Abstract
Background:
Inconsistent findings have been reported regarding the association of C-reactive protein to albumin ratio (CAR) with survival outcome in patients with pancreatic cancer. We conducted the current meta-analysis to assess the prognostic utility of elevated baseline CAR in predicting overall survival (OS) in pancreatic cancer patients.
Methods:
A comprehensively literature search was performed in the PubMed and Embase database until February 10, 2019. Studies evaluating the association between pretreatment CAR and OS among pancreatic cancer were selected. Study quality was evaluated by using the Newcastle-Ottawa Scale.
Results:
Nine retrospective studies involving 1534 pancreatic cancer patients were identified. A meta-analysis using a random-effect model indicated that elevated CAR was associated with poor OS (hazard ratio 1.98; 95% confidence interval 1.58–2.48). Subgroup analysis produced similar prognostic values for OS in different geographical regions, sample sizes, thresholds of CAR, treating methods, and Newcastle-Ottawa Scale points.
Conclusion:
Elevated pretreatment CAR may independently predict poor OS in pancreatic cancer patients. Pretreatment CAR is possibly a simple and cost-effective blood-derived indicator for predicting survival outcome in patients with pancreatic cancer.
Keywords: C-reactive protein/albumin ratio, meta-analysis, overall survival, pancreatic cancer
1. Introduction
Pancreatic cancer remains the sixth most frequent malignancy.[1] Inoperable advanced pancreatic cancer accounts for approximately 80% to 85% of patients.[2] Despite great advancement in chemotherapy and surgery, the 5-year survival rate of advanced pancreatic cancer is less than 5%.[3] Therefore, identification of new prognostic markers is very crucial for improved risk stratification in advanced pancreatic cancer patients.
Cancer-related inflammation play an important role in carcinogenesis and tumor progression.[4,5] Chronic systemic inflammation markers, such as Glasgow prognostic score (GPS) or modified GPS (mGPS), platelet-lymphocyte ratio (PLR), and neutrophil-to-lymphocyte ratio (NLR) have been linked to the survival of cancer patients.[6] The mGPS established on the C-reactive protein (CRP) and albumin level is recognized as a useful prognostic score for predicting the prognosis of cancer patients.[7] CRP/albumin ratio (CAR), a new developed predictive indicator, reflects both inflammatory and nutritional status of cancer patients. Both inflammation and malnutrition contribute to poor outcomes in cancer patients. An elevated CAR indicates a worse general condition for cancer patients.[8] CAR has been identified as an independent prognostic marker for survival outcomes in patients with solid tumors.[9] However, conflict results[10–15] have been reported regarding the association of elevated baseline CAR with survival outcome in pancreatic cancer patients. Thus, the predicting role of CAR in these patients remains inconclusive.
Among pancreatic cancer patients, no previous meta-analysis has specially focused on the use of pretreatment CAR in the prediction of overall survival (OS). We therefore performed the current meta-analysis to investigate the prognostic utility of elevated baseline CAR in predicting OS in patients with pancreatic cancer.
2. Materials and methods
2.1. Literature search
The current meta-analysis was followed by the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-analysis.[16] Ethical approval was not necessary because this study is a study-level analysis. A comprehensively literature search was performed in the PubMed and Embase database from their inceptions to February 10, 2019. The literature search applied the following strategy: C-reactive protein/albumin ratio or C-reactive protein to albumin ratio and pancreatic cancer or pancreatic tumor or pancreatic carcinoma or “pancreatic ductal adenocarcinoma”. Additionally, the reference lists of pertinent articles were also manually searched for any possible missing studies.
2.2. Inclusion and exclusion criteria
Studies satisfying the following inclusion criteria were eligible:
-
(1)
observational studies enrolling patients with confirmed diagnosis of pancreatic cancer;
-
(2)
exposure was baseline CAR;
-
(3)
reported multivariable adjusted hazard ratio (HR) and 95% confidence interval (CI) for OS associated with elevated CAR.
Exclusion criteria included
-
(1)
reported unadjusted risk estimates;
-
(2)
post-treatment CAR as exposure; and
-
(3)
meeting abstracts, reviews, or comments.
2.3. Data extraction and study quality
The following items were independently extracted by 2 authors from the included studies: surname of the first author, publication year, country, design of study, sample sizes, gender, mean/median age, cancer stage, type of treatment, cutoff value for CAR, duration of follow-up, multivariable adjusted risk HR with 95% CI, and variables in the adjustment model. Methodological quality was evaluated using the Newcastle–Ottawa Scale (NOS) for cohort studies by 2 independent authors.[17] Studies with NOS scores of 7 points or over were recognized as high-quality. Disagreement between 2 authors was resolved through consensus.
2.4. Data synthesis and analyses
The prognostic value of elevated baseline CAR was pooled by the multivariable adjusted HR and 95% CI. The Cochrane Q test and the I2 statistic were used to check the presence of heterogeneity between studies.
P value < .1 of Cochrane Q test or I2 statistic ≥50% indicates the presence of statistical significant heterogeneity. A random-effect model was selected for the pooling analysis in the presence of significant heterogeneity. Otherwise, a fixed-effect model was selected. Publication bias was quantitatively examined using the Begg rank correlation[18] and Egger linear regression test,[19] with significance level set at P < .10. Furthermore, a trim-and-fill approach was used to explore the influence of possible missing studies. Furthermore, we conducted the subgroup analysis according to sample sizes (≥100 vs <100), country (Japan vs South Korea vs China), treating methods (chemotherapy vs resection), thresholds of CAR (≤0.5 vs >0.5), and NOS points (<7 vs ≥7). Sensitivity analysis was conducted by sequentially omitting 1 study at each turn from the meta-analysis. All statistical analyses were carried out with STATA 12.0 (Stata Corp LP, TX).
3. Results
3.1. Search results and study features
Initially, 361 potentially pertinent articles were identified by searching the above-mentioned databases. Of which, 255 duplicates were removed. Eighty-six records were removed after scanning the titles or abstracts. The remaining 22 articles were retrieved for full-text assessment. Finally, 9 studies[10–13,20–24] were included in the meta-analysis (Fig. 1).
Figure 1.
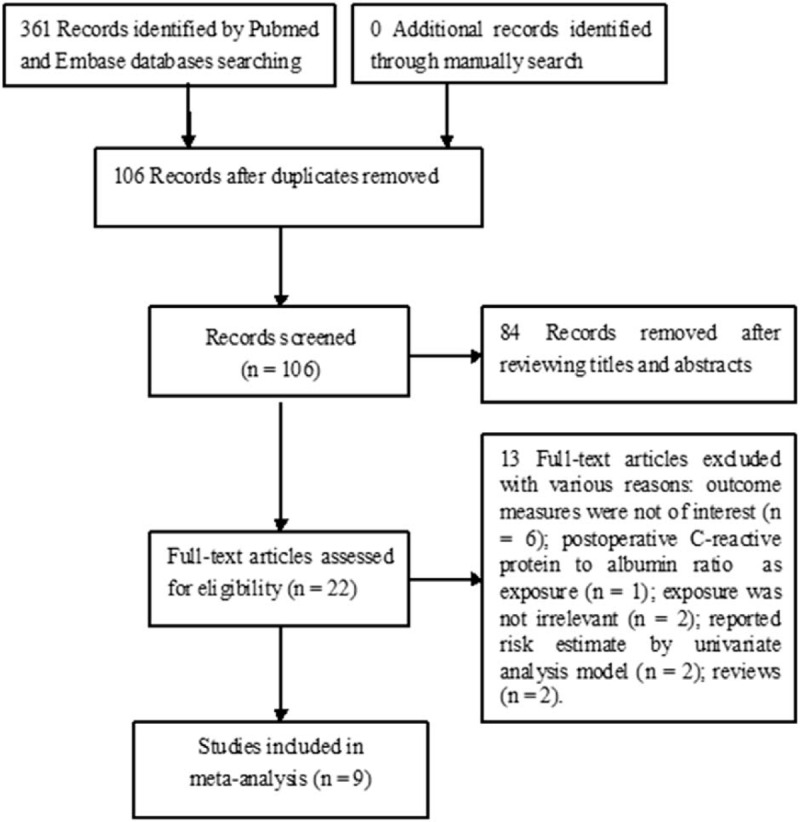
Flowchart showing the study selection process.
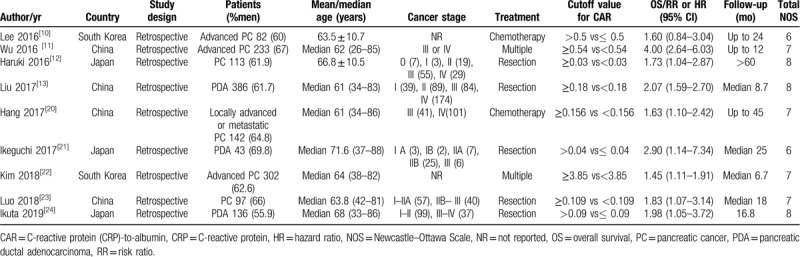
Table 1 shows the main feature of included studies. All eligible studies were retrospective designs. These included studies were published from 2016 to 2019 and conducted in China,[11,13,20,23] South Korea,[10,22] and Japan.[12,21,24] The patients number of included studies varied from 43 to 386, with a total of 1534 pancreatic cancer patients. Five studies only included surgical resection patients, 2 studies enrolled patients undergoing chemotherapy, and the remaining studies enrolled patients receiving multiple treatments. The thresholds of elevated baseline CAR varied between 0.03 and 3.85. Seven studies[11–13,20,22–24] achieving 7 to 8 NOS points were defined as high quality.
Table 1.
Summary of clinical studies included in meta-analysis.
3.2. Impact of CAR on overall survival
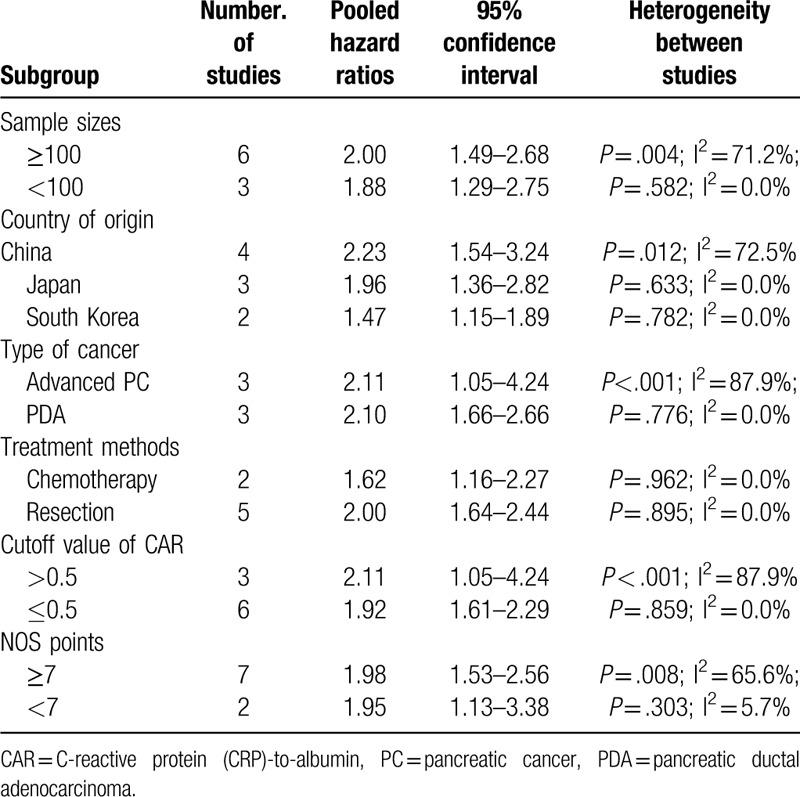
All the included studies reported results on serum CAR and OS in pancreatic cancer patients. As shown in Figure 2, there was significant heterogeneity across studies (I2 = 56.7%; P = .018). A random effect model meta-analysis indicated that elevated baseline CAR was associated with poor OS (HR 1.98; 95% CI 1.58–2.48) than those with lower CAR group. In the sensitivity analyses, removal of any individual studies had minimal impact on the pooled results (HR ranged from 1.76 to 2.10). P values of the Egger and Begg test were 0.585 and 0.466, respectively, which revealed no evidence of publication bias. In the trim-and-fill approach, there was 1 possible missing study in the funnel plot (Fig. 3). However, imputing this potential missing study did not significantly alter the original prognostic significance (HR 1.76; 95% CI 1.07–2.89; P = .027). Moreover, the significant prognostic roles were consistently observed in each named subgroup (Table 2).
Figure 2.
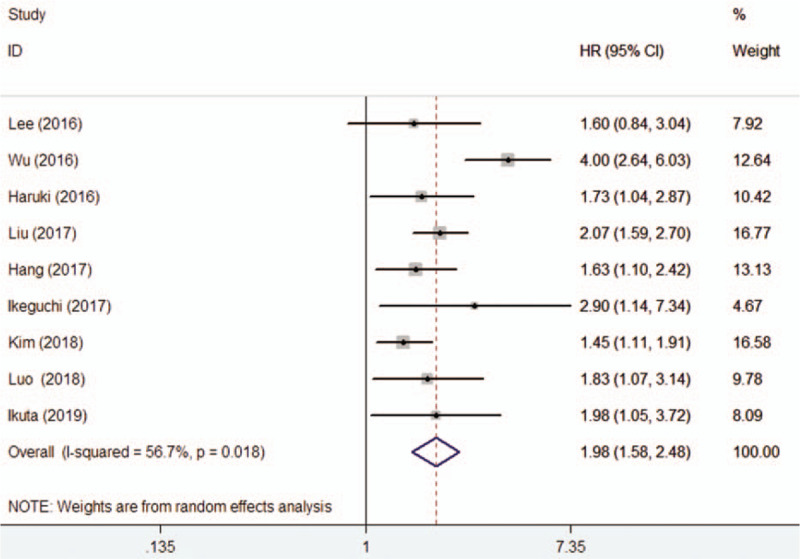
Forest plots showing HR and 95% CI of overall survival for high versus low C-reactive protein to albumin ratio in a random effect model. CI = confidence intervals, HR = hazard ratio.
Figure 3.
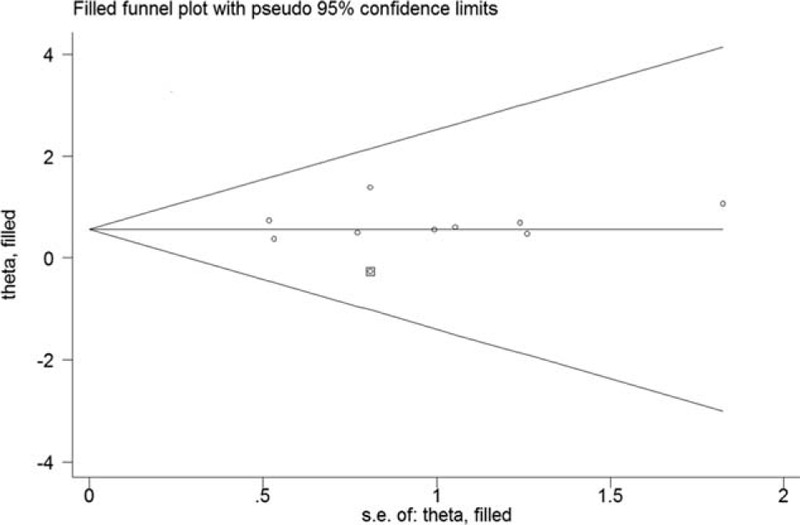
Funnel plot of high C-reactive protein to albumin ratio with overall survival. The circles alone are real studies and the circle enclosed in box is “filled” study.
Table 2.
Subgroup analyses of overall survival.
4. Discussion
Current results were obtained by analyzing data on 1534 pancreatic cancer patients in 9 retrospective studies. The main finding of this meta-analysis suggests that elevated pretreatment CAR may be independently predicted poor OS among pancreatic cancer patients. Pancreatic cancer patients with elevated CAR had a 98% higher risk of death compared with those with lower CAR level. Our subgroup analysis further confirmed the statistically significant prognostic value of elevated CAR in each named subgroup. Therefore, determination of CAR may be better defining the baseline risk in pancreatic cancer patients.
Several scoring systems based on the systemic inflammation have been developed to investigate the prognosis of pancreatic cancer patients.[25] Both mGPS and NLR are promising prognostic biomarkers for pancreatic cancer but PLR is less predictive than others.[26–28] In our analysis, the prognostic role of elevated baseline CAR in prediction of OS was superior to those of NLR and PLR. Jaundice is frequently observed in pancreatic cancer patients.[29] Malignant obstructive jaundice increases the circulation of inflammatory biomarkers.[30] Pancreatic cancer patients with jaundice may present high CAR and subsequently affect the prognostic value.
CAR was significantly associated with poor OS of pancreatic cancer patients, which is similar to the findings in lung cancer,[31] nasopharyngeal carcinoma,[32,33] colorectal cancer,[34] and various solid tumors.[35] In addition to OS, CAR also independently predicted the progression-free survival (HR 1.48; 95% CI 1.17–1.88)[22] and disease-free survival (HR 1.66; 95% CI 1.19–2.29)[15] in pancreatic cancer patients. There was no significant association between elevated baseline CAR and recurrence-free survival (HR 1.12; 95% CI 0.73–1.71).[14] However, elevated CAR at post-surgery day 14 but not baseline CAR independently predicted recurrence-free survival and OS.[14]
The cut-off values of CAR were lower in patients with surgical resection than in those without. This finding may be partly explained the better nutritional status in patients who received the resection. The prognostic mechanisms of CAR in pancreatic cancer are largely unknown. Cancer-related inflammatory responses may be associated with tumor progression and recurrence.[36] CRP has been demonstrated to independently predict poor survival in patients with pancreatic cancer.[37] Serum albumin level is an important biomarker of nutritional state. Meanwhile, serum level albumin also reflects a consequence of inflammatory status. Pretreatment hypoalbuminemia is also linked with poor survival in cancer patients.[38] CAR combined with the effects of CRP and albumin may continuously reflect the inflammatory and nutritional status of patients with different stages of pancreatic cancer. Therefore, CAR can improve the prognostic value than the single use of CRP or albumin.
Nevertheless, several limitations should be noted in this meta-analysis. First, all eligible studies were retrospective nature, which was more susceptible to recall bias and selection bias. Second, CRP is a non-specific inflammatory marker, and the coexistence of other systemic inflammatory diseases could influence CRP level. Third, cutoff values of high CAR were not unified and ranged between 0.04 and 3.85 across the included studies, which prevented us from determining the optimal cutoff value of CAR in predicting adverse outcomes. Fourth, we failed to perform a subgroup analysis based on tumor stages because the included studies did not report the risk estimate according to these characteristics. Future studies should investigate the prognostic utility of CAR according to the tumor stages. Fifth, all selected studies were from Asia, and the current results should be interpreted with caution in Western countries. Finally, the risk summary was established on the pooling multivariable adjusted risk estimates. Thus, prognostic value of CAR may have been overestimated because the lack of statistical significant risk estimate in the univariate analysis may be not included in the multivariable analysis.
5. Conclusions
In conclusion, the current meta-analysis indicates that elevated pretreatment CAR may independently predict poor OS in pancreatic cancer patients. Baseline CAR is possibly a simple and cost-effective blood-derived indicator for predicting survival outcome in patients with pancreatic cancer.
Author contributions
ZJ Gao contributed to study conception and design; Y Zang and Y Fan contributed to the literature search, data extraction, quality assessment, and statistical analysis. Y Zang drafted the manuscript.
Footnotes
Abbreviations: CAR = C-reactive protein to albumin ratio, CI = confidence intervals, GPS = Glasgow prognostic score, HR = hazard ratio, mGPS = modified Glasgow prognostic score, NLR = neutrophil-to-lymphocyte ratio, NOS = Newcastle–Ottawa Scale, OS = overall survival, PLR = platelet-lymphocyte ratio.
How to cite this article: Zang Y, Fan Y, Gao Z. Pretreatment C-reactive protein/albumin ratio for predicting overall survival in pancreatic cancer: a meta-analysis. Medicine. 2020;99:23(e20595).
The authors have no funding and conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files]. No funding support this manuscript.
References
- [1].Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- [2].Kamisawa T, Wood LD, Itoi T, et al. Pancreatic cancer. Lancet 2016;388:73–85. [DOI] [PubMed] [Google Scholar]
- [3].Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin 2016;66:271–89. [DOI] [PubMed] [Google Scholar]
- [4].Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature 2008;454:436–44. [DOI] [PubMed] [Google Scholar]
- [5].McKay CJ, Glen P, McMillan DC. Chronic inflammation and pancreatic cancer. Best practice & research. Clin Gastroenterol 2008;22:65–73. [DOI] [PubMed] [Google Scholar]
- [6].Bugada D, Allegri M, Lavand’homme P, et al. Inflammation-based scores: a new method for patient-targeted strategies and improved perioperative outcome in cancer patients. Biomed Res Int 2014;2014:142425.doi: 10.1155/2014/142425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].McMillan DC. The systemic inflammation-based Glasgow Prognostic Score: a decade of experience in patients with cancer. Cancer Treat Rev 2013;39:534–40. [DOI] [PubMed] [Google Scholar]
- [8].Miyamoto T, Fujitani M, Fukuyama H, et al. The C-reactive protein/albumin ratio is useful for predicting short-term survival in cancer and noncancer patients. J Palliat Med 2019;22:532–7. [DOI] [PubMed] [Google Scholar]
- [9].Cui X, Jia Z, Chen D, et al. The prognostic value of the C-reactive protein to albumin ratio in cancer: an updated meta-analysis. Medicine (Baltimore) 2020;99:e19165.doi: 10.1097/MD.0000000000019165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lee JM, Lee HS, Hyun JJ, et al. Prognostic value of inflammation-based markers in patients with pancreatic cancer administered gemcitabine and erlotinib. World J Gastro Oncol 2016;8:555–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wu M, Guo J, Guo L, et al. The C-reactive protein/albumin ratio predicts overall survival of patients with advanced pancreatic cancer. Tumour Biol 2016;37:12525–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Haruki K, Shiba H, Shirai Y, et al. The C-reactive protein to albumin ratio predicts long-term outcomes in patients with pancreatic cancer after pancreatic resection. World J Surg 2016;40:2254–60. [DOI] [PubMed] [Google Scholar]
- [13].Liu Z, Jin K, Guo M, et al. Prognostic value of the CRP/alb ratio, a novel inflammation-based score in pancreatic cancer. Ann Surg Oncol 2017;24:561–8. [DOI] [PubMed] [Google Scholar]
- [14].Arima K, Yamashita YI, Hashimoto D, et al. Clinical usefulness of postoperative C-reactive protein/albumin ratio in pancreatic ductal adenocarcinoma. Am J Surg 2018;216:111–5. [DOI] [PubMed] [Google Scholar]
- [15].Fujiwara Y, Haruki K, Shiba H, et al. C-reactive protein-based prognostic measures are superior at predicting survival compared with peripheral blood cell count-based ones in patients after curative resection for pancreatic cancer. Anticancer Res 2018;38:6491–9. [DOI] [PubMed] [Google Scholar]
- [16].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and. PLoS Med 2009;6:e1000097.doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wells G, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed October 15, 2020). [Google Scholar]
- [18].Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. [PubMed] [Google Scholar]
- [19].Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hang J, Xue P, Yang H, et al. Pretreatment C-reactive protein to albumin ratio for predicting overall survival in advanced pancreatic cancer patients. Sci Rep 2017;7:2993.doi: 10.1038/s41598-017-03153-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ikeguchi M, Hanaki T, Endo K, et al. C-reactive protein/albumin ratio and prognostic nutritional index are strong prognostic indicators of survival in resected pancreatic ductal adenocarcinoma. J Pancreat Cancer 2017;3:31–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kim HJ, Lee SY, Kim DS, et al. Inflammatory markers as prognostic indicators in pancreatic cancer patients who underwent gemcitabine-based palliative chemotherapy. Korean J Intern Med 2020;35:171–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Luo BY, Yang Y, Duan YF, et al. Preoperative C-reactive protein/albumin ratio predicts the prognosis of patients with resectable pancreatic cancer. Zhonghua wai ke za zhi 2018;56:712–7. [DOI] [PubMed] [Google Scholar]
- [24].Ikuta S, Aihara T, Yamanaka N. Preoperative C-reactive protein to albumin ratio is a predictor of survival after pancreatic resection for pancreatic ductal adenocarcinoma. Asia Pac J Clin Oncol 2019;15:e109–14. [DOI] [PubMed] [Google Scholar]
- [25].Ahmad J, Grimes N, Farid S, et al. Inflammatory response related scoring systems in assessing the prognosis of patients with pancreatic ductal adenocarcinoma: a systematic review. Hepatobiliary Pancreat Dis Int 2014;13:474–81. [DOI] [PubMed] [Google Scholar]
- [26].Chen H, Hu N, Chang P, et al. Modified Glasgow prognostic score might be a prognostic factor for hepatocellular carcinoma: a meta-analysis. Panminerva Med 2017;59:302–7. [DOI] [PubMed] [Google Scholar]
- [27].Zhou Y, Wei Q, Fan J, et al. Prognostic role of the neutrophil-to-lymphocyte ratio in pancreatic cancer: a meta-analysis containing 8252 patients. Clin Chim Acta 2018;479:181–9. [DOI] [PubMed] [Google Scholar]
- [28].Zhou Y, Cheng S, Fathy AH, et al. Prognostic value of platelet-to-lymphocyte ratio in pancreatic cancer: a comprehensive meta-analysis of 17 cohort studies. Onco Targets Ther 2018;11:1899–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sperti C, Frison L, Liessi G, et al. The management of obstructive jaundice in pancreatic cancer. Ann Ital Chir 2007;78:469–74. [PubMed] [Google Scholar]
- [30].Ljungdahl M, Osterberg J, Ransjo U, et al. Inflammatory response in patients with malignant obstructive jaundice. Scand J Gastroenterol 2007;42:94–102. [DOI] [PubMed] [Google Scholar]
- [31].Deng TB, Zhang J, Zhou YZ, et al. The prognostic value of C-reactive protein to albumin ratio in patients with lung cancer. Medicine (Baltimore) 2018;97:e13505.doi: 10.1097/MD.0000000000013505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Gao N, Yang RN, Meng Z, et al. The prognostic value of C-reactive protein/albumin ratio in nasopharyngeal carcinoma: a meta-analysis. Biosci Rep 2018;38:BSR20180686.doi: 10.1042/BSR20180686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Yang X, Liu H, He M, et al. Prognostic value of pretreatment C-reactive protein/albumin ratio in nasopharyngeal carcinoma: a meta-analysis of published literature. Medicine (Baltimore) 2018;97:e11574.doi: 10.1097/MD.0000000000011574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Fan Y, Xiang S, Dai Z, et al. Prognostic significance of C-reactive protein to albumin ratio in colorectal cancer patients: a meta-analysis. Int J Colorectal Dis 2019;34:1105–11. [DOI] [PubMed] [Google Scholar]
- [35].Wu J, Tan W, Chen L, et al. Clinicopathologic and prognostic significance of C-reactive protein/albumin ratio in patients with solid tumors: an updated systemic review and meta-analysis. Oncotarget 2018;9:13934–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010;140:883–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Stevens L, Pathak S, Nunes QM, et al. Prognostic significance of pre-operative C-reactive protein and the neutrophil-lymphocyte ratio in resectable pancreatic cancer: a systematic review. HPB (Oxford) 2015;17:285–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nut J 2010;9:69.doi: 10.1186/1475-2891-9-69. [DOI] [PMC free article] [PubMed] [Google Scholar]


