMRI of the human motor cortex using a dedicated multichannel MR coil. NeuroImage 150, 262–269. [DOI] [PubMed] [Google Scholar]
- de Weijer AD, Sommer IE, Bakker EJ, Bloemendaal M, Bakker CJ, Klomp DW, Bestmann S, Neggers SF, 2014. A setup for administering TMS to medial and lateral cortical areas during whole-brain FMRI recording. J. Clin. Neurophysiol 31, 474–487. [DOI] [PubMed] [Google Scholar]
- Feredoes E, Heinen K, Weiskopf N, Ruff C, Driver J, 2011. Causal evidence for frontal involvement in memory target maintenance by posterior brain areas during distracter interference of visual working memory. Proc. Natl. Acad. Sci 108, 17510–17515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Buckner RL, Liu H, Chakravarty MM, Lozano AM, Pascual-Leone A, 2014. Resting-state networks link invasive and noninvasive brain stimulation across diverse psychiatric and neurological diseases. Proc. Natl. Acad. Sci 111 E4367–E75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Halko MA, Eldaief MC, Pascual-Leone A, 2012. Measuring and manipulating brain connectivity with resting state functional connectivity magnetic resonance imaging (fcMRI) and transcranial magnetic stimulation (TMS). Neuroimage 62, 2232–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Liu H, Pascual-Leone A, 2013. Identification of reproducible individualized targets for treatment of depression with TMS based on intrinsic connectivity. Neuroimage 66, 151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser MF, Coalson TS, Robinson EC, Hacker CD, Harwell J, Yacoub E, Ugurbil K, Andersson J, Beckmann CF, Jenkinson M, 2016. A multi-modal parcellation of human cerebral cortex. Nature 536, 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon EM, Laumann TO, Gilmore AW, Newbold DJ, Greene DJ, Berg JJ, Ortega M, Hoyt-Drazen C, Gratton C, Sun H, 2017. Precision functional mapping of individual human brains. Neuron 95, 791–807 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett M, 2007. Transcranial magnetic stimulation: a primer. Neuron 55, 187–199. [DOI] [PubMed] [Google Scholar]
- Hanakawa T, Mima T, Matsumoto R, Abe M, Inouchi M, S-i U, Anami K, Honda M, Fukuyama H, 2009. Stimulus–response profile during single-pulse transcranial magnetic stimulation to the primary motor cortex. Cereb. Cortex 19, 2605–2615. [DOI] [PubMed] [Google Scholar]
- Hawco C, Voineskos AN, Steeves JK, Dickie EW, Viviano JD, Downar J, Blumberger DM, Daskalakis ZJ, 2018. Spread of activity following TMS is related to intrinsic resting connectivity to the salience network: a concurrent TMS-fMRI study. Cortex 108, 160–172. [DOI] [PubMed] [Google Scholar]
- Hu X, Le TH, 1996. Artifact reduction in EPI with phase‐encoded reference scan. Magn. Reson. Med 36, 166–171. [DOI] [PubMed] [Google Scholar]
- Hu Y, Glover GH, 2006. Partial‐k‐space acquisition method for improved SNR efficiency and temporal resolution in 3D fMRI. Magn. Reson. Med 55, 1106–1113. [DOI] [PubMed] [Google Scholar]
- In M-H, Cho S, Shu Y, Min H-K, Bernstein MA, Speck O, Lee KH, Jo HJ, 2017a. Correction of metal-induced susceptibility artifacts for functional MRI during deep brain stimulation. NeuroImage 158, 26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- In M-H, Posnansky O, Beall EB, Lowe MJ, Speck O, 2015. Distortion correction in EPI using an extended PSF method with a reversed phase gradient approach. PLoS One 10, e0116320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- In M-H, Posnansky O, Speck O, 2017b. High-resolution distortion-free diffusion imaging using hybrid spin-warp and echo-planar PSF-encoding approach. NeuroImage 148, 20–30. [DOI] [PubMed] [Google Scholar]
- In M-H, Speck O, 2012. Highly accelerated PSF-mapping for EPI distortion correction with improved fidelity. Magn. Reson. Mater. Phys. Biol. Med 25, 183–192. [DOI] [PubMed] [Google Scholar]
- In MH, Posnansky O, Speck O, 2016. PSF mapping‐based correction of eddy‐current‐induced distortions in diffusion‐weighted echo‐planar imaging. Magn. Reson. Med 75, 2055–2063. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, 2012. Smith SM. Fsl. Neuroimage 62, 782–790. [DOI] [PubMed] [Google Scholar]
- Kemna LJ, Gembris D, 2003. Repetitive transcranial magnetic stimulation induces different responses in different cortical areas: a functional magnetic resonance study in humans. Neurosci. Lett 336, 85–88. [DOI] [PubMed] [Google Scholar]
- Kleiner M, Brainard D, Pelli D, Ingling A, Murray R, Broussard C, 2007. What’s new in Psychtoolbox-3. Perception 36, 1. [Google Scholar]
- Laumann TO, Gordon EM, Adeyemo B, Snyder AZ, Joo SJ, Chen M-Y, Gilmore AW, McDermott KB, Nelson SM, Dosenbach NU, 2015. Functional system and areal organization of a highly sampled individual human brain. Neuron 87, 657–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefaucheur J-P, André-Obadia N, Antal A, Ayache SS, Baeken C, Benninger DH, Cantello RM, Cincotta M, de Carvalho M, De Ridder D, 2014. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin. Neurophysiol 125, 2150–2206. [DOI] [PubMed] [Google Scholar]
- Legon W, Sato TF, Opitz A, Mueller J, Barbour A, Williams A, Tyler WJ, 2014. Transcranial focused ultrasound modulates the activity of primary somatosensory cortex in humans. Nat. Neurosci 17, 322. [DOI] [PubMed] [Google Scholar]
- Leitão J, Thielscher A, Tuennerhoff J, Noppeney U, 2017. Comparing TMS perturbations to occipital and parietal cortices in concurrent TMS-fMRI studies—methodological considerations. PLoS One 12 e0181438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitão J, Thielscher A, Tünnerhoff J, Noppeney U, 2015. Concurrent TMS-fMRI reveals interactions between dorsal and ventral attentional systems. J. Neurosci 35, 11445–11457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitão J, Thielscher A, Werner S, Pohmann R, Noppeney U, 2012. Effects of parietal TMS on visual and auditory processing at the primary cortical level–a concurrent TMS-fMRI study. Cereb. Cortex 23, 873–884. [DOI] [PubMed] [Google Scholar]
- Liu Z, Ding L, He B, 2006. Integration of EEG/MEG with MRI and fMRI. IEEE Eng. Med. Biol. Mag 25, 46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK, Wandell BA, 2004. Interpreting the BOLD signal. Annu. Rev. Physiol 66, 735–769. [DOI] [PubMed] [Google Scholar]
- Loureiro JR, Hagberg GE, Ethofer T, Erb M, Bause J, Ehses P, Scheffler K, Himmelbach M, 2017. Depth‐dependence of visual signals in the human superior colliculus at 9.4 T. Hum. Brain Mapp 38, 574–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moisa M, Pohmann R, Ewald L, Thielscher A, 2009. New coil positioning method for interleaved transcranial magnetic stimulation (TMS)/functional MRI (fMRI) and its validation in a motor cortex study. J. Magn. Reson. Imaging 29, 189–197. [DOI] [PubMed] [Google Scholar]
- Navarro de Lara LI, Windischberger C, Kuehne A, Woletz M, Sieg J, Bestmann S, Weiskopf N, Strasser B, Moser E, Laistler E, 2015. A novel coil array for combined TMS/fMRI experiments at 3 T. Magn. Reson. Med 74, 1492–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh SH, Chung JY, In MH, Zaitsev M, Kim YB, Speck O, Cho ZH, 2012. Distortion correction in EPI at ultra‐high‐field MRI using PSF mapping with optimal combination of shift detection dimension. Magn. Reson. Med 68, 1239–1246. [DOI] [PubMed] [Google Scholar]
- Pérez-Bellido A, Anne Barnes K, Crommett LE, Yau JM, 2017. Auditory frequency representations in human somatosensory cortex. Cereb. Cortex 28, 3908–3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JC, Reithler J, Schuhmann T, De Graaf T, Uludağ K, Goebel R, Sack AT, 2012. On the feasibility of concurrent human TMS-EEG-fMRI measurements. J. Neurophysiol 109, 1214–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahnev D, Nee DE, Riddle J, Larson AS, D’Esposito M, 2016. Causal evidence for frontal cortex organization for perceptual decision making. Proc. Natl. Acad. Sci 113, 6059–6064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis J, Swayne OB, Vandermeeren Y, Camus M, Dimyan MA, Harris‐Love M, Perez MA, Ragert P, Rothwell JC, Cohen LG, 2008. Contribution of transcranial magnetic stimulation to the understanding of cortical mechanisms involved in motor control. J. Physiol 586, 325–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson MD, Gore JC, Constable RT, 1997. Measurement of the point spread function in MRI using constant time imaging. Magn. Reson. Med 38, 733–740. [DOI] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A, SoTC G, 2009. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin. Neurophysiol 120, 2008–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff CC, Bestmann S, Blankenburg F, Bjoertomt O, Josephs O, Weiskopf N, Deichmann R, Driver J, 2007. Distinct causal influences of parietal versus frontal areas on human visual cortex: evidence from concurrent TMS–fMRI. Cereb. Cortex 18, 817–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff CC, Blankenburg F, Bjoertomt O, Bestmann S, Freeman E, Haynes J-D, Rees G, Josephs O, Deichmann R, Driver J, 2006. Concurrent TMS-fMRI and psychophysics reveal frontal influences on human retinotopic visual cortex. Curr. Biol 16, 1479–1488. [DOI] [PubMed] [Google Scholar]
- Sack AT, Kohler A, Bestmann S, Linden DE, Dechent P, Goebel R, Baudewig J, 2007. Imaging the brain activity changes underlying impaired visuospatial judgments: simultaneous FMRI, TMS, and behavioral studies. Cereb. Cortex 17, 2841–2852. [DOI] [PubMed] [Google Scholar]
- Sack AT, Kohler A, Linden DE, Goebel R, Muckli L, 2006. The temporal characteristics of motion processing in hMT/V5+: combining fMRI and neuronavigated TMS. Neuroimage 29, 1326–1335. [DOI] [PubMed] [Google Scholar]
- Shastri A, George M, Bohning D, 1999. Performance of a system for interleaving transcranial magnetic stimulation with steady-state magnetic resonance imaging. Electroencephalogr. Clin. Neurophysiol 51 (Supplement), 55. [PubMed] [Google Scholar]
- Shitara H, Shinozaki T, Takagishi K, Honda M, Hanakawa T, 2011. Time course and spatial distribution of fMRI signal changes during single-pulse transcranial magnetic stimulation to the primary motor cortex. Neuroimage 56, 1469–1479. [DOI] [PubMed] [Google Scholar]
- Taylor AJ, Kim JH, Ress D, 2018. Characterization of the hemodynamic response function across the majority of human cerebral cortex. NeuroImage 173, 322–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Bovik AC, Sheikh HR, Simoncelli EP, 2004. Image quality assessment: from error visibility to structural similarity. IEEE Trans. Image Process 13, 600–612. [DOI] [PubMed] [Google Scholar]
- Weiskopf N, Hutton C, Josephs O, Deichmann R, 2006. Optimal EPI parameters for reduction of susceptibility-induced BOLD sensitivity losses: a whole-brain analysis at 3 T and 1.5 T. Neuroimage 33, 493–504. [DOI] [PubMed] [Google Scholar]
- Weiskopf N, Josephs O, Ruff CC, Blankenburg F, Featherstone E, Thomas A, Bestmann S, Driver J, Deichmann R, 2009. Image artifacts in concurrent transcranial magnetic stimulation (TMS) and fMRI caused by leakage currents: modeling and compensation. J. Magn. Reson. Imaging 29, 1211–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Opitz A, Craddock RC, Wright MJ, Zuo X-N, Milham MP, 2016. Assessing variations in areal organization for the intrinsic brain: from fingerprints to reliability. Cereb. Cortex 26, 4192–4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau JM, Hua J, Liao DA, Desmond JE, 2013. Efficient and robust identification of cortical targets in concurrent TMS–fMRI experiments. Neuroimage 76, 134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau JM, Jalinous R, Cantarero GL, Desmond JE, 2014. Static field influences on transcranial magnetic stimulation: considerations for TMS in the scanner environment. Brain Stimul. 7, 388–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaitsev M, Hennig J, Speck O, 2004. Point spread function mapping with parallel imaging techniques and high acceleration factors: fast, robust, and flexible method for echo‐planar imaging distortion correction. Magn. Reson. Med 52, 1156–1166. [DOI] [PubMed] [Google Scholar]
- Zeng H, Constable RT, 2002. Image distortion correction in EPI: comparison of field mapping with point spread function mapping. Magn. Reson. Med 48, 137–146. [DOI] [PubMed] [Google Scholar]