Abstract
Background:
This study will be proposed for investigating the effects of high-quality nursing intervention (HQNI) on the psychological disorder in patients with gastric cancer during perioperative period (GC-PPP).
Methods:
A cumulative search from inception up to the March 31, 2020 will be performed in the following databases: Cochrane Library, MEDLINE, EMBASE, Web of Science, VIP database, and China National Knowledge Infrastructure. We will search all potential studies from those electronic databases regardless their language and publication status. We will only consider randomized controlled trials (RCTs) for inclusion, which explores the effect of HQNI on the psychological disorder in patients with GC-PPP. Study identification, information extraction, and study quality appraisal will be independently and respectively done by 2 researchers. Any different opinions between 2 researchers will be disentangled by a third researcher after discussion. Cochrane risk of bias tool will be used for study quality assessment, and RevMan 5.3 software will be utilized for statistical analysis.
Results:
This study will provide a high-quality synthesis of psychological disorder outcomes to evaluate the effects and safety of HQNI for patients with GC-PPP.
Conclusion:
The findings of this study will provide reference and evidence to appraise whether HQNI is an effective on the psychological disorder in patients with GC-PPP
Study registration number:
INPLASY202040080.
Keywords: effect, gastric cancer, high-quality nursing intervention, psychological disorder, randomized controlled trial
1. Introduction
Gastric cancer (GC) remains one of the most common cancers, which is the leading cause of cancer-related death globally.[1–4] Previous epidemiological study reported more than 1,000,000 new cases and 783,000 deaths of GC around the world.[5,6] If it cannot be identified and treated at early stage, most patients result in very severe outcome results.[7–9] Thus, it is very important to diagnose and to treat this condition at early stage.
Surgery is the most widely treatment for GC.[10–12] However, most patients with gastric cancer during perioperative period (GC-PPP) experience psychological disorder.[13–18] Fortunately, high-quality nursing intervention (HQNI) is reported to mange this condition effectively.[19–24] It is still unclear whether HQNI on psychological disorder in patients with GC-PPP is effective and safe. Therefore, it is very necessary to systematically assess the effect of HQNI on psychological disorder in patients with GC-PPP, and to determine whether it is a good choice for such patients.
2. Methods
2.1. Study registration
We have registered this study through INPLASY202040080, and we have reported it following the guideline of the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols Statement.[25]
2.2. Ethics and dissemination
All data utilized in this study will be collected from previous trials. Thus, no ethic approval is needed. This study will be published through a peer-reviewed journal.
2.3. Criteria for included studies
2.3.1. Study types
This proposed study will include randomized controlled trials (RCTs) reported for assessing the effects of HQNI on the psychological disorder in patients with GC-PPP with no restrictions of language and publication status. We will exclude any uncontrolled trials, non-RCTs and quasi-RCTs.
2.3.2. Participants
All GC-PPP patients (18 years old or more) with psychological disorder, including depression and anxiety will be fully considered, regardless the race, gender, and country.
2.3.3. Interventions
Experimental group: We will include all patients who received HQNI for the management of psychological disorder.
Control group: We will consider participants who underwent any treatments. However, we will exclude patients who also received any forms of HQNI.
2.3.4. Outcomes
Primary outcomes are depression (as measured by any validated scales, such as Hamilton Depression Rating Scale), and anxiety (as assessed by any validated scores, such as Hamilton Anxiety Rating Scale).
Secondary outcomes consist of health-related quality of life (as checked by any relevant tools, such as 36-Item Short Form Health Survey), and incidence of any adverse events.
2.4. Strategy of literature searches
The following electronic databases will be retrieved cumulatively from inception up to the March 31, 2020: Cochrane Library, MEDLINE, EMBASE, Web of Science, VIP database, and China National Knowledge Infrastructure. The RCTs of HQNI for the management of psychological disorder in patients with GC-PPP will be searched from above databases. The literatures included will not subject to any language and publication status. The Cochrane Library search strategy is presented in Table 1.
Table 1.
Search strategy of Cochrane Library.
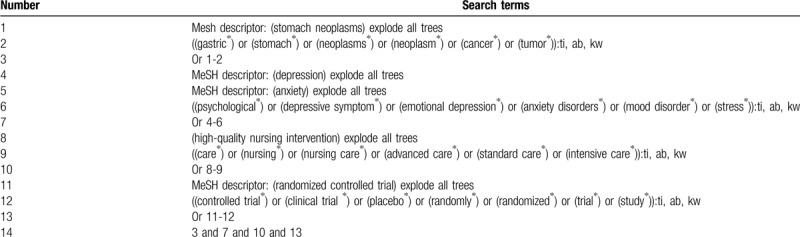
In addition, a reference list of included RCTs and related reviews will be examined. We will search relevant conference abstracts and new trials from the clinical trial registry.
2.5. Data collection
2.5.1. Study selection
Two researchers will import all searched records into Endnote X7 and all duplicate citations will be eliminated. The titles and abstracts of all searched literatures will be identified according to the eligibility criteria. All irrelevant studies will be excluded. The potential trials will be furthered assessed by reading the full-text papers. All excluded studies will be noted and listed in the table with specific reasons. Any differences will be solved by a third researcher through consultation. The selection of study process is presented in a flow diagram.
2.5.2. Data extraction
All essential data will be collected by 2 independent researchers using a predefined data extraction sheet. Any inconsistent views will be figured out through discussion by a third researcher. This data extraction sheet will consist of study information, time of publication, first author, participants, diagnostic criteria, inclusion and exclusion criteria, randomization details, blind, allocation, interventions, comparators, indicators, findings, and adverse events. If any unclear or missing data is identified, we will contact the trial authors to obtain such information.
2.6. Assessment of risk of bias for included trials
Two independent researchers will assess the risk of bias for each included trial using
Cochrane Collaboration Tool through 7 items. Each item is graded as low, unclear, and high risk of bias. If any different opinions occur between 2 researchers, we will invite a third researcher to solve them by discussion.
2.7. Statistical analysis
We will apply ReMan 5.3 software to perform statistical analysis. We will express continuous values using mean difference or standardized mean difference and 95% confidence intervals, and dichotomous values using risk ratio and 95% confidence intervals. I2 test will be utilized to assess inconsistencies and heterogeneity across included trials. I2 ≤ 50% means a minor heterogeneity, and a fixed-effect model will be used to synthesize the outcome indicator data. I2 > 50% exerts substantial heterogeneity, and a random-effect model will be utilized to pool the outcome indicator data. If ample data are included for specific types of intervention and control, a meta-analysis will be undertaken if trials are sufficiently similar with respect to the study information, patient characteristics, interventions, controls, and outcome indicators. Otherwise, we will carry out subgroup analysis to identify possible sources of the significant heterogeneity.
2.7.1. Subgroup analysis
Subgroup analysis will be conducted based on the different interventions, comparators, and outcome indicators to explore any possible sources of significant heterogeneity among included trials.
2.7.2. Sensitivity analysis
If necessary, sensitivity analysis will be undertaken to investigate the robustness and stability of study findings by removing studies with high risk of bias.
2.7.3. Reporting bias
We will also perform funnel plot[26] and Egger regression test[27] to check any possible reporting bias if at least 10 included trials are included.
2.7.4. Grading the quality of evidence
We will evaluate the quality of evidence for each outcome indicator through the Grading of Recommendations Assessment Development and Evaluation approach.[28] It rates as 5 levels of very low, low, medium, and high. Its results will be summarized in the "Summary of Findings” table.
3. Discussion
The purpose of this study is to assess the effect of HQNI on psychological disorder in patients with GC-PPP. To our best knowledge, this is the first study to investigate the effect of HQNI on psychological disorder in patients with GC-PPP. The conclusions drawn from this study may be beneficial to both patient and clinicians, and health-related policy makers. However, there are some potential limitations in this study. First, there may be a risk of heterogeneity in study quality, and an insufficient number of high quality RCTs. Second, there may be a small sample size of included studies. Finally, there may be missing potential eligible studies, although we try our best to search more electronic databases and grey literatures.
Author contributions
Conceptualization: Xiu-Li He, Zhi-Min Cao.
Data curation: Xiu-Li He, Zhi-Min Cao.
Formal analysis: Xiu-Li He, Zhi-Min Cao.
Investigation: Zhi-Min Cao.
Methodology: Xiu-Li He.
Project administration: Zhi-Min Cao.
Resources: Xiu-Li He.
Software: Xiu-Li He.
Supervision: Zhi-Min Cao.
Validation: Xiu-Li He, Zhi-Min Cao.
Visualization: Xiu-Li He, Zhi-Min Cao.
Writing – original draft: Xiu-Li He, Zhi-Min Cao.
Writing – review & editing: Xiu-Li He, Zhi-Min Cao.
Footnotes
Abbreviations: GC = Gastric cancer, GC-PPP = gastric cancer during perioperative period, HQNI = high-quality nursing intervention, RCTs = randomized controlled trials.
How to cite this article: He XL, Cao ZM. Effect of high-quality nursing intervention on the psychological disorder in patients with gastric cancer during perioperative period: a protocol of systematic review and meta-analysis. Medicine. 2020;99:23(e20381).
This study is supported by Research Fund of Shaanxi Provincial Department of Health (2012E-06). The funder had no role in this study.
The authors have no conflicts of interests to disclose.
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
References
- [1].Kinoshita T. Minimally invasive approaches for early gastric cancer in East Asia: current status and future perspective. Transl Gastroenterol Hepatol 2020;5:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Jiang F, Shen X. Current prevalence status of gastric cancer and recent studies on the roles of circular RNAs and methods used to investigate circular RNAs. Cell Mol Biol Lett 2019;24:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Roberts SE, Morrison-Rees S, Samuel DG, et al. Review article: the prevalence of Helicobacter pylori and the incidence of gastric cancer across Europe. Aliment Pharmacol Ther 2016;43:334–45. [DOI] [PubMed] [Google Scholar]
- [4].Yoshida Y, Sasako M, Kato H. Bannasch P, et al. Early detection of gastrointestinal cancers: recent progress in endoscopy and surgical results. Cancer Diagnosis. Berlin: Springer Verlag; 1992. 33–41. [Google Scholar]
- [5].Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- [6].Merchant SJ, Kim J, Choi AH, et al. A rising trend in the incidence of advanced gastric cancer in young Hispanic men. Gastric Cancer 2017;20:226–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Zhu L, Qin J, Wang J, et al. Early gastric cancer: current advances of endoscopic diagnosis and treatment. Gastroenterol Res Pract 2016;2016:9638041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wang Y, Li Z, Shan F, et al. Current status of diagnosis and treatment of early gastric cancer in China--data from China Gastrointestinal Cancer Surgery Union. Zhonghua Wei Chang Wai Ke Za Zhi 2018;21:168–74. [PubMed] [Google Scholar]
- [9].Sawaki K, Kanda M, Kodera Y. Review of recent efforts to discover biomarkers for early detection, monitoring, prognosis, and prediction of treatment responses of patients with gastric cancer. Expert Rev Gastroenterol Hepatol 2018;12:657–70. [DOI] [PubMed] [Google Scholar]
- [10].Gu L, Khadaroo PA, Chen L, et al. Comparison of long-term outcomes of endoscopic submucosal dissection and surgery for early gastric cancer: a systematic review and meta-analysis. J Gastrointest Surg 2019;23:1493–501. [DOI] [PubMed] [Google Scholar]
- [11].Bausys R, Bausys A, Stanaitis J, et al. Propensity score-matched comparison of short-term and long-term outcomes between endoscopic submucosal dissection and surgery for treatment of early gastric cancer in a Western setting. Surg Endosc 2019;33:3228–37. [DOI] [PubMed] [Google Scholar]
- [12].Hu J, Zhao Y, Ren M, et al. The comparison between endoscopic submucosal dissection and surgery in gastric cancer: a systematic review and meta-analysis. Gastroenterol Res Pract 2018;2018:4378945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Cao XL, Wang X, Li P, et al. Psychological effects of advanced care on patients received endoscopic gastric cancer resection. Medicine (Baltimore) 2019;98:e17497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Huang T, Zhou F, Yuan X, et al. Reactive oxygen species are involved in the development of gastric cancer and gastric cancer-related depression through abl1-mediated inflammation signaling pathway. Oxid Med Cell Longev 2019;2019:5813985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Baudry AS, Anota A, Mariette C, et al. The role of trait emotional intelligence in quality of life, anxiety and depression symptoms after surgery for esophageal or gastric cancer: A French national database FREGAT. Psychooncology 2019;28:799–806. [DOI] [PubMed] [Google Scholar]
- [16].Jeong A, An JY. The moderating role of social support on depression and anxiety for gastric cancer patients and their family caregivers. PLoS One 2017;12:e0189808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Xu L, Pan Q, Lin R. Prevalence rate and influencing factors of preoperative anxiety and depression in gastric cancer patients in China: Preliminary study. J Int Med Res 2016;44:377–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Choi JH, Kim ES, Lee YJ, et al. Comparison of quality of life and worry of cancer recurrence between endoscopic and surgical treatment for early gastric cancer. Gastrointest Endosc 2015;82:299–307. [DOI] [PubMed] [Google Scholar]
- [19].Li Y. Application effect of high-quality nursing intervention in perioperative period of laparoscopic radical gastrectomy. Capital Food Med 2019;26:113. [Google Scholar]
- [20].Xu XM. The significance of perioperative nursing intervention on blood glucose and complications in patients with gastric cancer and diabetes. New World Diabetes 2017;20:144–6. [Google Scholar]
- [21].Zhang FX. Nursing care of elderly patients with gastric cancer during perioperative period. World 's Latest Med Inf Dig 2017;17:280–2. [Google Scholar]
- [22].Feng Y, Ji JB. The effect of high-quality nursing service model on complications of radical gastrectomy for gastric cancer. Med Theory Pract 2015;28:729–31. [Google Scholar]
- [23].Li YJ. The effect of perioperative nursing on the nursing quality of patients with gastric cancer eradication. West Med 2012;25:113–5. [Google Scholar]
- [24].Rao Y, Zhu JM, Cheng L, et al. The effect of nursing intervention of information knowledge belief behavior model on psychological status of patients with gastric cancer during perioperative period. Chin J Behav Med Sci 2004;6:92–3. [Google Scholar]
- [25].Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sutton AJ, Duval SJ, Tweedie RL, et al. Empirical assessment of effect of publication bias on meta-analyses. BMJ 2000;320:1574–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Phi L, Ajaj R, Ramchandani MH, et al. Expanding the Grading of Recommendations Assessment, Development, and Evaluation (Ex-GRADE) for evidence-based clinical recommendations: validation study. Open Dent J 2012;6:31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]


