To discuss outcomes with patients in daily clinical practice and to facilitate outcome comparisons between institutions, a standardized outcome set for patients with multiple myeloma (MM) is needed. The goal of this study was to align outcome measurement in MM by defining a set of outcomes most relevant for patients with MM and defining instruments to measure these outcomes. The outcome set was developed in 4 hospitals in the Netherlands. To ensure feasibility, panelists with expertise or experience in MM management across clinical specialties, patients and scientific researchers, participated in in-depth group meetings. Various information sources were used to identify a comprehensive list of outcomes. The list of potential outcomes was refined through consensus discussions by focusing on outcomes that had direct impact on patients, reflected clinical care, and were feasible to measure in daily clinical practice. The defined MM outcome set includes both clinical (eg, overall survival, complications) as well as patient-reported outcomes (eg, neuropathy, fatigue). Additional sociodemographic, clinical and treatment characteristics were defined to allow for case-mix risk-adjusted analyses. Recommended time-points for data collection were determined. This study resulted in a standard set of outcomes and accompanying instruments for use in daily clinical practice for management and evaluation of MM patient care. Implementation has started in five hospitals in the Netherlands and will be evaluated. Future goal is to enroll an outcome set in all hospitals in the Netherlands and abroad, in order to carry out continuous and measurable improvement of outcomes for patients with MM.
MM is a cancer of plasma cells and accounts for 13% of the hematological malignancies.1,2 Because of widespread adoption of new anti-cancer therapies, the survival of MM has increased considerably in the last decades.3–5 In the Netherlands, the five-year relative survival increased from 32% in the period 1996 to 2000 to 54% in 2011 to 2015, resulting in a 20-year prevalence of 7100 patients in January 2018.1 Similar increases in survival and prevalence have been reported worldwide.2 Many new drugs, including monoclonal antibodies, next-generation proteasome inhibitors and a next-generation immunomodulatory agent were introduced in the past few years,3 providing more options but adding complexity to the treatment of the disease. Treatment strategies include longer treatment duration and increasing number of treatment lines, accompanied by a higher risk of side effects such as polyneuropathy, acute renal failure, cardiac toxicity and pneumonia/infections. In addition, as many patients with MM live longer, patients are also at risk for long-term consequences of MM and/or MM treatment. Symptoms such as fatigue, pain, neuropathy, cognitive problems and depressive feelings are reported6–11 and are experienced more frequently by patients with MM as compared to people without cancer of the same age and sex.12 These symptoms have shown to negatively impact patients’ health-related quality of life (HRQoL).12–17
With the continuing changes in treatment, related (long-term) side effects and the complexity and costs of treatment of patients with MM, the goals of treatment and therapeutic decision making need to be expanded besides improvement in overall survival to include several previously overlooked items such as HRQoL, managing (long-term) side effects and refining duration of therapy. Although there are efforts to measure clinical outcomes (eg, survival, response status) a standard approach is lacking,18 resulting in significant variation in methods of measuring and reporting outcomes. In addition, patient-reported outcomes (PROs) are very infrequently assessed in daily clinical practice. PROs however, can be more meaningful to patients compared to clinical outcomes18 and can contribute to improving shared decision making and management of patients with MM19.
Since an agreed standard approach is missing, the ability to discuss outcomes with individual patients and to perform comparisons of outcomes between institutions that could lead to improvement of care is limited. Defining standardized and patient-centered outcome measurement sets are therefore essential to improve care. The International Consortium for Health Outcomes Measurement (ICHOM) focuses on developing such standard sets for various diseases,20 whereby according to the framework of value-based healthcare (VBHC) the key is measuring outcomes that matter most to patients21 in addition to clinical outcomes.
The goal of this study was to develop a standard set of outcomes in MM by 1) defining a set of outcomes that are most relevant to patients with MM, and 2) defining instruments to measure these outcomes for use in daily clinical practice. This standard set of outcomes will enable discussion with individual patients and compare outcome between institutions and health-care professionals with the ultimate goal to improve MM patient care within the Netherlands and abroad.
Working team and process
The development of an outcome set was undertaken by four hospitals in the Netherlands. Panelist consisted of patients (N = 4 of which 3 with partner) and experts in the field of MM, including hematologists (N = 4), nurse practitioners specialized in hematology (N = 6), a social worker (N = 1), a physical therapist (N = 1), hospital care organizers (N = 3), scientific researchers from the Netherlands Cancer Registry (N = 3) participated in in-depth group meetings. The participating patients varied in age, sex, phase in their care cycle, clinical experience and came from different geographical regions of the Netherlands. The detailed work process is provided in supplement (Supplement 1).
Defined population
Panelists agreed that a set of standardized outcome measures should be defined for patients with ‘symptomatic Multiple Myeloma’ ≥18 years of age who fulfill the International Myeloma Working Group (IMWG) criteria.22 The precise definition of the medical condition that was defined is displayed in Figure 1. Exclusion criteria that were defined are: patients aged < 18, patients with treatment contra-indication and patients with MGUS (Monoclonal gammopathy of undetermined significance) or with Smouldering (asymptomatic) MM. Furthermore, the starting and end point of care were defined and a care pathway most ideal for MM patients was described.
Figure 1.
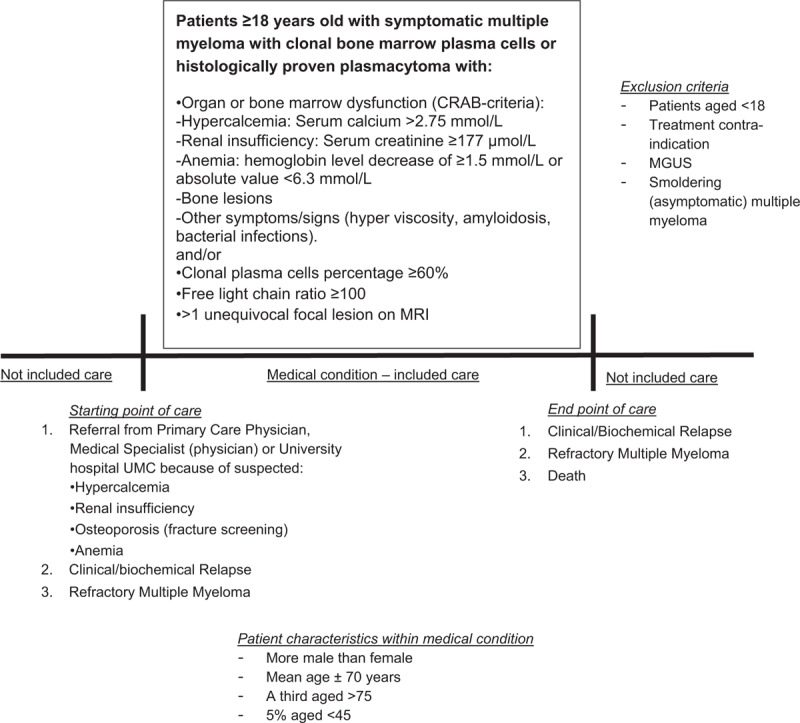
Definition of medical condition for which a set of standardized outcome measures is defined.
The standard set: outcome domains and measures
Many potential outcomes were considered by the panelists before agreement on the standard set of outcomes recommended for patients with MM. It was decided that the clinical outcome “time to relapse” which was selected in step 1A was excluded from the preliminary outcome set, to keep the set as compact and feasible to measure as possible. Quality of death, relationship/marital problems, sexual problems and fear of physical exercise were selected in step 1B, but not in 1A. They were included in the final set as they were indicated to be very important to patients. The standard outcome set includes both clinical outcomes and PROs.
Clinical outcomes
-
1.
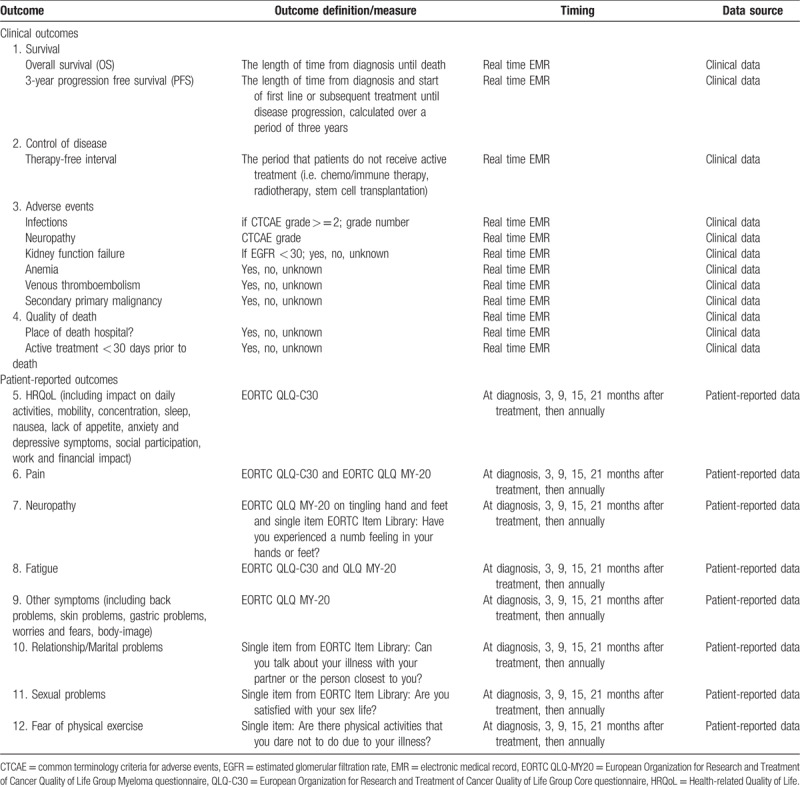
Survival: MM is a disease of the elderly with a relative short life expectancy. Therefore, overall survival (OS, defined as the length of time from diagnosis until death) captures the ultimate impact of care and was included in the outcome set. In addition, the working team decided to also include the three-year progression free survival (PFS, defined as the length of time from diagnosis and start of first line or subsequent treatment until disease progression; Table 1).
-
2.
Control of disease: Disease remission is one of the major goals of MM treatment. Especially the period without needing treatment was indicated as important by patients. Therefore, therapy free interval was included in the outcome set, with the definition listed in Table 1.
-
3.
Adverse events: Side effects of treatment were listed as important outcomes as they impact patients’ survival as well as HRQoL. Although patients with MM are always treated prophylactically with antiviral/bacterial therapy, many of them still present themselves with infections.23 Also, motoric and sensory neuropathy are frequently reported by patients as a consequence of chemotherapy.11,16 For that reason, infections and neuropathy were included in the outcome set and scored according to the Common Terminology Criteria for Adverse Events (CTCAE, version 5). In addition, kidney failure, anemia, and venous thromboembolism were selected for inclusion in the outcome set (Table 1).
-
4.
Quality of death: Quality of death was indicated by patients as an important outcome and will be evaluated with place of death and active treatment < 30 days prior to death.
Table 1.
Outcome Domains and Definitions Included in the Standard Set.
Patient-reported outcomes (PROs)
Fatigue, (bone) pain, neuropathy, quality of life, dyspnea, and being able to work were the most frequently mentioned symptoms or subjects by patients participating in the discussion groups. These themes were, amongst others, also found in the literature that focused on PROs among patients with MM, both internationally and in the Netherlands.6–17 HRQoL and symptoms can most appropriately be captured by patients using self-reported patient-reported outcome measures (PROMs). PROMs used for patients with MM are the generic European Organization for Research and Treatment of Cancer Quality of Life Group Core questionnaire (EORTC QLQ-C30)24 and the disease-specific EORTC QLQ Multiple Myeloma questionnaire (EORTC QLQ-MY20).25 The EORTC QLQ-C30 includes five scales on physical, role, emotional, cognitive, and social functioning; a global health status/quality of life scale; three symptom scales on fatigue, nausea and vomiting, and pain; and 6 single items assessing dyspnea, sleeping problems, appetite loss, constipation, diarrhea, and financial problems. The EORTC QLQ-MY20 contains items on pain (bone, back, hip, shoulder, chest) increase during activity, other disease symptoms and side effects of treatment (e.g. tingling hands/feet, heartburn, burning/sore eyes), one functional scale on future perspective, and one single item on body image. Answer categories for the EORTC QLQ-C30 and the EORTC QLQ-MY20 range from 1 (not at all) to 4 (very much).24,25
These 2 questionnaires cover almost all symptoms that were highlighted by patients and are translated and validated in many languages and have been and are widely used internationally.26 It was therefore decided to use these questionnaires. However, some outcomes that were indicated to be important were not covered by these questionnaires. For the following topics a single item was selected: relationship/marital problems, sexual problems and fear of physical exercise. One item on neuropathy about tingling hands and feet is part of the MY-20 questionnaire, however as this problem is sometimes not experienced as tingling but as a numb feeling an item about numbness was added. All except one (fear of physical exercise) of these items were available in the EORTC QLG Item Library27 and the wording and response scale are therefore similar as the questions of the EORTC QLQ-C30 and MY-20. All PROs that were included in the standard set are listed in Table 1.
Timing and measurement process
The timing of measurement was determined based on clinical relevance and feasibility. All clinical- and patient-reported outcomes will be collected at time of diagnosis and 3, 9, 15, and 21 months after treatment, and subsequently annually. To be able to discuss outcomes during visits, it is necessary to collect PRO-data aligned with individual clinical care.
Case-mix factors
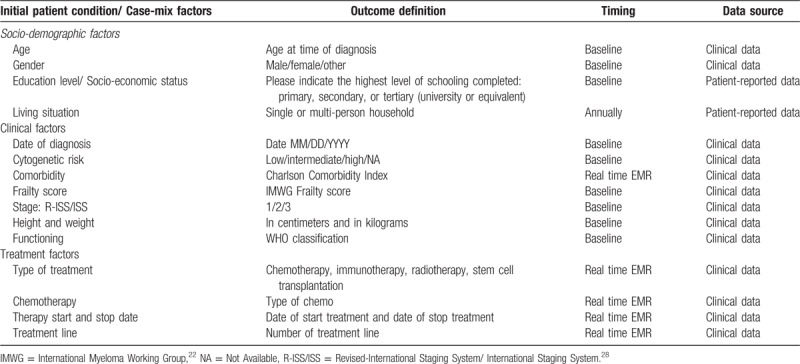
Several patient characteristics and risk factors are known to influence patients’ outcomes. In order to make meaningful comparisons, we therefore defined key patient characteristics to be included in the set, that is, socio-demographic characteristics: age, sex, education level/socio-economic status, health status; clinical factors: cytogenetic risk, date of diagnosis, IMWG frailty score, comorbidity, (Revised) International Staging System (R-ISS/ISS) stage,28 height, weight; treatment factors: type of therapy, type of chemotherapy, therapy start and stop date and treatment line. These selected factors are viewed to have a strong and independent effect on the outcomes included in the set. All case-mix factors and definitions are presented in Table 2.
Table 2.
Case-Mix Factors and Definitions Included in the Outcome Set.
This study resulted in a set of outcomes and accompanying definitions or measures for patients with MM for use in routine clinical practice. The outcomes represent important indicators that are most relevant to patients. Additional sociodemographic, clinical and treatment characteristics were defined to allow for case-mix risk-adjusted analyses. With measuring the outcomes of this set, we expect to be able to focus on meaningful results for patients and health-care professionals, improve communication between health-care professionals and patients, help to make informed decisions about treatment options, assess performance and guide improvements in clinical practice, and allow for benchmarking.
Recently, an outcome set for patients with MM was defined in Spain.29 At time of their publication, our development of an outcome set had already started. When comparing the Spanish and Dutch outcome sets, we observed a clear overlap. With respect to the clinical outcomes, OS and PFS were included in both sets. In Spain, minimal residual disease (MRD) and treatment response were also included in this domain. MRD was not included in our set as it is not yet a routine test in the Netherlands. Treatment response was not included to limit the number of outcomes. In addition, complications and PROs were defined as outcomes in both sets, with both including HRQoL (functioning and symptoms), pain, fatigue, body image and sexual problems. In the Spanish set, outcomes on treatment adherence and preferences and satisfaction were included, which were not defined in our set. On the contrary, we included neuropathy, relationship/marital problems and symptoms frequently reported by patients with MM (such as specific areas of the body with pain, aches and worries about health and future as measured by the EORTC QLQ MY-2025) and a single item on fear of physical exercise that were not included in the Spanish set. The overlap in these two outcome sets suggests that outcomes that matter most to patients with MM are similar for patients living in a Northern European or Southern European country. In the future, efforts to harmonize both the Spanish and the Dutch outcome set to an international standardized set will be made to compare results in Europe or even worldwide, in which cultural differences will be considered.
As a next step we are implementing the outcome set in 5 pilot hospitals in the Netherlands which will be coordinated by a steering committee. Barriers such as time, logistics and unfamiliarity with PROs will challenge use in daily clinical practice in a first introduction period. With respect to the clinical outcomes, the majority is registered in routine clinical practice, although not in a standardized manner. Standardized sets in the electronic medical record may help facilitate structured recording of outcomes such as infections and neuropathy via the CTCAE criteria in a standardized manner. The accuracy and completeness of data, as well as the response of patients to PROMs, is crucial for the relevance of the data and will be evaluated after the implementation in the pilot hospitals. Since data will be collected of every individual patient this will result in population-based information on outcomes, which is of merit compared to outcomes collected in selected trial populations. By understanding that the outcomes of the questionnaires will be discussed during clinical visits and if needed treatment adjustments will be made, patients will feel more involved in their care and are hopefully more likely to complete PROMs.
The strength of this study is reflected by the joint effort of a large group of panelists with diverse expertise from four hospitals. However, there were several limitations to the development of the standardized outcome set. The defined outcome set has been derived from consensus opinions of the panel, based on the currently available scientific evidence and experience, and not been developed using a structured method (for example a Delphi method). As the implementation phase of the set of outcomes for use in daily clinical practice is ongoing, results about the feasibility of outcome measurement using the set will follow in the near future. There will be an annual update in the HOVON MM working group to evaluate and discuss its relevance for patients and health care professionals.
In conclusion, we have set out to produce relevant outcomes that matter most to patients with MM and implementation is currently ongoing in 5 pilot hospitals. Future goal is to enroll an outcome set in all hospitals in the Netherlands and abroad, in order to carry out continuous and measurable improvement of outcomes for patients with MM.
Supplementary Material
Acknowledgments
The authors would like to acknowledge Sara Sprinkhuizen, Department of Internal Medicine, Máxima Medical Centre, Eindhoven and Veldhoven, and Mirian Brink, Department of Research and Development, Netherlands Comprehensive Cancer Organisation, Utrecht for their contribution to this research and manuscript.
Appendix A: Overview of potential outcomes for patients with MM.

Footnotes
MD Levin has received research support of Janssen, Amgen, Takeda and Celgene; AB has received research support of Janssen, Celgene, Takeda and Amgen; PS has received research support of Amgen, Celgene, Janssen, Karyopharm and SkylineDX and takes place in the advisory boards of Amgen, Celgene, Janssen, Karyopharm, SkylineDx and BMS.
For the remaining authors, no relevant conflicts of interest were declared.
References
- 1. Figures Netherlands Cancer Registry. 2018. http://www.cijfersoverkanker.nl. [Accessed March 11, 2019]. [Google Scholar]
- 2. SEER Cancer Stat Facts: Myeloma. National Cancer Institute. Bethesda M. https://seer.cancer.gov/statfacts/html/mulmy.html. [Accessed March 11, 2019]. [Google Scholar]
- 3.Rollig C, Knop S, Bornhauser M. Multiple myeloma. Lancet. 2015;385:2197–2208. [DOI] [PubMed] [Google Scholar]
- 4.Kumar SK, Rajkumar SV, Dispenzieri A, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111:2516–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brenner H, Gondos A, Pulte D. Expected long-term survival of patients diagnosed with multiple myeloma in 2006-2010. Haematologica. 2009;94:270–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uyl-de Groot CA, Buijt I, Gloudemans IJ, et al. Health related quality of life in patients with multiple myeloma undergoing a double transplantation. Eur J Haematol. 2005;74:136–143. [DOI] [PubMed] [Google Scholar]
- 7.Sirohi B, Powles R, Lawrence D, et al. An open, randomized, controlled, phase II, single centre, two-period cross-over study to compare the quality of life and toxicity experienced on PEG interferon with interferon-alpha2b in patients with multiple myeloma maintained on a steady dose of interferon-alpha2b. Ann Oncol. 2007;18:1388–1394. [DOI] [PubMed] [Google Scholar]
- 8.Sherman AC, Simonton S, Latif U, et al. Changes in quality-of-life and psychosocial adjustment among multiple myeloma patients treated with high-dose melphalan and autologous stem cell transplantation. Biol Blood Marrow Transplant. 2009;15:12–20. [DOI] [PubMed] [Google Scholar]
- 9.Parsons JA, Greenspan NR, Baker NA, et al. Treatment preferences of patients with relapsed and refractory multiple myeloma: a qualitative study. BMC Cancer. 2019;19:264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee SJ, Richardson PG, Sonneveld P, et al. Bortezomib is associated with better health-related quality of life than high-dose dexamethasone in patients with relapsed multiple myeloma: results from the APEX study. Br J Haematol. 2008;143:511–519. [DOI] [PubMed] [Google Scholar]
- 11.Beijers AJ, Oerlemans S, Mols F, et al. The magnitude of neurotoxicity in patients with multiple myeloma and the impact of dose modifications: results from the population-based PROFILES registry. Ann Hematol. 2017;96:653–663. [DOI] [PubMed] [Google Scholar]
- 12.Mols F, Oerlemans S, Vos AH, et al. Health-related quality of life and disease-specific complaints among multiple myeloma patients up to 10 yr after diagnosis: results from a population-based study using the PROFILES registry. Eur J Haematol. 2012;89:311–319. [DOI] [PubMed] [Google Scholar]
- 13.Nielsen LK, Jarden M, Andersen CL, et al. A systematic review of health-related quality of life in longitudinal studies of myeloma patients. Eur J Haematol. 2017;99:3–17. [DOI] [PubMed] [Google Scholar]
- 14.Sonneveld P, Verelst SG, Lewis P, et al. Review of health-related quality of life data in multiple myeloma patients treated with novel agents. Leukemia. 2013;27:1959–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramsenthaler C, Osborne TR, Gao W, et al. The impact of disease-related symptoms and palliative care concerns on health-related quality of life in multiple myeloma: a multi-centre study. BMC Cancer. 2016;16:427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beijers AJ, Vreugdenhil G, Oerlemans S, et al. Chemotherapy-induced neuropathy in multiple myeloma: influence on quality of life and development of a questionnaire to compose common toxicity criteria grading for use in daily clinical practice. Support Care Cancer. 2016;24:2411–2420. [DOI] [PubMed] [Google Scholar]
- 17.van der Poel MW, Oerlemans S, Schouten HC, et al. Elderly multiple myeloma patients experience less deterioration in health-related quality of life than younger patients compared to a normative population: a study from the population-based PROFILES registry. Ann Hematol. 2015;94:651–661. [DOI] [PubMed] [Google Scholar]
- 18.Osborne TR, Ramsenthaler C, Siegert RJ, et al. What issues matter most to people with multiple myeloma and how well are we measuring them? A systematic review of quality of life tools. Eur J Haematol. 2012;89:437–457. [DOI] [PubMed] [Google Scholar]
- 19.Kvam AK, Fayers P, Wisloff F. What changes in health-related quality of life matter to multiple myeloma patients? A prospective study. Eur J Haematol. 2010;84:345–353. [DOI] [PubMed] [Google Scholar]
- 20. International Consortium for Health Outcome Measurement (ICHOM). https://www.ichom.org. [Accessed August 22, 2019]. [Google Scholar]
- 21.Porter ME. What is value in health care? N Engl J Med. 2010;363:2477–2481. [DOI] [PubMed] [Google Scholar]
- 22. International Myeloma Working Group (IMWG). https://www.myeloma.org/international-myeloma-working-group-imwg-criteria-diagnosis-multiple-myeloma. [Accessed January 15, 2019]. [Google Scholar]
- 23.Blimark C, Holmberg E, Mellqvist UH, et al. Multiple myeloma and infections: a population-based study on 9253 multiple myeloma patients. Haematologica. 2015;100:107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. [DOI] [PubMed] [Google Scholar]
- 25.Cocks K, Cohen D, Wisloff F, et al. An international field study of the reliability and validity of a disease-specific questionnaire module (the QLQ-MY20) in assessing the quality of life of patients with multiple myeloma. Eur J Cancer. 2007;43:1670–1678. [DOI] [PubMed] [Google Scholar]
- 26. European Organisation of Research and Treatment of Cancer Quality of Life Group Website. https://qol.eortc.org. [Accessed August 22, 2019]. [Google Scholar]
- 27. European Organisation of Research and Treatment of Cancer Quality of Life Group Item Library. https://www.eortc.org/app/uploads/sites/2/2018/09/IL-manual-20180305.pdf. [Accessed August 22, 2019]. [Google Scholar]
- 28.Palumbo A, Avet-Loiseau H, Oliva S, et al. Revised international staging system for multiple myeloma: a report from international myeloma working group. J Clin Oncol. 2015;33:2863–2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blade J, Calleja MA, Lahuerta JJ, et al. Defining a set of standardised outcome measures for newly diagnosed patients with multiple myeloma using the Delphi consensus method: the IMPORTA project. BMJ Open. 2018;8:e018850. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


