Philadelphia (Ph)-negative myeloproliferative neoplasms (MPN) are acquired hematologic diseases with increased production of mature blood cells. They include polycythemia vera (PV), essential thrombocythemia (ET) and myelofibrosis (MF). The most frequent molecular abnormality found in Ph negative MPN is JAK2V617F, an activating mutation of JAK2 which is responsible for constitutive signaling of various cytokine receptors. Arterial and venous thromboses are the main complications of these diseases and are responsible for high rates of morbidity and mortality. Of note there is a disproportionate incidence of thrombosis at unusual sites including splanchnic vein thrombosis.1 Splanchnic vein thromboses (SVT) involve one or more abdominal veins, the two most frequent are Portal Vein Thrombosis (PVT) and Budd Chiari Syndrome (BCS). Pathophysiology of thrombosis in MPN is complex and involves abnormalities in blood cells, plasma factors, and endothelial cells (ECs). Several groups, using different techniques, have shown JAK2V617F expression in endothelial cells (Supplemental Fig. 1). Using laser capture microdissection, JAK2V617F was demonstrated in ECs from hepatic venules in 2 of 3 patients with PV and BCS.2JAK2V617F endothelial cells were demonstrated in microdissected splenic capillaries and in ECs cultured from splenic vein in patients with myelofibrosis but without SVT.3 Although these teams performed experiments to ensure that the DNA they obtained originated from ECs, it is difficult to completely rule out a possible contamination by blood cells. Analysis of endothelial progenitor cells, specifically endothelial colony forming cells (ECFCs), is an alternative way to look for JAK2V617F ECs. Indeed, ECFCs are reported to be the only “true” endothelial progenitor cells, as they are the only ones able to generate blood vessels in vivo: they display clonogenic potential, endothelial but not myeloid cell surface markers, and pronounced postnatal vascularisation ability in vivo.4,5 ECFCs are a unique tool to investigate endothelial molecular dysfunction in disease, as they give access to endothelial cells from patients in a non-invasive way and a promising tool for vascular regenerative approaches and gene therapy.6 Yoder et al studied 11 JAK2V617F MPN patients and reported 3 JAK2V617F ECFCs derived from only 1 of 11 patients. Of note, this patient presented with thrombosis and later developed PV.4 In another study, the JAK2V617F mutation was not detected in any of 75 ECFCs obtained from 57 patients with JAK2V617F MPN but no thrombosis.7 Teofili et al reported JAK2V617F ECFCs in 5 of 22 MPN patients, all with thrombotic complications including 1 with BCS and 1 with PVT.8 Lastly, 4 of 5 JAK2V617F-positive patients with BCS but without overt MPN had JAK2V617F ECFCs cultured from the bone marrow.9 Taken together, these results suggest that the presence of JAK2V617F ECFCs in patients is associated with thrombosis, even in the absence of overt MPN. Our groups have previously demonstrated (a) that the presence of JAK2V617F in ECs modifies their phenotype and makes them prothrombotic,10 highlighting the importance of looking for JAK2V617F ECs in patients; (b) the importance of using correctly characterized ECFCs in investigating this.6 Confirming that JAK2V617F positive ECFCs are associated with previous thrombosis in MPN patients would suggest that ECFCs culture and JAK2V617F genotyping may be used as a marker of thrombotic risk in MPN patients, before they develop thrombosis.
The aim of our study was to analyze ECFCs isolated from peripheral blood of patients with Ph-negative JAK2V617F-positive MPN and to compare the results to the conclusions of previous studies. We focused on patients with JAK2V617F MPN and SVT, as these thrombotic complications are known to be closely associated with MPN. We describe results from 31 patients from Bordeaux, France and then London, United Kingdom (cohort 1: 20 patients) and London (cohort 2: 11 patients). (Table 1). All patients gave informed consent.
Table 1.
Characteristics of the 12 patients with SVT and ECFCs analyzed.
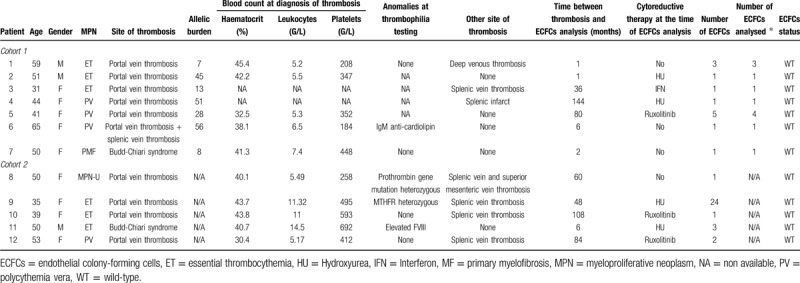
Cohort 1: ECFCs were cultured from 25 to 60 ml of peripheral blood (PB) as previously published.4,11–13 ECFCs appeared between 5 and 20 days of culture in 9/20 patients. In 7/9 patients, there was amplification to passage 4 (P4). ECFCs were characterized using morphologic criteria (monolayers of cobblestone-appearing cells) and their capacity to proliferate and to amplify until P4. Cloning cylinders were used to isolate each colony. After amplification of each colony separately, DNA and in 3 samples RNA were extracted. Reverse transcription quantitative polymerase chain reaction experiments (RT-qPCR) confirmed that the isolated ECFCs expressed endothelial markers (VWF, KDR, CD31, CD146) but not hematopoietic factors (CD45, CD14) (Supplemental Fig. 2A). The presence of JAK2V617F was then investigated using quantitative allele-specific PCR techniques.14,15 Given that we analyzed each colony separately in cohort 1, we considered that a JAK2V617F positive colony would have an allele burden of 50% if heterozygous or 100% if homozygous.
Cohort 2: PB samples (60 ml) were collected from 11 patients for ECFCs isolation.4,11–13 ECFCs appeared between 8 and 28 days of culture in 9/11 patients. In 5/9 patients there was successful colony expansion until P4. ECFC were characterized in 3 patients using immunofluorescence for endothelial markers (VE-cadherin and vWF)13 and clonogenic capability for at least P4 (Supplemental Fig. 2B).
Using identical methods in the 2 cohorts, we successfully isolated ECFCs in 18/31 patients. In 12 patients (38.7%) we could grow highly proliferative ECFCs colonies and expanded them for at least 4 passages. EC were characterized by immunofluorescence or RT-PCR in 5 patients. We obtained DNA of adequate quality from these cells (Fig. 1). In all 12 patients, the ECFCs carried the JAK2 wild-type allele but not the mutated one (Table 1).
Figure 1.
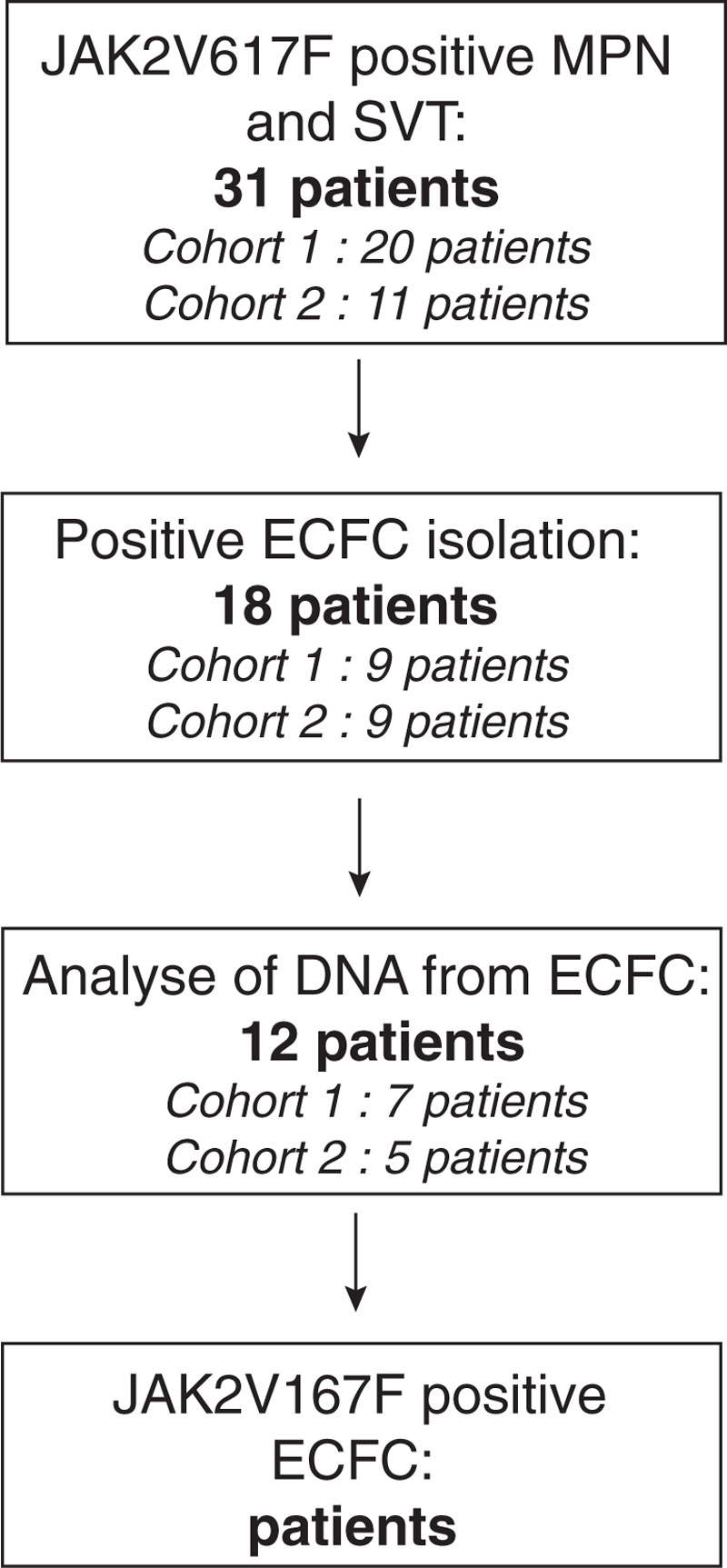
Flow-chart of patients.
In summary, we studied 31 patients with MPN-SVT for JAK2V617F in ECFCs. ECFCs were grown using rigorous laboratory methods. Of the 12 patients where DNA from ECFCs was extracted and studied, none demonstrated the presence of JAK2V617F mutation in endothelial cells. This is in contradiction with previously published results.8,9 These differences may be explained by several reasons.
-
(1)
Culture conditions and definition of ECFCs could be different between our study and the others. Helman et al used BM as a source of endothelial cells and a methodology different of the standard procedure. Interestingly, they observed the appearance of colonies only after 7 days in culture, earlier than what observed from peripheral blood ECFCs. Thus, their method may have resulted in the isolation of an earlier or different type of endothelial progenitor cell, compared to circulating ECFCs. However, the culture conditions and definitions in our study are similar to those used in Teofili et al, and Yoder et al.
-
(2)
The JAK2V617F genotyping technique and threshold for JAK2V617F positivity can also have influenced the interpretation of the data. Indeed, in cohort 1, we considered that an ECFC carried JAK2V617F if the allele burden was 50% or 100%, as described above, given that the cells were isolated from single colonies. This same reasoning had been used by the Yoder group who reported that the 3 JAK2V617F ECFC they found in the same patient carried the mutation at the heterozygous state.4 In cohort 2, where we did not isolate single clones, we used a threshold of 1%. It may be that other reports used a lower JAK2V617F positive threshold, which can lead to the risk of detecting “contaminating” JAK2V617F positive blood cells that do not belong to the ECFCs clone.
-
(3)
The differences may also be due to patients’ characteristics. Indeed, although all our patients had overt MPN and SVT, the analysis of ECFCs occurred at various time points after diagnosis of MPN and initiation of cytoreductive treatment. Notably our cohort comprised a range of MPN diagnoses and cytoreductive agent. However, a third of our patients were assessed before initiation of cytoreductive therapy, none of them presenting JAK2V617F positive ECFCs.
-
(4)
We could only grow highly proliferative ECFCs (up to passage 4) for 12/31 patients (38.7%), all being JAK2 wild type. Recently, the Vascular Biology Standardization Subcommittee from the International Society on Thrombosis and Hemostasis published standardization of methods to quantify and culture ECFCs. They reported a success rate of 70% in isolating ECFC from peripheral blood of healthy subjects, keeping in mind that ECFC colonies were defined as well-circumscribed colonies of cobblestone appearance with more than 50 adherent cells. Here, our success rate is lower; but we only reported the growth of ECFC that had undergone at least 4 passages, as we aimed to have sufficient number of ECFCs to obtain DNA of good enough quality. Besides, we cannot completely rule out that, for unexplained reasons, we could not grow JAK2V617F ECFCs but only JAK2 wild type ECFCs.
-
(5)
Among the 12 informative MPN-SVT patients, 10 had Portal Vein thrombosis and only 2 had Budd Chiari Syndrome. Whereas we can confidently conclude that JAK2V617F ECFCs are not a hallmark of PVT, it is hard to draw the same conclusion for BCS as we only analyzed 2 patients.
In conclusion, using well-established methods across two cohorts of MPN-SVT patients we did not find any circulating JAK2V617F ECFCs in these patients. The differences between this and other studies raise the question of whether the methodology for isolation and characterization of ECFCs may influence the findings. The presence of the JAK2V617F mutation in ECs could have a significant role in the thrombotic pathophysiology of the disease; hence ECFCs from peripheral blood might be used as a marker of thrombotic risk in MPN patients. Our results suggest that this is not always the case and that this technique is currently not sufficiently reproducible to define patients with thrombotic risk. Our results do not exclude the presence of endothelial-like cells derived from the hematopoietic lineage, expressing JAK2V617F, which can integrate into the vessel wall. This would explain the presence of JAK2V617F endothelial cells found using microdissection in the spleen and liver.
Acknowledgements
Bordeaux: We wish to thank Axelle Lascaux, Jean-François Viallard, Pierre Duffau for their help in patient recruitment. We are also grateful to the French Intergroup Myeloproliferative (FIM). This study was supported by research grants from INSERM, ANR-DFG JAKPOT and The Fondation Bettencourt Schueller. AG was supported by research grant from INSERM (Poste Accueil INSERM).
London: We wish to thank Dr Anna Randi of Imperial College London, for support and advice in growth and characterization of ECFC; Steve Hart and Robert Baker of Health Services Laboratories for their assistance in DNA analysis; Koval Smith (Imperial College London, now at Autolus) for his help with immunofluorescence analysis. The study and AD were supported by a Research grant from Novartis to fund the MASCOT study. KP was supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Imperial College Healthcare NHS Trust and Imperial College London.
Supplemental methods
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Sekhar M, McVinnie K, Burroughs AK. Splanchnic vein thrombosis in myeloproliferative neoplasms. Br J Haematol. 2013;162:730–747. [DOI] [PubMed] [Google Scholar]
- 2.Sozer S, Fiel MI, Schiano T, et al. The presence of JAK2V617F mutation in the liver endothelial cells of patients with Budd-Chiari syndrome. Blood. 2009;113:5246–5249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosti V, Villani L, Riboni R, et al. Spleen endothelial cells from patients with myelofibrosis harbor the JAK2V617F mutation. Blood. 2013;121:360–368. [DOI] [PubMed] [Google Scholar]
- 4.Yoder MC, Mead LE, Prater D, et al. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Medina RJ, Barber CL, Sabatier F, et al. Endothelial progenitors: a consensus statement on nomenclature: endothelial progenitors nomenclature. Stem Cells Transl Med. 2017;6:1316–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paschalaki KE, Randi AM. Recent advances in endothelial colony forming cells toward their use in clinical translation. Front Med. 2018;5:295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piaggio G, Rosti V, Corselli M, et al. Endothelial colony-forming cells from patients with chronic myeloproliferative disorders lack the disease-specific molecular clonality marker. Blood. 2009;114:3127–3130. [DOI] [PubMed] [Google Scholar]
- 8.Teofili L, Martini M, Iachininoto MG, et al. Endothelial progenitor cells are clonal and exhibit the JAK2V617F mutation in a subset of thrombotic patients with Ph-negative myeloproliferative neoplasms. Blood. 2011;117:2700–2707. [DOI] [PubMed] [Google Scholar]
- 9.Helman R, Pereira W, de O, et al. Granulocyte whole exome sequencing and endothelial JAK2V617F in patients with JAK2V617F positive Budd-Chiari Syndrome without myeloproliferative neoplasm. Br J Haematol. 2018;180:443–445. [DOI] [PubMed] [Google Scholar]
- 10.Guy A, Gourdou-Latyszenok V, Lay NL, et al. Vascular endothelial cell expression of JAK2V617F is sufficient to promote a pro-thrombotic state due to increased P-selectin expression. Haematologica. 2019;104:70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ingram DA, Mead L, Tanaka H, et al. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104:2752–2760. [DOI] [PubMed] [Google Scholar]
- 12.Starke RD, Ferraro F, Paschalaki KE, et al. Endothelial von Willebrand factor regulates angiogenesis. Blood. 2011;117:1071–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paschalaki KE, Starke RD, HU Y, et al. Dysfunction of endothelial progenitor cells from smokers and chronic obstructive pulmonary disease patients due to increased DNA damage and senescence. Stem Cells. 2013;31:2813–2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larsen TS, Christensen JH, Hasselbalch HC, et al. The JAK2 V617F mutation involves B- and T-lymphocyte lineages in a subgroup of patients with Philadelphia-chromosome negative chronic myeloproliferative disorders. Br J Haematol. 2007;136:745–751. [DOI] [PubMed] [Google Scholar]
- 15.Lippert E, Boissinot M, Kralovics R, et al. The JAK2-V617F mutation is frequently present at diagnosis in patients with essential thrombocythemia and polycythemia vera. Blood. 2006;108:1865–1867. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


