Abstract
The current absence of a disease-modifying treatment for Alzheimer disease highlights the necessity for the benefits of nonpharmacological approaches. We aimed to investigate the effect of exercise in older patients with Alzheimer dementia.
This is an observational, prospective cohort study in medical center. Eighty older patients with Alzheimer dementia, including 54 with mild dementia and 26 with moderate dementia, were followed up over 2 years. Patients were divided into exercise and no-exercise groups according to their weekly exercise habit. Mini-Mental State Examination (MMSE), clinical dementia rating (CDR), and senior fitness test were checked initially. We defined death and unexpected hospitalization as the outcomes.
Age, sex, education years, and MMSE showed no significant differences between the groups (P > .05) in all patients. All the patients of the exercise group had significantly better left upper body strength, higher aerobic endurance, and left and right balance maintenance time than those of the no-exercise group (P < .05). There were no changes in hospitalization and mortality between the exercise and non-exercise groups during the 2-year follow-ups in all participants. However, in the mild and moderate dementia subgroups, age, sex, education years, and MMSE showed no significant differences between the groups (P > .05). The exercise group had significantly better lower body strength, left upper body strength, aerobic endurance, right upper body flexibility, lower body flexibility, balance maintenance, and agility than the no-exercise group in patients with mild dementia (P < .05). Moreover, the exercise group had significantly lesser unexpected hospitalization than the no-exercise group in the patients with mild dementia (P = .037).
Despite the similarity in the status of dementia, exercise habit was found to be associated with a better senior fitness test score status. Hence, exercise can decrease unexpected hospitalization in patients with mild dementia but not those with total dementia.
Keywords: Alzheimer dementia, hospitalization, physical fitness, senior fitness test
1. Introduction
Owing to the fact that there is 1 new case of dementia detected every 4 seconds, it was identified as a “global public health priority" by the World Health Organization in 2015.[1] In 2015, the number of people affected by Alzheimer dementia (AD) and other dementias worldwide was estimated to be 47 million, and this number is expected to reach 75 million by 2030 and 131 million by 2050. There is no cure for dementia yet; thus, intervening with lifestyle strategies on known risk factors for cognitive impairment is an important strategy for reducing dementia risk—or at least delaying its onset.[2] However, it is difficult to maintain prolonged lifestyle changes involving diet, exercise, and social activity in the elderly to prevent dementia or cognitive function decline.
Poor physical function and muscle strength coexist in patients with cognitive impairment.[3] Ageing and dementia result in a loss of physical function, quality of life, and adverse events such as increased risk of falls, falls-related fracture, and other comorbidities. The loss of physical function is a combination of a high incidence together with a high susceptibility to injury, because of a high prevalence of clinical diseases (eg, osteoporosis), age- and dementia-related physiological changes (slowed protective reflexes) that make even a relatively mild fall particularly dangerous.[4] Evidence supports the increased risk of falls in individuals even in the early stages of dementia or mild cognitive impairment (MCI). The normal gait depends on several biomechanical structure, including leg joints, and appropriate timing and intensity of muscle action, normal vision, vestibular system, sensory ability, and proprioception. The changes in gait and balance may be related to this increased fall risk.[5]
Some exercises may be useful in reducing the loss of physical function and the risk of fall or even help recover from fall-related injuries. Cognitive function in elderly adults can be normal, mildly impaired, or complete dementia, but the effect of exercise on this population is diverse. Passive finger exercises can be integrated into physical exercise programs for older people with dementia to improve their urinary control, defecation function, and activities of daily living (ADL).[6] Intensive, dementia-adjusted training is feasible and improves clinically meaningful gait variables in people with dementia. The exercise program may represent a model for preventing and rehabilitating gait deficits in the target group.[7]
Increasing evidence[8] supports the claim that exercise and mental activity are strategies to improve cognitive function among people with and without cognitive complaints or impairment. But a randomized controlled trial[9] with moderate- to high-intensity exercise training for people with dementia showed exercise does not slow cognitive impairment in people with mild to moderate dementia. However, the exercise training program improved physical fitness.[9] Anyhow, exercise is recommended by the American College of Sports Medicine as it improves functional independence and the quality of life and reduces the risk of chronic disease in all elderly people.[10]
To understand the effect of regular exercise in patients with AD, we investigated the impact of regular exercise habit and the initial physical fitness evaluation to see whether it could predict the outcome of the patients with mild and moderate dementia and did follow-ups for 2 years.
2. Material and methods
2.1. Patients
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of Chang Gung Memorial Hospital and written informed consent was obtained from patients. A total of 80 patients (27 males, 53 females) diagnosed with AD were prospectively recruited from Kaohsiung Chang Gung Memorial Hospital multidisciplinary dementia outpatient clinic from 2015 June to 2016 March. The patients were followed up for 2 years (till 2018 March). The clinical diagnosis of each patient was reached by consensus of a panel composed of neurologists, psychiatrists, and neuroradiologists in a dementia-integrated outpatient clinic. A trained neuropsychologist administered the neurobehavioral tests, including the Mini-Mental State Examination (MMSE)[11] and clinical dementia rating (CDR) scores[12] for global assessment of cognitive and functional status in the dementia integrated outpatient clinic.
Demographic data including age, sex, education years, comorbidities and eating habits including milk, soymilk, calcium, multivitamins, and vitamin B complex of the patients were checked with a formal questionnaire. Theses data were collected by the case manager. The demographic factor represented independent variables in the statistical analysis. For disease comorbidities, we recorded the history of surgery in the knee, hip, and spine and systemic diseases, including diabetes mellitus, hypertension, hyperlipidemia, and gout.
2.2. Anthropometric measurements
Height and weight were measured with patients wearing a disposable gown provided to them. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Waist circumference (WC) (narrowest diameter between xiphoid process and iliac crest) was measured using a Lufkin measuring tape. Hip circumference was measured at the level of maximum extension of the hip. Tetrapolar body electrical bioimpedance was used to determine body composition, including protein and fat mass (InBody 3.0 body composition analyzer; Biospace Co Ltd, Seoul, South Korea).[13] We also checked vital signs, including heart rate and systolic and diastolic blood pressure.
2.3. Senior fitness test
The senior fitness test was checked by a physical activity trainer. The senior fitness test includes measurement of lower body strength (chair stands for 30 seconds: people need to repeatedly stand up from and sit down on a chair for 30 seconds and the number of stands is recorded.), upper body strength (this is determined according to the number of time a person lifts a 5-lb [2.27 kg] [for women] or an 8-lb [3.63 kg] weight [for men] in 30 seconds), aerobic endurance (determined based on the number of full steps (high knee steps) completed in 2 minutes), lower body flexibility (as determined by the Chair Sit and Reach Test; it is measured in distance [centimeters]), upper body flexibility (as determined by the Back Scratch Test; it is measured in distance [centimeters]), and agility (the 2.45-m Up-and-Go test and this is measured in time [seconds]).[14] Functional balance was assessed based on 1-leg standing time with eyes open, up to a maximum of 30 seconds. Participants were required to carry out single-leg stands in 2 trials for each foot and the mean time was recorded. Muscle strength was measured with the grip strength by using a Jamar hand dynamometer (Sammons Preston, Bolingbrook, IL). This test was assessed by an experienced physiotherapist (KH), and testing was conducted in rooms with adequate space and lighting in the hospitals where the patients were recruited to ensure optimal performance. The advantages of these tests are following: security, simplicity, and no specific equipment requirements for its implementation.
2.4. Exercise questionnaire
Their exercise habits were defined through our questionnaire. We modified the question previous were used in another study for survey exercise habit in our study.[15] The patients were asked the following question regarding past physical activity during the interview“Did you practice sports or physical exercise sufficient to produce sweating or shortness of breath?”. Those who answered “≥2 hours per week” were defined as having exercise habits. The following question was asked regarding current physical activity: “Do you practice walking more than 30 minutes every day?” Those who answered “yes” were defined as having a current walking habit but not exercise habit. To reduce information bias of exercise habits, we confirmed the exercise habits by double checking with the patients and caregivers. Unexpected hospitalization was defined as an unscheduled admission after completing the above examination and we followed up each patient through medical visits for 2 years and recorded the outcomes using case manager.
Statistical analyses were performed using the Statistical Product and Service Solutions (SPSS) software (version 22; SPSS Inc, Chicago, IL). Clinical data were expressed as mean ± standard deviation. Pearson χ2 test was used for analysis of categorical variables such as sex, diet habit, presence of systemic disease and operation history, unexpected hospitalization, and death. Mann–Whitney U test was used, as appropriate, to compare continuous variables between groups because the sample size was small and not normally distributed.
3. Results
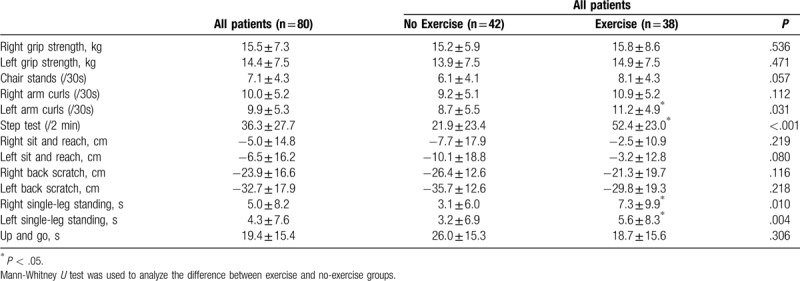
Of the 80 patients, 54 were diagnosed with mild AD (CDR = 1) and 26 with moderate AD (CDR = 2). The mean age was 76.0 ± 8.9 years, the mean years of education was 5.6 ± 4.9. In Table 1, details regarding demographic characteristics are presented. The patients were further divided into the exercise group and the no-exercise group. There were no statistically significant differences in age, sex, education years, MMSE results, blood pressure, heart rate, body height, body weight, body mass index, waist circumference, hip circumference, body fat percentage, or bilateral hand grip strength in the 2 groups. The vital signs, including blood pressure and heart rate, were not different in the 2 groups. There were no differences in eating behavior and systemic disease in the exercise and no-exercise groups. With regard to the senior fitness test in Table 2, the exercise group had significantly better scores in 4 tests including left arm curls (11.2 ± 4.9 vs 8.7 ± 5.5, P = .031), step tests (52.4 ± 23.0 vs 21.9 ± 23.4, P < .001), right single-leg standing (7.3 ± 9.9 vs 3.1 ± 6.0, P = .010), and left single-leg standing (5.6 ± 8.3 vs 3.2 ± 6.9, P = .004).
Table 1.
Demographic data of the participants with dementia (n = 80).
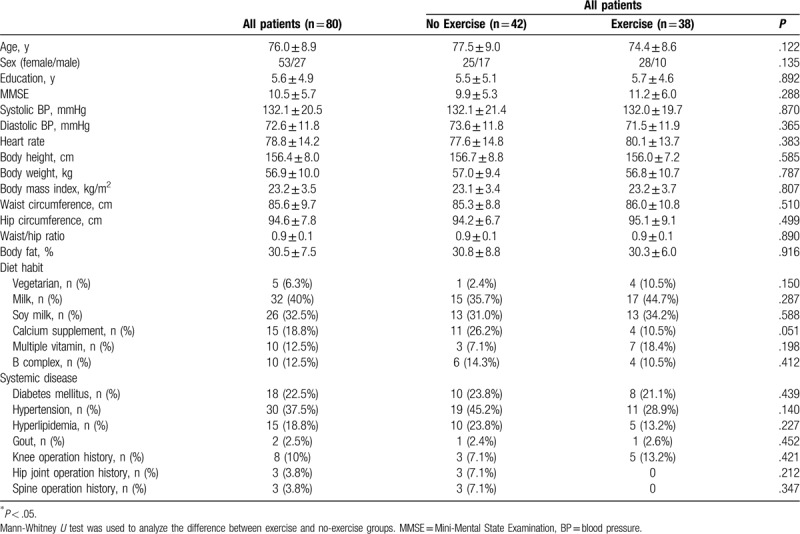
Table 2.
Physical fitness of the participants with dementia (n = 80).
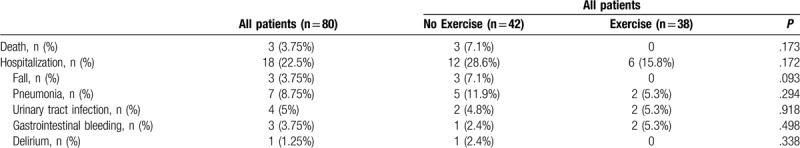
In Table 3, there were no statistically significant differences in unexpected hospitalization, etiology, or death in patients with exercise and patients with no exercise (P > .05).
Table 3.
Outcome of exercise in patients with dementia with and without exercise habit.
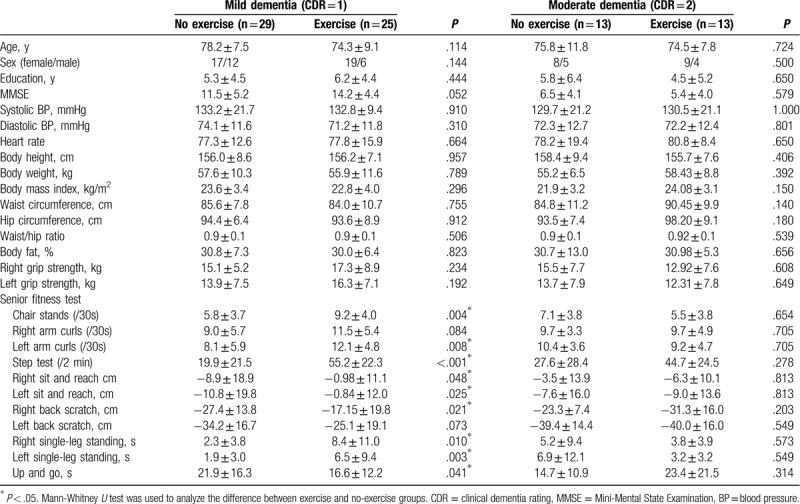
Further, we analyzed patients with exercise and no-exercise according to dementia severity (Table 4). In mild and moderate patients with dementia too there were no statistically significant differences in age, sex, education years, MMSE results, body height, body weight, body mass index, waist circumference, hip circumference, body fat percentage, and bilateral hand grip strength in exercise and no-exercise groups. In patients with MCI, the exercise group had significantly better performance in 9 of 11 fitness test domains, including 30 second chair stands, upper body strength (left arm curls), 2-minute high knee steps, right and left sit and reach, right back scratch test, single-leg standing (right and left), and timed up and go test compared to the no-exercise group. In patients with moderate MCI, the exercise group had no difference among the 11 test domains when compared to the no-exercise group.
Table 4.
Demographic data of subgroup analysis.
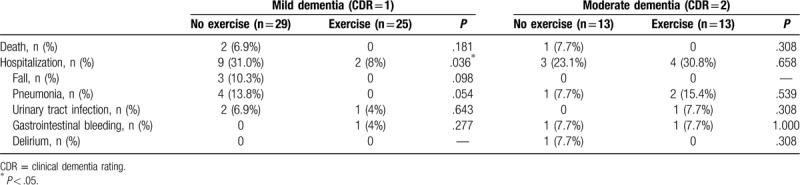
In Table 5, we further analyzed the unexpected hospitalization, etiology, and death in mild and moderate subgroups of patients with dementia. In mild dementia, there were significantly higher rates of unexpected hospitalization in the no-exercise group than in the exercise group (31.0% vs 8%, P = .036). There were no statistically significant differences in etiology or death in patients with mild dementia in the exercise and no exercise (P > .05) groups. In patients with moderate dementia, there were no statistically significant differences in unexpected hospitalization, etiology, or death in patients of the exercise and no exercise groups (P > .05).
Table 5.
Outcome of exercise in patients with dementia with and without exercise habit.
4. Discussion
This study explored the benefits related to exercise in patients with AD. There were 4 main findings. Frist, in all patients with dementia, initial exercise was associated with better upper body strength, aerobic endurance, and balance. Second, in all participants, there were no statistically significant differences in unexpected hospitalization, etiology (fall, pneumonia, urinary tract infection, gastrointestinal bleeding, and delirium), or death between patients with exercise and no exercise. Third, exercise was not associated with better physical fitness in patients with moderate dementia. However, exercise helped patients with mild dementia perform better in lower body strength, left upper body strength, aerobic endurance, right upper body flexibility, bilateral lower body flexibility, balance maintenance, and agility tests than the same subcategory in the no-exercise group (P < .05). Fourth, in subgroup analysis, exercise decreased the unexpected hospitalization in patients with mild dementia. Indeed, the shorter duration of hospitalization might be attributed to initial exercise habits or associated with initial better fitness despite similar dementia status.
Musculoskeletal fitness appears to be particularly important for elderly people and their ability to maintain functional independence. In fact, many activities of daily living do not require a large aerobic output but depend on one or more of the musculoskeletal fitness components. Exercise training improves many elements of physical wellness among older adults, including aerobic fitness and functional mobility and reduces the risk of chronic disease.[4,16] In this study, exercise habit was associated with better upper body strength, aerobic endurance, and balance in all patients with dementia. However, there were no improvements in senior fitness function ability in patients with moderate dementia with regular exercise behavior. In subgroup analysis, we found that exercise contributed to better senior fitness test scores in patients with mild dementia but no such trend was observed in patients with moderate dementia. Why the effect of exercise could not have associated with better physical fitness? There are 3 possible reasons for this. First, the relatively smaller number of cases we used might lead to less conclusive results. Second, the drugs for moderate and severe dementia treatment may mask the effect of better physical fitness in patients. Third, the effect of exercise could be masked by the moderate to severe dementia status itself.
The outcomes of the cohort studies included death and hospitalization, which had no significant differences in their rates between the exercise and no exercise groups in all enrolled patients with dementia. When we performed subgroup analysis in patients with mild dementia, we found that the exercise group had significantly lower unexpected hospitalization rates than the no exercise group. The etiology of admission included falling down accidents, pneumonia, and urinary tract infection.
Exercise is beneficial to patients with AD and falls seemed to decrease in the exercise group.[17,18] In our study, there were 3 falls in the no-exercise group and none in the exercise group in patients with mild dementia. We found that balancing ability was better in the exercise group in patients with dementia. Fall-related injury is a common reason for admission to hospitals among people with dementia. The effect of exercise on falls remained a trend. Aspiration pneumonia is a dominant form of community-acquired and healthcare-associated pneumonia, and a leading cause of death among aging populations. In our study, there were 4 cases of pneumonia in the no-exercise group and none in the exercise group in patients with mild dementia. Evidence is still lacking regarding the prevention of aspiration pneumonia and improvement in the swallowing function. Because the normal swallowing process is performed involuntarily by the pharyngeal phase of the swallowing reflex, it is not easy for exercise therapy to improve this reflex. However, the activity of abdominal muscles is important for the cough function, which is an important defense reaction to preventing aspiration.[19] However, our results revealed that exercise is associated with lower hospitalization in patients with mild dementia.
It is apparent that physical activity is essential in the prevention of chronic disease and premature death.[20] Our results also revealed that exercise is associated with beneficial effects in the mild dementia group. However, doubt remains over the optimal amount (frequency, duration, and intensity of exercise) and the minimum amount of exercise required for health benefits, in particular, the effects of intensity (eg, moderate vs vigorous) on health status. There is evidence that the intensity of physical activity is inversely and linearly associated with mortality.[20] Our study merely investigated patients with dementia who already maintained an exercise habit for at least 1 year. We asked these patients whether they exercised >150 minutes per week for at least 1 year. Those who answered that they have regular exercise habit showed statistically better performance in the hand grip strength and senior fitness test than those without exercise habit, among patients with mild dementia. In our 38 patients with regular exercise habits, their exercise methods included fitness classes (3), running (7), fast walking (2), Tai Chi (4), dance (3), swimming (4), and others (16). Recently, investigators have postulated that even lower levels of weekly energy expenditure may be associated with health benefits.[21–23] This means that our results confirmed that exercise, even at low intensities, could possibly improve physical fitness too.
In the future aging society, it incurs a significant economic burden of direct health care and nonhealth care costs to take care of patients with dementia. Because dementia is a long deteriorating process,[13,24] the patients will live for 8 to 10 years or longer. A growing number of studies have linked dementia with physical deterioration which might reduce muscles mass, and result in higher risks of falls, fractures, and hospitalization.[5,18] The loss of their ability to cope with activities of daily living could decrease their quality of life,[25–26] institutionalization[27] and increase the risk of death.[28] Besides, people with dementia are more frequently hospitalized due to infectious diseases, fractures, or nutritional disorders than those without dementia.[29] However, the most possible preventable factors of hospitalization factor is trauma, falling, and fracture. Hence, it is necessary for them to keep their daily functions during their late life. The evidence that muscle loss and bone absorption can occur within days of inactivity among older adults have been well documented.[30] The resultant weakness and instability associated with inactivity can lead to a higher risk of falls and fractures. Muscle tissue deterioration is a main determinant of functional independence in the elderly years. Evidence has documented that exercise programs are associated with a lower risk of falls.[31] An individualized, multicomponent exercise intervention performed during a short period provides a significant benefit over usual care and can help to reverse the functional decline.[32,33] Early mobilization with exercise programs including resistance exercises using variable resistance training machines and exercises involving mainly lower-limb muscles (squats rising from a chair, leg press, and bilateral knee extension), the upper-body musculature (seated bench [chest] press) progressive resistance, balance, and walking training exercises, balance training, and gait training with light loads (ie,0.5- to 1-kg anklets and handgrip ball) has proven beneficial in improving the functional recovery and counteract the muscle weakness in very elderly patients.[34] As in elderly patients, we need a thorough caring plan and help maintain their independence such as walking, or carrying out day-to-day functions on their own in dementia patients. The benefit of exercise was launching medical/nursing care protocols for patients with AD owing to its significant benefits on the upper and lower body muscle strength and flexibility, agility and dynamic balance, and endurance fitness (from the senior fitness test).[25] Exercise could improve physical function to preserve the ability of independence and quality of life in patients with dementia.[9,25–26] As in our Table 5, our result showed a trend exercise has a lower incidence of falling down, pneumonia, and urinary tract infection in mild dementia subgroup. Aging is also associated with a decline in the normal functioning of the immune system. Exercise has a profound effect on the normal functioning of the immune system through reductions in inflammation, maintenance of thymic mass, alterations in the composition of “older” and “younger” immune cells, enhanced immunosurveillance, and/or the amelioration of psychological stress.[35–37] However, the intensive training, aerobic, or resisted exercise have different effect in immune system.[35–37] To prevent unexpected hospitalizations, exercise is encouraged because of its low cost and the improvement it brings in their physical fitness which could possibly prevent hospitalization.
The main strengths of this analysis are that the cohort study was prospectively designed for an outcome analysis using individual-level data to reach a confirmatory conclusion with respect to exercise habit as an effect in people with dementia. Furthermore, we used scientific measurement of the patients’ fitness and regular follow-ups were done. The data are valid. There are several limitations to this study. First, we could not demonstrate the exercise frequency and severity. However, we confirmed the exercise habits by double checking with the patients and caregivers. Second, we focused on patients with mild and moderate dementia only, and our findings may not apply to people with no dementia. Third, the physical performance test appears less suitable to monitor clinically relevant intra-individual performance changes because of large standard deviation.[38] However, the physical performance tests evaluated are useful for detecting differences in performance between older people with mild to moderate dementia by cross-sectional analysis.[39]
5. Conclusion
In conclusion, exercise had benefits in senior fitness in patients with mild dementia and decreased unexpected hospitalization rates. However, the importance of exercise in moderate dementia is still unclear and needs further study.
Acknowledgments
The authors wish to thank the patients and their caregivers for their time and commitment to this research and all team members from the cognitive and aging center for study executive coordination.
Author contributions
Conceptualization: Chien-Liang Chen, NaiChing Chen.
Data curation: Lin Li, Hui-chen Lin, NaiChing Chen.
Methodology: Ke-Hau Chen, Hsiu-Hui Chen.
Supervision: Chien-Liang Chen.
Writing – review & editing: Chien-Liang Chen, NaiChing Chen.
Footnotes
Abbreviations: AD = Alzheimer dementia, ADL = activities of daily living, BMI = body mass index, CDR = clinical dementia rating, GS = grip strength, MCI = mild cognitive impairment, MMSE = Mini-Mental State Examination, SPSS = Statistical Product and Service Solutions, WC = waist circumference.
How to cite this article: Chen KH, Chen HH, Li L, Lin Hc, Chen CL, Chen NC. The impact of exercise on patients with dementia: A 2-year follow-up. Medicine. 2020;99:23(e20597).
Source of support: This work was supported by the grant CMRPG8F1041-3 from Chang Gung Memorial Hospital, Taiwan. The funder had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
The authors report no conflict of interest.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].First WHO Ministerial Conference on Global Action Against Dementia: Meeting Report 2015. World Health Organization, Geneva. Available at: www.who.int. Accessed April 2017. [Google Scholar]
- [2].Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet 2015;385:2255–63. [DOI] [PubMed] [Google Scholar]
- [3].Auyeung TW, Kwok T, Lee J, et al. Functional decline in cognitive impairment—the relationship between physical and cognitive function. Neuroepidemiology 2008;31:167–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Rubenstein LZ, Josephson KR. The epidemiology of falls and syncope. Clin Geriatr Med 2002;18:141–58. [DOI] [PubMed] [Google Scholar]
- [5].Lach HW, Harrison BE. Phongphanngam. Falls and fall prevention in older adults with early-stage dementia: an integrative review. Res Gerontol Nurs 2017;10:139–48. [DOI] [PubMed] [Google Scholar]
- [6].Liu B, Chen X, Li Y, et al. Effect of passive finger exercises on grip strength and the ability to perform activities of daily living for older people with dementia: a 12-week randomized controlled trial. Clin Interv Aging 2018;13:2169–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Schwenk M, Zieschang T, Englert S, et al. Improvements in gait characteristics after intensive resistance and functional training in people with dementia: a randomised controlled trial. BMC Geriatr 2014;14:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Heyn P, Abreu BC, Ottenbacher KJ. The effects of exercise training on elderly persons with cognitive impairment and dementia: a meta-analysis. Arch Phys Med Rehabil 2004;85:169–704. [DOI] [PubMed] [Google Scholar]
- [9].Lamb SE, Sheehan B, Atherton N, et al. Dementia And Physical Activity (DAPA) trial of moderate to high intensity exercise training for people with dementia: randomised controlled trial. BMJ 2018;361:k1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chodzko-Zajko WJ, Proctor DN, Fiatarone Singh MA, et al. American College of Sports Medicine. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med Sci Sports Exerc 2009;41:1510–30. [DOI] [PubMed] [Google Scholar]
- [11].Folstein MF, Folstein SE, McHugh PR. Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–98. [DOI] [PubMed] [Google Scholar]
- [12].Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993;43:2412–4. [DOI] [PubMed] [Google Scholar]
- [13].Bergman RN, Stefanovski D, Buchanan TA, et al. A better index of body adiposity. Obesity (Silver Spring) 2011;19:1083–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lin PS, Hsieh CC, Cheng HS, et al. Association between physical fitness and successful aging in Taiwanese older adults. PLoS One 2016;11:e0150389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Akune T, Muraki S, Oka H, et al. Exercise habits during middle age are associated with lower prevalence of sarcopenia: the ROAD study. Osteoporos Int 2014;25:1081–8. [DOI] [PubMed] [Google Scholar]
- [16].Larson EB, Bruce RA. Health benefits of exercise in an aging society. Arch Intern Med 1987;147:353–6. [PubMed] [Google Scholar]
- [17].Teri L, McCurry SM, Buchner DM, et al. Exercise and activity level in Alzheimer's disease: a potential treatment focus. J Rehabil Res Dev 1998;35:411–9. [PubMed] [Google Scholar]
- [18].Roitto HM, Kautiainen H, Öhman H, et al. Relationship of neuropsychiatric symptoms with falls in Alzheimer's disease—does exercise modify the risk? J Am Geriatr Soc 2018;66:2377–81. [DOI] [PubMed] [Google Scholar]
- [19].Abe T, Kusuhara N, Yoshimura N, et al. Differential respiratory activity of four abdominal muscles in humans. J Appl Physiol 19851996;80:1379–89. [DOI] [PubMed] [Google Scholar]
- [20].Lee IM, Skerrett PJ. Physical activity and all-cause mortality: what is the dose-response relation? Med Sci Sports Exerc 2001;33:S459–71. [DOI] [PubMed] [Google Scholar]
- [21].Paffenbarger RS, Jr, Hyde RT, Wing AL, et al. The association of changes in physical-activity level and other lifestyle characteristics with mortality among men. N Engl J Med 1993;328:538–45. [DOI] [PubMed] [Google Scholar]
- [22].Kushi LH, Fee RM, Folsom AR, et al. Physical activity and mortality in postmenopausal women. JAMA 1997;277:1287–92. [PubMed] [Google Scholar]
- [23].Leon AS, Connett J, Jacobs DR, Jr, et al. Leisure-time physical activity levels and risk of coronary heart disease and death. The Multiple Risk Factor Intervention Trial. JAMA 1987;258:2388–95. [PubMed] [Google Scholar]
- [24].Prince M, Ali GC, Guerchet M, et al. Recent global trends in the prevalence and incidence of dementia, and survival with dementia. Alzheimers Res Ther 2016;8:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Prince M, Ali GC, Guerchet M, et al. Exercise training is beneficial for Alzheimer's patients. Int J Sports Med 2008;29:845–50. [DOI] [PubMed] [Google Scholar]
- [26].Andersen CK, Wittrup-Jensen KU, Lolk A, et al. Ability to perform activities of daily living is the main factor affecting quality of life in patients with dementia. Health Qual Life Outcomes 2004;2:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Knopman DS, Berg JD, Thomas R, et al. Nursing home placement is related to dementia progression: experience from a clinical trial. Alzheimer's Disease Cooperative Study. Neurology 1999;52:714–8. [DOI] [PubMed] [Google Scholar]
- [28].Van Dijk PT, Mehr DR, Ooms ME, et al. Comorbidity and 1-year mortality risks in nursing home residents. J Am Geriatr Soc 2005;53:660–5. [DOI] [PubMed] [Google Scholar]
- [29].Pinkert C, Holle B. People with dementia in acute hospitals. Literature review of prevalence and reasons for hospital admission. Z Gerontol Geriatr 2012;45:728–34. [DOI] [PubMed] [Google Scholar]
- [30].Van Ancum JM, Scheerman K, Jonkman NH, et al. Change in muscle strength and muscle mass in older hospitalized patients: a systematic review and meta-analysis. Exp Gerontol 2017;92:34–41. [DOI] [PubMed] [Google Scholar]
- [31].Martínez-Velilla N, Cadore L, Casas-Herrero Á, et al. Physical activity and early rehabilitation in hospitalized elderly medical patients: systematic review of randomized clinical trials. J Nutr Health Aging 2016;20:738–51. [DOI] [PubMed] [Google Scholar]
- [32].Schaller SJ, Anstey M, Blobner M, et al. International Early SOMS-guided Mobilization Research Initiative. Early, goal-directed mobilisation in the surgical intensive care unit: a randomised controlled trial. Lancet 2016;388:1377–88. [DOI] [PubMed] [Google Scholar]
- [33].Schweickert WD, Pohlman MC, Pohlman AS, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet 2009;373:1874–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Martínez-Velilla N, Casas-Herrero A, Zambom-Ferraresi F, et al. Effect of exercise intervention on functional decline in very elderly patients during acute hospitalization: a randomized clinical trial. JAMA Intern Med 2019;179:28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Simpson RJ, Kunz H, Agha N, et al. Exercise and the regulation of immune functions. Prog Mol Biol Transl Sci 2015;135:355–80. [DOI] [PubMed] [Google Scholar]
- [36].Simpson RJ, Lowder TW, Spielmann G, et al. Exercise and the aging immune system. Ageing Res Rev 2012;11:404–20. [DOI] [PubMed] [Google Scholar]
- [37].Sellami M, Gasmi M, Denham J, et al. Effects of acute and chronic exercise on immunological parameters in the elderly aged: can physical activity counteract the effects of aging? Front Immunol 2018;9:2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Blankevoort CG, van Heuvelen MJ, Scherder EJ. Reliability of six physical performance tests in older people with dementia. Phys Ther 2013;93:69–78. [DOI] [PubMed] [Google Scholar]
- [39].Fox B, Henwood T, Neville C, et al. Relative and absolute reliability of functional performance measures for adults with dementia living in residential aged care. Int Psychogeriatr 2014;26:1659–67. [DOI] [PubMed] [Google Scholar]


