Abstract
Background:
To assess the association of the interaction between the rs9619311 and rs402007 polymorphisms and smoking with essential hypertension (EH) in a Chinese Han population.
Method:
Peripheral blood samples were extracted from 422 EH patients and 280 normotensive (NT) patients in a Chinese Han population. A whole blood genomic DNA extraction kit was used to extract genomic DNA from the blood samples. Polymerase chain reaction restriction fragment length polymorphism was used to detect the rs402007 polymorphism of a disintegrin and metalloproteinase with thrombospondin type motifs 1 gene and the rs9619311 polymorphism of the tissue inhibitor of metalloproteinase-3 gene. The distributions of the genotypes and alleles between the 2 study groups (EH and NT) were compared. The main risk factors for EH were determined by using logistic regression analysis. The effects of gene-gene and gene-smoking interactions on EH were analyzed using multifactor dimensional reduction.
Results:
The frequencies of the rs402007 GC + CC genotype and the C allele were significantly different between the EH and NT groups (0.68 vs 0.57, χ2 = 8.99a, P = .003, odds ratio [OR] = 1.19; 0.45 vs 0.32, χ2 = 22.16a, P < .001, OR = 1.38). The frequencies of the rs9619311 TC + CC genotype and the C allele were also significantly different between the 2 groups (0.33 vs 0.25, χ2 = 4.51a, P = .04, OR = 1.44; 0.18 vs 0.13, χ2 = 7.03a, P = .01, OR = 1.50). Logistic regression analysis suggests that the rs402007 and rs9619311 polymorphisms are independent risk factors for EH (OR = 2.37, 1.86; P < .001, respectively). The multifactor dimensionality redundant analysis results showed that the interaction among rs402007, rs9619311, and smoking was statistically significant (P = .001).
Conclusions:
A disintegrin and metalloproteinase with thrombospondin type motifs 1 rs402007 and tissue inhibitor of metalloproteinase-3 rs9619311 polymorphisms are associated with EH in a Chinese Han population, and there was a positive interaction among rs402007, rs9619311, and smoking.
Keywords: a disintegrin and metalloproteinase with thrombospondin type motifs 1, essential hypertension, gene polymorphism, smoking, tissue inhibitor of metalloproteinase-3
1. Introduction
Essential hypertension (EH) is a polygenic disease caused by the interaction between genetic and environmental factors.[1,2] Rs402007 is located in the first exon of a disintegrin and metalloproteinase with thrombospondin type motifs 1 (ADAMTS1) gene. ADAMTS1 is a newly discovered Zn2+-dependent metalloproteinase that is structurally related to the matrix metalloproteinase (MMP) family and has been implicated in a number of pathophysiological conditions, including osteoarthritis and more recently, atherosclerosis.[3–5] A great deal of research has shown that the rs402007 polymorphism is associated with acute cerebral infarction, coronary heart disease, and the efficacy of statins. In addition, we previously showed that the rs402007 polymorphism is associated with EH in Han Chinese.[6,7] The rs9619311 locus is located in the promoter region of the tissue inhibitor of metalloproteinase-3 (TIMP3) gene, which is a specific inhibitor of MMPs.[8,9] Therefore, we hypothesized that there may be some interaction between these polymorphisms (rs9619311 in ADAMTS1 and rs402007 in TIMP3) and environmental factors, specifically smoking, in EH in the Chinese Han population.
2. Materials and methods
2.1. Study patients
A total of 702 unrelated patients aged 34 to 86 (60.59 ± 9.16) years, who were hospitalized for the first time at Zhejiang Xinhua Hospital, were enrolled from June 2013 to June 2016. The patients were sorted into groups based on the presence or absence of EH according to the criteria of the 2018 European Society of Cardiology and European Society of Hypertension Guidelines for the Management of Arterial Hypertension. Group 1, which was classified as the EH group, consisted of 422 patients aged 40 to 86 (61.90 ± 8.95) years. Group 2, which was classified as the normotensive group, consisted of 280 patients aged 34 to 84 (58.63 ± 9.13) years. The exclusion criteria were acute and chronic glomerulonephritis, Cushing syndrome, renal arterial stenosis, sleep apnea, primary aldosteronism, coarctation of aorta, chromaffin tumor, acromegaly, long-term use of hormones, and the use of central nervous system and nonsteroidal anti-inflammatory drugs. The Ethics Committee of Zhejiang Xinhua Hospital approved our study, and informed consent was obtained from all study participants, who were fully informed about the purpose and procedures of this study. The study subjects had no consanguineous relationships with each other.
2.2. Methods
2.2.1. Extraction of genomic DNA
Blood samples were collected from the elbow vein (basilic vein or median vein) of fasting subjects, and genomic DNA was extracted by using the blood genomic DNA extraction kit (Terri Bioengineering Co., Ltd., Shanghai). The A260/A280 ratio was determined by using a quantitative nucleic acid analysis instrument. DNA specimens with an A260/A280 ratio between 1.7 and 2.0 were used as templates for polymerase chain reaction (PCR) amplification. All genomic DNA samples were stored at −80°C until use.
2.2.2. Primer design and PCR
The sequence of the target fragments were obtained from the Ensembl database, and primers were designed by using Primer 5.0 software. The sequences of the primers used for fragment amplification were as follows: TIMP-3 forward 5′-CCCCAAATCCCTTGCTGA-3′ and reverse 5′-TTGACTGTGCTTGGTGGA-3′, and ADAMTS-1 forward 5′-GGCGTCTTTGGGATGGAA-3′ and reverse 5′-CAGGAGACACCGCTCGTAG-3′. The PCR mixture consisted of 0.25 μL of 5 U/μL Taq DNA polymerase, 1.0 μL of DNA template, 0.3 μL of 20 μM primers (forward and reverse), 0.5 μL of 10 mM deoxy-ribonucleoside triphosphate Mix, 1 μL of 25 mM MgCl2, 2.5 μL of 10× buffer, and sterile water to bring the total reaction volume to 25 μL. The PCR conditions were as follows: pre-denaturation for 2 minutes (95°C), followed by 35 cycles of denaturation for 45 seconds (94°C), annealing for 45 seconds (53°C), and extension for 1 minute (72°C), with a final extension step at 72°C for 10 minutes. The PCR products were visualized by a gel imaging analyzer after 1.8% agarose gel electrophoresis and ethidium bromide staining.
2.2.3. Sequencing and mutation analyses
The amplified target DNA fragments in the agarose gel were recovered and purified by gel exaction, and DNA sequencing was carried out.
2.3. Statistical analysis
The data were analyzed by using SPSS, version 22.0 statistical software. Numerical data were analyzed by using the R × C Chi-squared test (χ2) and partitions of the χ2 method. Measurement data were compared by the t-test or nonparametric rank sum test. Risk factors for EH were screened by logistic regression analysis. Multifactor dimensionality redundant (MDR) software was used to analyze gene-gene and gene-environment interactions. All statistical tests were 2-tailed, and P-values less than .05 were considered statistically significant.
3. Results
3.1. Comparison of patient data between the 2 groups
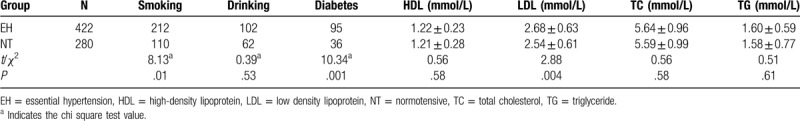
There was no significant difference between the 2 groups in the levels of high-density lipoprotein-cholesterol, total cholesterol (TC), triglyceride, and alcohol consumption (P > .05). The following variables showed statistically significant differences between the EH and NT groups: age, smoking, low-density lipoprotein-cholesterol (LDL-C), and diabetes (P < .05). See Table 1 for details.
Table 1.
Comparison of patient characteristics between the normotensive and essential hypertension groups.
3.2. Comparison of the genotypes and alleles between the 2 groups
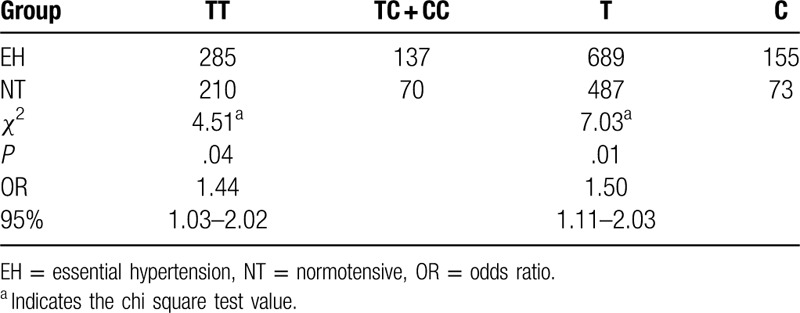
The results of the genotyping of the rs402007 (G/C) and rs9619311 (T/C) loci are shown in Figure 1. The frequencies of GG, GC, and CC at the rs402007 (G/C) locus were 0.36, 0.47, and 0.17, respectively, and the genotypic distribution was in Hardy–Weinberg equilibrium (χ2 = 0.27, P = .60). The frequencies of the G and C alleles were 0.60 and 0.40, respectively. The frequency of the GC + CC genotype was 0.682 in the EH group and 0.57 in the NT group, and this difference was statistically significant (χ2 = 8.99a, P = .003, odds ratio [OR] = 1.19). The frequencies of the C allele in the EH and NT groups were 0.45 and 0.32, respectively, and this difference was statistically significant (χ2 = 22.16a, P < .001, OR = 1.39). The genotypic distribution between the groups was in Hardy–Weinberg equilibrium (χ2 = 2.20, 0.14, P = 3.51, .06). See Table 2 for details. The frequencies of TT, TC, and CC at the rs9619311 (T/C) locus were 0.71, 0.27, and 0.03, respectively, and the genotypic distribution was in Hardy–Weinberg equilibrium (χ2 = 0.48, P = .49). The frequencies of the T and C alleles were 0.84 and 0.16. The frequency of the TC + CC genotype was 0.33 in the EH group and 0.25 in the NT group, and this difference was statistically significant (χ2 = 4.51a, P = .04, OR = 1.44). The frequencies of the C allele in the EH and NT groups were 0.18 and 0.13, respectively, and this difference was statistically significant (χ2 = 7.03a, P = .01, OR = 1.50). The genotypic distribution between the 2 groups was in Hardy–Weinberg equilibrium (χ2 = 1.50, 0.86, P = .22, .35, respectively). See Table 3 for details.
Figure 1.
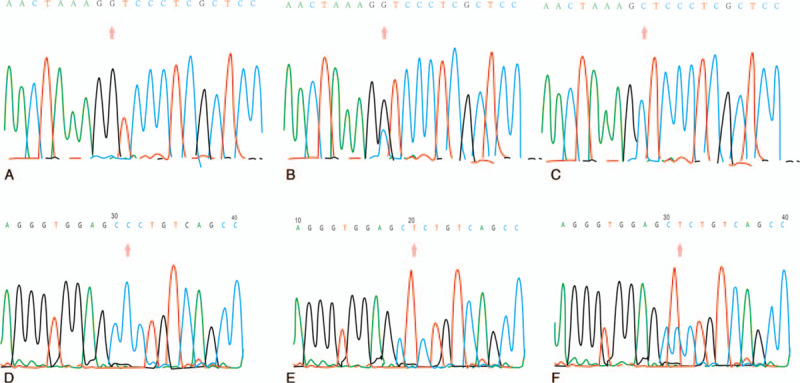
Sequencing of the ADAMTS1 rs402007 loci: (A) GG genotype; (B) GC genotype; (C) CC genotype. Sequencing of the TIMP3 rs9619311 loci: (D) CC genotype; (E) TT genotype; (F) TC genotype.
Table 2.
Comparison of genotype and allele frequencies at the rs402007 locus of the ADAMTS1 gene between the essential hypertension and normotensive groups.
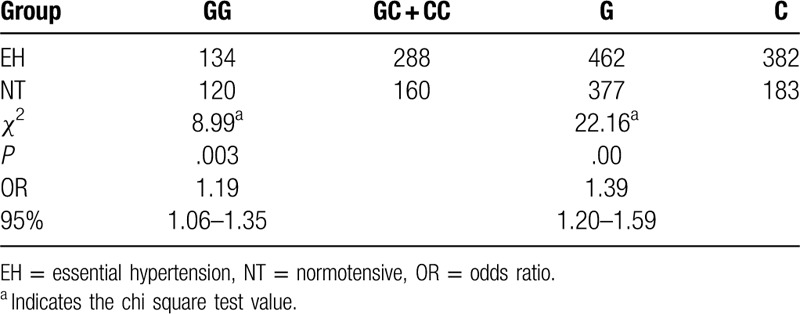
Table 3.
Comparison of genotype and allele frequencies of the rs9619311 locus of the TIMP3 gene between the essential hypertension and normotensive groups.
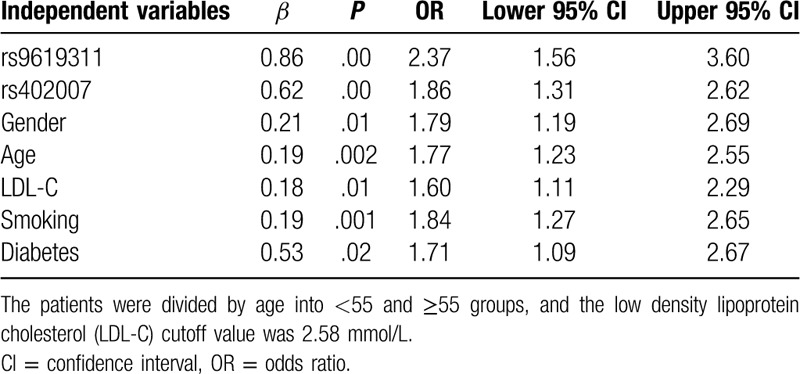
3.3. Logistic regression analysis of risk factors associated with EH
After adjusting for gender, age, smoking, LDL-C, and other risk factors, there were statistically significant differences in the rs9619311 and rs402007 genotypes between the 2 groups (OR = 2.37, 1.86; P < .001, respectively). See Table 4 for details.
Table 4.
Logistic regression analysis of the risk factors associated with essential hypertension.
3.4. Interaction between gene polymorphisms and smoking
The rs402007 and rs9619311 polymorphisms and smoking were analyzed by MDR, and the results showed that there was interaction among rs402007, rs9619311, and smoking (P = .001). The interaction model between rs402007 and rs9619311 had the highest validation sample accuracy and cross-validation consistency, and the results were statistically significant (P = .001). See Figures 2 and 3 for details.
Figure 2.
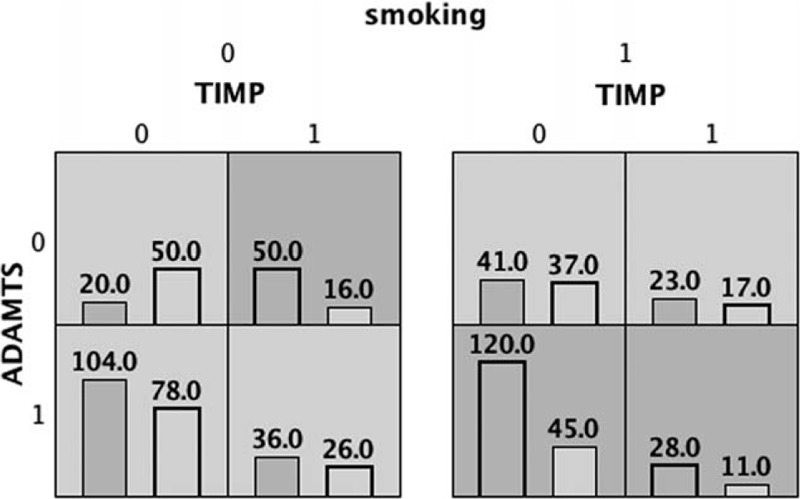
The left column in each cell represents the number of EH cases, and the right column represents the number of NT cases. EH = essential hypertension, NT = normotensive.
Figure 3.
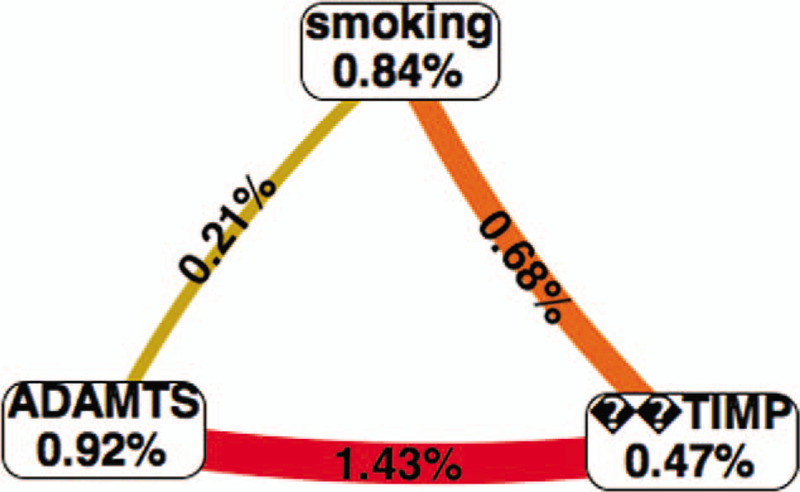
Red indicates strong synergy.
4. Discussion
EH is a major risk factor for coronary heart disease, cerebral infarction, and other cardiovascular and cerebrovascular diseases. Every year, approximately 7.5 million people die of EH worldwide.[10] Changes in the composition of the peripheral vascular wall, especially structural remodeling of the extracellular matrix in the vascular wall, play an important role in the pathogenesis of EH.[11] As an inflammatory related protein, ADAMTS1 is associated with the occurrence and development of EH. Ren et al suggested that ADAMTS1 is involved in regulating the proliferation of vascular smooth muscle cells and plays an important role in the structural remodeling of the vascular wall by degrading polyglycan.[12] Weiss et al also suggested that ADAMTS1 regulates the growth of fibroblasts by binding to FGF-2, and promotes the formation and maturation of collagen fibers, which has an effect on components of the extracellular matrix in the vascular wall.[13] The results of the present study showed that the frequencies of the GC + CC genotype and C allele at the rs402007 locus of the ADAMTS1 gene were higher in the EH group than in the NT group. Logistic regression analysis indicated that it is an independent risk factor for EH. C allele carriers were 1.385 times more likely to develop EH than non-C allele carriers, which is consistent with the results of our previous study, suggesting that the C allele is a susceptibility allele for EH. TIMP-3 is located in the q12.1-q13.2 region of chromosome 22, and it contains 5 exons and 4 introns. A study of 1000 people aged 50 to 60 years showed a significant association between the C allele at the rs9619311 locus of TIMP-3 gene and hypertension.[14] In their report, Schrimpf et al suggested that TIMP3 has a variety of biological activities that may contribute to blood vessel stability, and most importantly, it inhibits the activity of many metalloproteinases, including ADAMTS1 and MMPs.[15] Our study showed that the frequencies of the T and C alleles at the rs9619311 locus of TIMP-3 were 0.84 and 0.16, respectively, and that C allele carriers are 1.50 times more likely to develop EH, which is consistent with the genotype frequency previously reported in a southern Chinese population. Therefore, we speculated that the C allele at the rs9619311 locus of TIMP-3 gene might alter protein expression by changing a transcription factor-binding site, which may upset the balance of TIMP-3, ADAMTS1, and MMPs and promote remodeling of the extracellular matrix in the peripheral vascular wall, thus increasing the risk of EH.
EH is a polygenic hereditary disease that is affected by both genes and environmental factors. Gene-gene and/or gene-environment interactions play an important role in the occurrence and development of EH. Studies by Sun et al showed that the I/D and A2350 G locus in the angiotensin converting enzyme gene interact with age, sex, LDL-C, triglyceride, and other factors. Zawilla et al studied the I/D and A2350 G polymorphism of the angiotensin converting enzyme gene. Their results showed that smoking increased the prevalence of EH in the population carrying the AG, GG, and DD genotypes.[16] Smoking is an important risk factor for EH. In studying the relationship between the gene-smoking interaction and EH through an analysis of 6889 patients in the Frampham Heart Research Center, Sung et al identified 7 significant loci as well as 21 additional possible loci that interact with smoking.[17] Basson et al showed that the interaction of the rs9399633 and rs11717948 loci with smoking has a significant effect on systolic blood pressure.[17] Taylor et al found that 2 genetic polymorphisms, rs11158609 and rs8078051, may interact with smoking in African Americans with EH.[18] The relationship between EH and the interaction of the polymorphisms in the ADAMTS1 and TIMP-3 genes and smoking were assessed by using the interaction model theory and MDR software. The results showed that there was an interaction between these 3 components (P = .001); however, further research is needed.
There are many limitations to this study. First, in this study, only 2 single nucleotide polymorphisms were studied, and many other EH-related single nucleotide polymorphisms were omitted. Second, the protein expression levels of ADAMTS1 and TIMP in each group were not assessed. Third, this study is an association study conducted at a single center and single population. Due to the influence of ethnicity and region, the results of this study may not be replicated in other populations. Finally, the sample size of this study was 702, which is smaller than that of the large association study. Therefore, our team will try to improve these limitations in future studies.
5. Conclusions
The rs402007 ADAMTS-1 and rs9619311 TIMP-3 polymorphisms may be associated with susceptibility to EH, and the interaction between these polymorphisms and smoking may increase the risk of EH.
Author contributions
Conceptualization: Ming Yang.
Data curation: Liping Dou.
Formal analysis: Dongming Lin.
Supervision: Shuwei Huang.
Writing – original draft: Chao Chen.
Writing – review and editing: Ming Yang.
Footnotes
Abbreviations: EH = essential hypertension, MDR = multifactor dimensionality reduction, NT = normotensive, PCR = polymerase chain reaction.
How to cite this article: Chen C, Yang M, Dou LP, Ling DM, Huang S. Association of the interaction between the rs9619311 and rs402007 polymorphisms and smoking with essential hypertension in Chinese Han population. Medicine. 2020;99:23(e20552).
CC and MY contributed equally to this work.
This work was supported by grants from the Traditional Chinese Medicine Key Research Project (grant number 2014ZZ004 to SW Huang).
The authors have no conflicts of interest.
References
- [1].Sun F, Zhang K, Li X, et al. Effects of angiotensin-converting enzyme gene and environment interaction on essential hypertension in the Han nationality. Wei Sheng Yan Jiu 2017;46:378–83. [PubMed] [Google Scholar]
- [2].Chen ML, Huang TP, Chen TW, et al. Interactions of genes and sodium intake on the development of hypertension: a cohort-based case-control study. Int J Environ Res Public Health 2018;15:E1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Salter RC, Ashlin TG, Kwan AP, et al. ADAMTS proteases: key roles in atherosclerosis? J Mol Med (Berl) 2010;88:1203–11. [DOI] [PubMed] [Google Scholar]
- [4].Porter S, Clark IM, Kevorkian L, et al. The ADAMTS metalloproteinases. Biochem J 2005;386:15–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Tortorella MD, Malfait F, Barve RA, et al. A review of the ADAMTS family, pharmaceutical targets of the future. Curr Pharm Des 2009;15:2359–74. [DOI] [PubMed] [Google Scholar]
- [6].Peters BJ, Rodin AS, Klungel OH, et al. Variants of ADAMTS1 modify the effectiveness of statins in reducing the risk of myocardial infarction. Pharmacogenet Genomics 2010;20:766–74. [DOI] [PubMed] [Google Scholar]
- [7].Xue W, Zhang Z, Zeng S, et al. Expression and clinical significance of tissue inhibitor of metalloproteinases-1 (TIMP-1) and a disintegrin and metalloproteinase with thrombospondin type 1 motif 1(ADAMTS1) in post-kidney-transplant bladder tumors. Ann Transplant 2017;22:622–30. [DOI] [PubMed] [Google Scholar]
- [8].Donghao Z, Bo X, Min Z, et al. Uncontrolled hypertension increases risk of all-cause and cardiovascular disease mortality in US adults: the NHANES III Linked Mortality Study. Sci Rep 2018;8:9418.1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res 2003;92:827–39. [DOI] [PubMed] [Google Scholar]
- [10].Galis ZS, Khatri JJ. Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad, and the ugly. Circ Res 2002;90:251–62. [PubMed] [Google Scholar]
- [11].Ren P, Zhang L, Xu G, et al. ADAMTS-1 and ADAMTS-4 levels are elevated in thoracic aortic aneurysms and dissections. Ann Thorac Surg 2013;95:570–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Weiss G, Goldsmith LT, Taylor RN, et al. Inflammation in reproductive disorders. Reprod Sci 2009;16:216–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Basu R, Lee J, Morton JS, et al. TIMP3 is the primary TIMP to regulate agonist-induced vascular remodelling and hypertension. Cardiovasc Res 2013;98:360–71. [DOI] [PubMed] [Google Scholar]
- [14].Schrimpf C, Koppen T, Duffield JS, et al. TIMP3 is regulated by pericytes upon shear stress detection leading to a modified endothelial cell response. Eur J Vasc Endovasc Surg 2017;54:524–33. [DOI] [PubMed] [Google Scholar]
- [15].Zawilla N, Shaker D, Abdelaal A, et al. Angiotensin-converting enzyme gene polymorphisms and hypertension in occupational noise exposure in Egypt. Int J Occup Environ Health 2014;20:194–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Yun JS, Fuentes LDL, Schwander KL, et al. Gene–smoking interactions identify several novel blood pressure loci in the Framingham heart study. Am J Hypertens 2015;28:343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Basson J, Sung YJ, Fuentes LL, et al. Influence of smoking status and intensity on discovery of blood pressure loci through gene-smoking interactions. Genet Epidemiol 2015;39:480–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Taylor JY, Sun YV, Barcelona VDM, et al. The combined effects of genetic risk and perceived discrimination on blood pressure among African Americans in the Jackson Heart Study. Medicine 2017;96:e8369. [DOI] [PMC free article] [PubMed] [Google Scholar]


