In this issue of HemaSphere, Øbro et al expand our current knowledge on the role of cytokines in patients with the Philadelphia-negative chronic myeloproliferative neoplasms (MPNs).1 For several reasons, their study is of utmost importance. First, this is the largest study on circulating cytokines reported in MPNs: a comprehensive cytokine profiling study, including more than 400 MPN patients. Second and highly interesting, the authors have identified an “essential thrombocythemia (ET)-specific inflammatory cytokine signature" encompassing 3 biomarkers: Eotaxin, GRO-α and EGF. Third, in ET patients, this cytokine signature predicted disease transformation based upon either the initial blood sample (for GRO-α) or on blood samples drawn later during the course of ET, (EGF), implying circulating cytokines are to be used not only in disease stratification but potentially also in disease monitoring. Fourth, in ET patients with extensive genomic profiling data, levels of inflammatory cytokines added significant value in predicting myelofibrotic transformation. Fifth, another most interesting and novel observation addressed a subset of monocytes (CD56+CD14+ proinflammatory monocytes), which were shown as a novel source of increased GRO-α levels.1
In recent years, interest in immune deregulation and chronic inflammation has increasingly been recognized as the driving force for clonal evolution and disease progression in MPNs (Fig. 1).2–6 Thus, in 2015, Sylvie Hermouet was the lead of a theme issue on “Mediators of Inflammation in Myeloproliferative Neoplasms: State of the Art.7” In this issue, several important papers were published on clinical, biochemical, immunologic and molecular aspects of chronic inflammation in MPNs, including the role and mechanisms of chronic inflammation in the pathogenesis of MPNs and associations between circulating cytokines and MPN diagnosis, disease stage, progression and prognosis.8–10 In the perspective of chronic inflammation and immune deregulation as highly significant pathogenic factors for clonal evolution and disease progression in MPNs, the study by Øbro et al raises several questions and issues to be pursued and validated in future prospective studies.
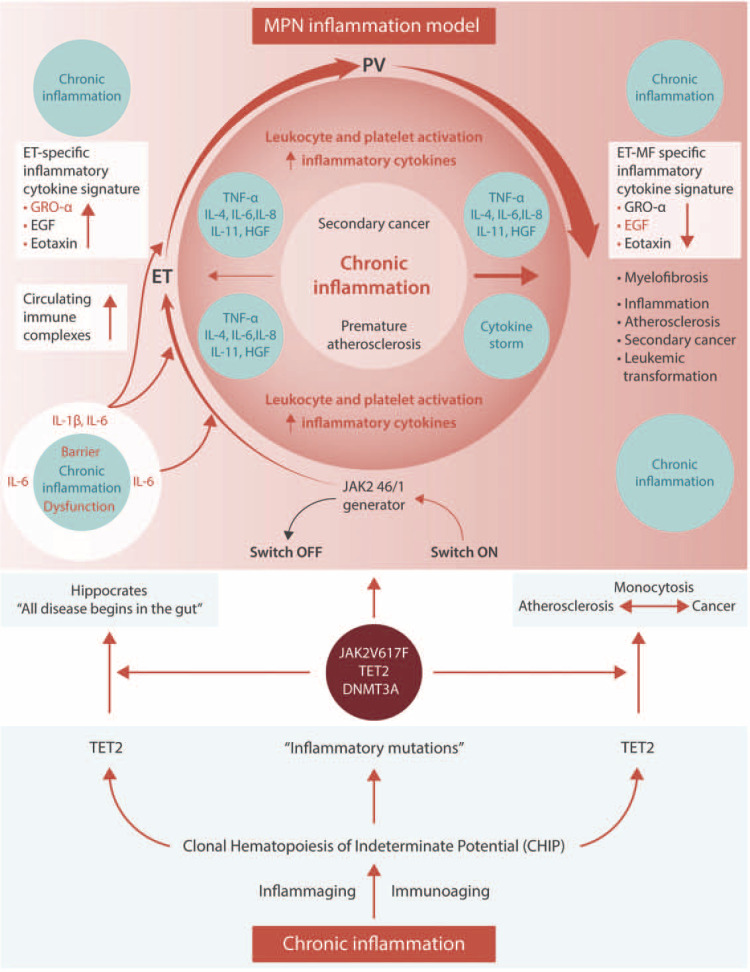
Figure 1.
Clonal hematopoiesis of indeterminate potential (CHIP) is common and increases in prevalence with age. Somatic mutations in JAK2, TET2, DNMT3A are among the most common and are associated with an increased risk of hematological and non-hematological malignancies as well as an increased risk of cardiovascular diseases. It is increasingly being recognized that the common denominator for development of cancer and cardiovascular diseases is chronic inflammation. The Philadelphia-negative chronic myeloproliferative neoplasms (MPNs) are “inflammatory” diseases, which have been described as “A Human Inflammation Model”, since chronic inflammation is likely both a trigger and driver of clonal evolution and determinant for disease progression, cardiovascular disease burden (early inflamma-aging and immune-aging) and the increased risk of second cancers. Small-intestinal barrier dysfunction may be highly important for MPN-development in patients with the TET2 mutation in the context of microbial-mediated inflammatory IL-6 signaling, having a crucial role for clonal evolution. Thus, the TET2 mutation is also associated with upregulation of several “inflammatory” cytokines, including IL1beta, IL-6 and CXCL1 (GRO-α). Together with EGF and Eotaxin, GRO-α constitutes an “essential thrombocythemia (ET)-specific inflammatory cytokine signature”, which predicts myelofibrotic transformation and adds significant value in prognostication of MPNs. In this self-perpetuated vicious circle from early MPN-stages (ET, PV) to the advanced myelofibrosis stage – driven by chronic inflammation – circulating levels of EGF and GRO-α steadily decrease and signal transformation to myelofibrosis. It is proposed that circulating IgA immune complexes, being elicited by bacterial intestinal translocation with antibody production against IgA, trigger a chronic inflammatory state with the production of inflammatory cytokines from several immune cells and consequently the development of MPNs from CHIP. Hereby, Hippocrates statement “All disease begins in the gut” holds true for MPNs as well.
Which are the cell sources for the elevated levels of cytokines in MPNs?
As pointed out in Øbro's paper, cytokines in MPNs—as in all other cancers and inflammatory diseases—are derived from both the malignant cells (in the bone marrow and in the circulation), from non-clonal stromal cells in the bone marrow and from circulating non-clonal immune cells, which are activated as part of “Tumor Immune Surveillance.” Considering the biological continuum from early stage MPN cancers (ET/polycythemia vera (PV)) towards the advanced myelofibrosis stage and current knowledge on chronic inflammation as a highly important driving force for clonal evolution and disease progression,2–12 one might anticipate the highest levels of inflammatory cytokines in patients with classic myelofibrosis, which clinically and biochemically is characterized by a hyperinflammatory state.4–12 Indeed, previous studies of circulating cytokine levels in MPNs generally show moderately elevated cytokines levels in the early stages (ET/PV) and the highest levels in myelofibrosis.11 Mathematical modeling studies have substantiated a steady increase in inflammatory cytokines in the biological MPN-continuum.12
Highly interesting, Øbro et al found CD56+CD14+ monocytes as a potential source of GRO-α. There is growing evidence that monocytes and macrophages carrying clonal mutations increase the risk for inflammation-related diseases such as atherosclerosis and cancer. In this regard, the TET mutation is of particular interest, since this mutation associates with monocytosis and increased IL-1beta and IL-6 production. In this context, monocytes may also be the link between atherosclerosis and cancer. The role of monocytosis for MPN-disease development and progression is being increasingly investigated. Several studies have shown monocytosis to be associated with a more “aggressive” clinical phenotype in patients already diagnosed with MPN. Since a proportion of the ET patients had a substantially higher number of GRO-α producing cells, one might speculate an increased number of these cells to be associated with monocytosis as well, implying that a subset of the ET-patients actually had myelofibrosis. Indeed, the findings of fibrosis grades 2 or 3 in some of the ET-patients are supportive.1
As also noted by Øbro et al, GRO-α has been associated with high platelet counts and implicated in vascular diseases, which are both closely linked to chronic inflammation.1 Indeed, it is worth considering whether elevated circulating GRO-α levels in patients with MPNs might also be derived from platelets, since GRO-α is stored in platelet granules and can be secreted by platelet activation. Furthermore, thrombopoietin can induce GRO production by megakaryocytes and stimulate platelet activation and granule release. Accordingly, there are reasons to believe that elevated plasma GRO-α levels in MPN-patients might be explained by several other mechanisms, including release from circulating activated platelets, increased GRO production by megakaryocytes induced by raised levels of TPO and the chronic inflammatory state as well.
Since elevated platelet counts are most prominent in the early MPN-stages (ET/PV), with decreasing levels when patients transform into myelofibrosis, the observation of high GRO-α levels in early MPNs might be partly attributed to higher platelets and lower GRO-α in myelofibrosis to hypoactive and “exhausted” circulating platelets. Similar mechanisms might explain the declining levels of epidermal growth factor (EGF) during myelofibrotic transformation, taking into account that this cytokine is also stored in platelets, which are decreased in advanced myelofibrosis. Together with decreased synthesis of EGF by defective and dysplastic megakaryocytes, these mechanisms might contribute to the lower circulating levels of EGF in myelofibrosis patients. The lower levels of GRO-α in primary myelofibrosis (PMF) is also surprising when considering that elevated expression of GRO-α in tumor and stromal cells or in the circulation actually indicates unfavorable prognosis in several other cancers. Since GRO-α is also considered an inflammatory cytokine and a marker for age-related pathology (inflammaging) and cancer correlating proportionally with IL-6 and IL-8, their findings seem at first glance counterintuitive to current concepts but certainly also emphasizing the need for further studies to substantiate their findings. Until then, it is intriguing to consider if lower levels of GRO-alpha during transformation to myelofibrosis actually is explained by a reduced number of circulating platelets, consequent to cytoreductive treatment (hydroxyurea) and/or platelets, which are defective and exhausted with less amounts of GRO-alpha in their alpha-granules.
How might cytoreductive agents, including hydroxyurea, interferon-alpha, anagrelide and immunosuppressive treatment impact circulating levels of inflammatory cytokines?
Hydroxyurea lowers elevated leukocyte-and platelets count within days and thereby also the number of those cells, which are significant sources of cytokine production. The impact of hydroxyurea upon elevated circulation levels of cytokines has not been studied systematically in MPNs. However, another “inflammation model” for thrombosis development—sickle cell anemia (SCA)—shares several thrombosis promoting mechanisms with MPNs, including in vivo activation of leukocytes and platelets and in vivo activation of endothelial cells as well. Hydroxyurea reduces the thrombosis risk including the risk of SC crisis—a hyperinflammatory syndrome—which is tightly associated with exacerbation of the chronic inflammatory state in SCA. Several studies have shown that circulating inflammatory cytokines (eg, TNF-α, IL-8, IL-1β, and IL-6) are markedly elevated in SCA and hydroxyurea significantly lowers them all. Hydroxyurea not only affects monocyte subsets, but also the ability of the cells to produce pro-inflammatory cytokines.13 Surprisingly, Øbro et al did not find any significant changes in individual cytokine levels due to hydroxyurea treatment; for the other treatments (interferon-alpha, ruxolitinib), they did not have a sufficient number of patients to make robust statistical comparisons. It is intriguing to speculate whether the elevated GRO-α and EGF levels in ET and PV patients might partly be explained by low-risk patients (<60 years, no prior thrombosis and platelets <1500 × 109/L) not receiving cytoreductive therapy (“watch and wait strategy”). If so, declining levels during disease progression towards myelofibrosis might associate with an increasing number of patients being treated with hydroxyurea with concurrent reduction in elevated inflammatory cytokines. Therefore, in future studies, the impact of treatment—potentially lowering elevated cytokine levels in MPNs—should also be carefully addressed (eg, hydroxyurea, glucocorticoids, methotrexate, danazol, JAK1/2 inhibitors, and statins).
How to differentiate “genuine” ET from early stage myelofibrosis without a bone marrow biopsy?
In the Øbro et al study, the specific inflammatory cytokine signature predicted disease transformation to myelofibrosis. Of note, baseline fibrosis grades were only given in 44 patients (from the PT-1 Trial), compromising the differentiation between “genuine” ET, early prefibrotic myelofibrosis and “hyperproliferative” MF in the remaining patients in their large cohort of “ET” patients. Although fibrosis grade was not correlated with GRO-α or EGF levels, one might speculate whether elevated GRO-α in the initial blood sample indeed reflected patients already having transformed and accordingly being more advanced towards the myelofibrosis stage. Thus, bone marrow fibrosis grading is challenging, taking into account the heterogeneous distribution of fibrosis pattern in a single bone marrow biopsy and likely therefore also in the skeleton in MPNs. Therefore, the interpretation of any correlation between circulating inflammatory cytokine levels and fibrosis grade should be cautiously addressed.
Conclusion
The findings in the present study by Øbro et al are highly exciting and of utmost importance, underscoring the significant role of chronic inflammation for MPN disease development and how simple tools such as measurement of circulating inflammatory cytokines might complement genomic profiling in assessing prognosis and monitoring of disease transformation to myelofibrosis. Therefore, their comprehensive study opens the avenue for similar future prospective studies, highlighting the impact of current therapies (eg, hydroxyurea, interferon-alfa, JAK 1/2 inhibitors) upon clonal evolution, and the mutational landscape and how it is shaped by the chronic inflammatory state as assessed by cytokine profiling studies. On this journey, we should not forget history, but look back and link previous studies on circulating immune complexes (IC) and complement activation in MPNs 30 years ago8,11 to current and future studies on the role of cytokines in disease pathogenesis, stratification, and prognostication of MPNs. This is even more important when considering that circulating IC are not only able to induce complement activation but production of several inflammatory cytokines.8,14 In this perspective, it is intriguing to consider the impact of dysfunction of the small-intestinal barrier for MPN-development.15 Bacterial translocation with antibody production against IgA might elicit a huge amount of IgA containing IC,14 triggering a chronic hyperinflammatory state with the production of inflammatory cytokines from several immune cells and thereby ultimately also triggering development of MPNs. If so, important MPN research on inflammation and immune deregulation during the last 30 years2–12 will be supportive of the Hippocrates statement “All disease begins in the gut” and this may hold true for MPNs as well.15
Footnotes
Citation: Hasselbalch HC, Hasselbalch HC, Hasselbalch HC, Hasselbalch HC, Hasselbalch HC, Hasselbalch HC. Cytokine Profiling as a Novel Complementary Tool to Predict Prognosis in MPNs? HemaSphere, 2020;00:00. http://dx.doi.org/10.1097/HS9.0000000000000407
The author has no conflicts of interest to disclose.
References
- 1.Øbro N, Grinfeld J, Belmonte M, et al. Longitudinal cytokine profiling identifies GRO-α and EGF as potential biomarkers of disease progression in essential thrombocythemia. HemaSphere. 2020;4:e371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barosi G. An immune dysregulation in MPN. Curr Hematol Malig Rep. 2014;9:331–339. [DOI] [PubMed] [Google Scholar]
- 3.Hermouet S, Vilaine M. The JAK2 46/1 haplotype: a marker of inappropriate myelomonocytic response to cytokine stimulation, leading to increased risk of inflammation, myeloid neoplasm, and impaired defense against infection? Haematologica. 2011;96:1575–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hasselbalch HC. Perspectives on chronic inflammation in essential thrombocythemia, polycythemia vera, and myelofibrosis: is chronic inflammation a trigger and driver of clonal evolution and development of accelerated atherosclerosis and second cancer? Blood. 2012;119:3219–3225. [DOI] [PubMed] [Google Scholar]
- 5.Koschmieder S, Mughal TI, Hasselbalch HC, et al. Myeloproliferative neoplasms and inflammation: whether to target the malignant clone or the inflammatory process or both. Leukemia. 2016;30:1018–1024. [DOI] [PubMed] [Google Scholar]
- 6.Craver BM, El Alaoui K, Scherber RM, et al. The critical role of inflammation in the pathogenesis and progression of myeloid malignancies. Cancers (Basel). 2018;10:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hermouet S, Hasselbalch HC, Čokić V. Mediators of inflammation in myeloproliferative neoplasms: state of the art. Mediators Inflamm. 2015;2015:964613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hasselbalch HC, Bj⊘rn ME. MPNs as inflammatory diseases: the evidence, consequences, and perspectives. Mediators Inflamm. 2015;2015:102476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hermouet S, Bigot-Corbel E, Gardie B. Pathogenesis of myeloproliferative neoplasms: role and mechanisms of chronic inflammation. Mediators Inflamm. 2015;2015:145293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mondet J, Hussein K, Mossuz P. Circulating cytokine levels as markers of inflammation in philadelphia negative myeloproliferative neoplasms: diagnostic and prognostic interest. Mediators Inflamm. 2015;2015:670580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hasselbalch HC. The role of cytokines in the initiation and progression of myelofibrosis. Cytokine Growth Factor Rev. 2013;24:133–145. [DOI] [PubMed] [Google Scholar]
- 12.Andersen M, Sajid Z, Pedersen RK, et al. Mathematical modelling as a proof of concept for MPNs as a human inflammation model for cancer development. PLoS One. 2017;12:e0183620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guarda CC, Silveira-Mattos PSM, Yahouédéhou SCMA, et al. Hydroxyurea alters circulating monocyte subsets and dampens its inflammatory potential in sickle cell anemia patients. Sci Rep. 2019;9:14829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hansen IS, Hoepel W, Zaat SAJ, et al. Serum IgA immune complexes promote proinflammatory cytokine production by human macrophages, monocytes, and kupffer cells through FcαRI-TLR cross-talk. J Immunol. 2017;199:4124–4131. [DOI] [PubMed] [Google Scholar]
- 15.Meisel M, Hinterleitner R, Pacis A, et al. Microbial signals drive pre-leukaemic myeloproliferation in a Tet2-deficient host. Nature. 2018;557:580–584. [DOI] [PMC free article] [PubMed] [Google Scholar]