Infections represent the most common and potentially life-threatening complication in patients with haematological malignancies, particularly in subjects with a lymphoid neoplasm.1 The increased risk of infections is ascribed to inherent disease-related immunological impairment, and can be worsened by chemo-immunotherapy and targeted agents.2–4 While bacterial and mycotic infections5,6 are known to be recurrent in haematological patients, viral infections were often overlooked but recently they became a world-wide concern.
West Nile virus (WNV) is an arthropod-borne virus commonly transmitted to humans mainly by Culex mosquitoes, although transmission through blood transfusion or organ transplantation has been reported.7 WNV is maintained in a continuous cycle within mosquitos and birds, wherein mosquitos are the vectors and the birds are the reservoir. Humans, just like horses and other mammalians, act as dead-end hosts and do not contribute to the spreading of the infection. In most immunocompetent patients WNV infection is usually asymptomatic, 20% of infected people develops a flu-like syndrome, while almost 1% experiences WNV neuroinvasive disease (WNVND).7 Symptoms, when occurring, generally develop after an incubation period typically lasting 2 to 6 days, but may extend to 14 days, or even longer in immunocompromised subjects. WNVND can occur as meningitis, encephalitis, or acute flaccid paralysis.8
So far, the knowledge on the clinical course, the rate of central nervous system (CNS) involvement and the outcome of WNV infection in patients with haematological malignancies is scanty, being limited to only a few reports.9 The aim of this multicentre study was to analyse the clinical features and the outcome of WNV infection in patients with malignancies of B-cell lineage.
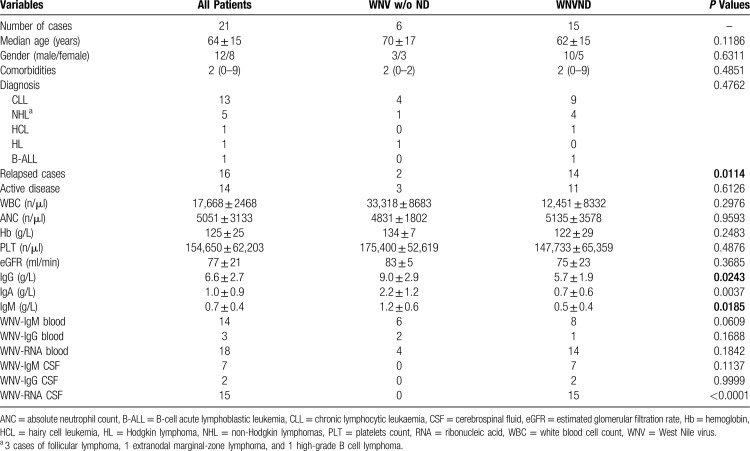
For this purpose, we retrospectively collected clinical data from 21 patients diagnosed with a B-cell lymphoid neoplasm who experienced WNV infection during the last 7 years at 8 Italian institution. Thirteen patients had chronic lymphocytic leukaemia (CLL), 5 non-Hodgkin lymphomas (3 follicular lymphomas, 1 high-grade lymphoma, and 1 extranodal marginal zone lymphoma), 1 hairy cell leukaemia, 1 Hodgkin lymphoma, and 1 B-cell precursor acute lymphoblastic leukaemia. Anti-WNV antibody and WNV-ribonucleic acid (RNA) were assessed in blood and cerebrospinal fluid (CSF) in all patients. CNS imaging studies (ie brain computer tomography scan and/or magnetic resonance immaging) were performed in all the patients with WNVND, in order to rule out other causes of neurological involvement such as bleeding or lymphoma/leukaemia localization. CNS symptoms associated with the presence of WNV-RNA and/or WNV-IgM in the CSF were applied as diagnostic criteria for WNVND, according to the current guidelines.8
The primary endpoint of the study was to evaluate the rate of WNVND. The secondary endpoints included the median overall survival (OS), calculated as time from WNV infection to death (event) or last known follow-up (censored), and WNV-related survival. Mann-Whitney and Fisher exact tests were used to compare continuous and categorical variables. This multicentre retrospective study was approved by the local research ethics committee of Padua Hospital and carried out according to Helsinki declaration. Informed consent was obtained from all alive patients. Authors can share patients’ data upon reasonable request.
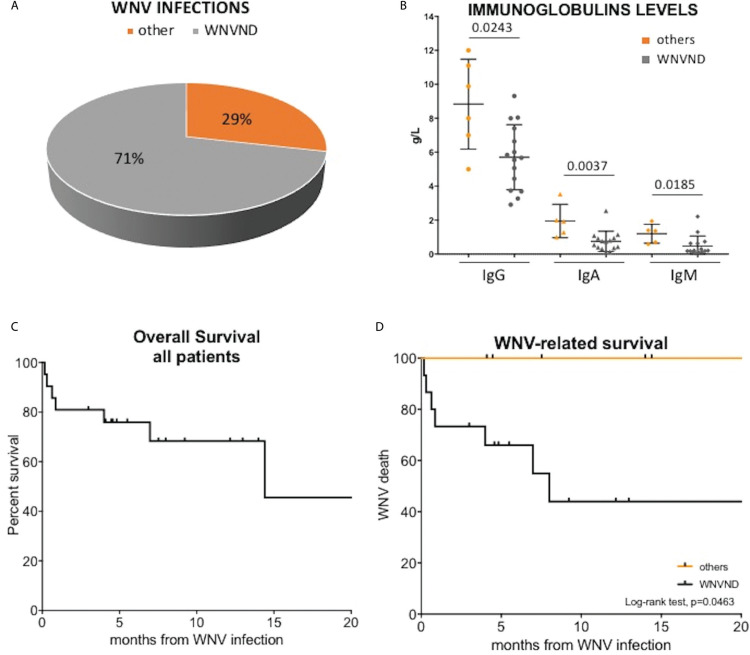
Clinical and laboratory features of the 21 patients are reported in Table 1 and in Figure 1A. Sixteen (76%) participants had received one previous anti-leukaemia/lymphoma treatment (0–3) and 10 (57%) had an active haematological disease at the time of WNV infection, including previously untreated cases and those with relapsed diseases. The median time from lymphoid neoplasm diagnosis to WNV infection was 6.5 ± 4.5 years, being longer in patients with WNVND (3.5 ± 2.9 vs 7.4 ± 4.4 in cases without and with WNVND, P = 0.0358). Patients who developed WNVND were more likely relapsed cases and had significantly lower serum immunoglobulin levels (IgG, IgA, and IgM; Figure 1B) as compared to subjects without neurological involvement (Table 1).
Table 1.
Clinical and Microbiological Characteristics of the Patients.
Figure 1.
Descriptive graphs and survival curves. (A) The upper left panel shows the apple-pie graph of WNV infection; 71% of patients had a WNVND, while 29% did not have a documented spread to central nervous system. (B) The upper right panel reports a scatter plot of serum IgG, IgA, and IgM immunoglobulin levels in patients with (n = 15) and without WNVND (n = 6). Immunoglobulins were measured once for each patient within 30 days before WNV infection. Cases with WNVND had significantly lower immunoglobulin levels as assessed by Mann-Whiteney U test. the lower panels report the overall survival of the whole cohort (C) and the WNV-related survival in patients with and without WNVND (D). Patients with WNVND have a short WNV-related survival (P = 0.0463). WNV = West Nile virus, WNVND = West Nile virus neuroinvasive disease.
All the patients presented fever (max value range 38.2–40°C), while 17 (81%) reported fatigue, 9 (42%) arthralgia, and 4 (19%) dyspnoea. As shown in Table 1, anti-WNV IgM was detected in the blood of 14 patients (67%), with a clear difference between patients with or without WNVND (53% vs 100%), suggesting that impaired humoral immunity may favour viral diffusion to CNS. Consistently, anti-WNV IgM were negative in CSF of half of WNVND cases. The presence of WNV-RNA in urine was identified in 4 of 11 assessed patients. Twenty (95%) subjects developed neurological symptoms, such as confusion, amnesia, or headache, but only 15/21 (71%, Figure 1A) fulfilled the criteria for WNVND. All the six patients without WNVND and 6 of 15 (40%) with WNVND showed complete resolution of the infection without any sequalae. The remaining 9 of 15 with WNVND manifested gait instability, depression, or amnesia at 1 year from infection occurrence. Given the lack of a standard therapeutic approach, our patients received different treatments, including polyclonal intravenous immunoglobulins (57%), corticosteroids (33%), antiviral drugs (29%: 24% acyclovir and 5% ganciclovir), and levetiracetam (29%: 15% as prophylaxis and 14% for seizure treatment). Most cases were managed in an inpatient setting, due to high-grade fever and neurological symptoms or clinical manifestations such as tremor and dizziness seizures and coma, requiring intravenous fluids or respiratory support.
Among the CLL subgroup, accounting for 62% of all cases, 8 of 13 patients had a relapsed disease and 4 harboured high-risk cytogenetics (ie 17p abnormalities or 11q deletion) (Table 1). WNVND was diagnosed in 9 (69%) CLL patients, 3 of whom were receiving a kinase inhibitor (2 ibrutinib and 1 idelalisib-rituximab) at the time of the infection onset. Both patients taking ibrutinib as first-line therapy mounted protective anti-WNV IgM, contributing to WNV clearance and complete recovery, without any permanent neurological sequelae. Conversely, the heavily pre-treated patient receiving idelalisib-rituximab showed persistent WNR-RNA load in blood and CSF, resulting in progressive neurological impairment. In the latter case, it is conceivable that rituximab and previous therapies, rather than idelalisib, blunted humoral immunity, compromising the host response.
After a median follow-up of 12.4 months, 8 patients (37%) died [6 cases of WNVND and 2 non-WNV-related deaths (1 Richter syndrome and 1 stroke)]. Among the patients with WNVND, the mortality rate was 40%. The 12-month OS of the whole cohort was 68% and the median OS was 14.4 months (Figure 1C). The median WNV-related survival was 8 months for patients with WNVND, while no death due to WNV infection was reported in subjects without neuro-invasive disease (P = 0.0463, Figure 1D).
Since 2008, the incidence of WNV infection increased substantially in South Europe, with peaks of cases registered in 2013 and 2015,10 likely due to environmental changes that favour mosquitos breeding and propagation. While most of the infected patients are asymptomatic, some of them can experience severe neurological illness. Older age and impaired adaptive immunity have been supposed to be the most relevant risk factors for CNS involvement.11 These conditions commonly coexist in subjects with lymphoid cancers, arousing concerns on WNV outbreaks in this population. Furthermore, the lack of approved vaccine or specific antiviral drugs narrow the therapeutic possibilities for the management of the disease, these latter being mainly based on supportive care. Overall, only 17 cases of WNVND in the context of a haematological neoplasm have so far been reported in the literature, mostly published as case reports.9 Interestingly, a paper reported 1 CLL patient developing WNVND while receiving ibrutinib.9
To our knowledge, this is the largest study that described clinical and laboratory features of WNV infection in patients with B-cell malignant lymphoid disorders, evaluating, for the first time, the rate of WNVND and the survival outcomes in this setting.
In our series, WNVND appeared to be very common, involving 71% of cases and causing 6 of 8 deaths. The risk of WNVND is estimated to be 0.6–0.7% in the immunocompetent individuals and 40% among solid transplant-recipients.12 Notably, in our study WNVND was associated with a dismal outcome: only 1 of 4 patients survived longer than 12 months after infection onset (with a median OS of 8 months) and mortality was as high as 40%, approximately four-fold higher as compared to unselected populations.7,11 Not surprisingly, hypogammaglobulinemia and the presence of a relapsed disease emerged as risk factors for CNS involvement.
The present study provides clinically relevant information on a potentially life-threatening viral disease that significantly worsens the outcome in patients with lymphoid neoplasms, accounting for high mortality and morbidity. It should be noted that, as mildly symptomatic patients are unlikely investigated for possible WNV infection, the incidence of non-neuroinvasive infection is underestimated. Moreover, the diagnostic process may be tricky in rituximab-treated patients, whose serologic tests are often negative.13 Therefore, haematologists are urged to start a timely and proper diagnostic workup, consisting of both serologic and molecular tests, in patients with unexplained fever and/or with mild neurological symptoms, especially those receiving chemo-immunotherapy or targeted therapy in endemic areas and during summer period. Currently, the implementation of dedicated policies for the prevention, surveillance, and control of WNV infection represents the most effective measure against virus outbreaks. Thus, future efforts should be directed to empower national surveillance systems and accomplish specific screening and treatment recommendations for haematological patients. Moreover, the protective effect of immunoglobulin replacement therapy remains to be elucidated.
Sources of Funding
This work was supported by funds from Gilead fellowship program 2017 and 2018 to LT, Fondo di Ateneo per la Ricerca 2016, 2017 of the University of Ferrara to GMR and FC, Fondo di Incentivazione alla Ricerca 2017 of the University of Ferrara to GMR, Ministero dell’Istruzione, dell’Universitàe della Ricerca PRIN 2015 to AC (2015ZMRFEA). AV received a research fellowship from the University of Padua supported by Ricerca per Credere nella Vita (RCV-ODV), Padua, Italy.
Disclosures
AV received honoraria from Janssen, Gilead, and Abbvie. LT received research funding by Gilead and Janssen, advisory board for Roche, Takeda, and Abbvie. GMR received research funding by Gilead. AC advisory board and speaker bureau for Roche, Abbvie, Gilead, and Janssen. GS board member of Abbvie, Roche, Janssen, and Celgene. ML received honoraria from Gilead, MSD, Pfizer, Novartis, Abbvie, Sanofi, Daiichi Sankyo, Jazz Pharmaceuticals. RM advisory board Abbvie, and Janssen. FP advisory board Roche. VN, MM, IF, RP, RS, FC, SI, MR, CB, SM, FG, MK, and RB have nothing to disclose.
Footnotes
Citation: Visentin A, Nasillo V, Marchetti M, Ferrarini I, Paolini R, Sancetta R, Rigolin GM, Cibien F, Riva M, Briani C, Marinello S, Piazza F, Gherlinzoni F, Krampera M, Bassan R, Cuneo A, Luppi M, Semenzato G, Marasca R, Trentin L. Clinical Characteristics and Outcome of West Nile Virus Infection in Patients with Lymphoid Neoplasms: An Italian Multicentre Study. HemaSphere, 2020;00:00. http://dx.doi.org/10.1097/HS9.0000000000000395
Authors’ contributions: AV designed the study, performed statistical analysis, evaluated patients, and wrote the article; VN evaluated patients and wrote the article; IF, SI, RF, RS, FC, MR, CB, SM, and FC provided intellectual inputs and evaluated patients; MM, IF, FP, FG, MK, RB, GMR, GS, RF, AC, ML, RM, and LT evaluated patients, provided intellectual inputs, and reviewed the article.
Andrea Visentin and Vincenzo Nasillo equally contributed to the work.
References
- 1.Visentin A, Imbergamo S, Gurrieri C, et al. Major infections, secondary cancers and autoimmune diseases occur in different clinical subsets of chronic lymphocytic leukaemia patients. Eur J Cancer. 2017;72:103–111. [DOI] [PubMed] [Google Scholar]
- 2.Forconi F, Moss P. Perturbation of the normal immune system in patients with CLL. Blood. 2015;126:573–581. [DOI] [PubMed] [Google Scholar]
- 3.Maffei R, Maccaferri M, Arletti L, et al. Immunomodulatory effect of ibrutinib: reducing the barrier against fungal infections. Blood Rev. 2020;40:100635. [DOI] [PubMed] [Google Scholar]
- 4.Visentin A, Compagno N, Cinetto F, et al. Clinical profile associated with infections in patients with chronic lymphocytic leukemia. Protective role of immunoglobulin replacement therapy. Haematologica. 2015;100:e515–e518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Visentin A, Gurrieri C, Imbergamo S, et al. Epidemiology and risk factors of invasive fungal infections in a large cohort of patients with chronic lymphocytic leukemia. Hematol Oncol. 2017;35:925–928. [DOI] [PubMed] [Google Scholar]
- 6.Tisi MC, Hohaus S, Cuccaro A, et al. Invasive fungal infections in chronic lymphoproliferative disorders: a monocentric retrospective study. Haematologica. 2017;102:e108–e111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petersen LR, Brault AC, Nasci RS. West Nile virus: review of the literature. JAMA. 2013;310:308–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Popescu CP, Florescu SA, Hasbun R, et al. Prediction of unfavorable outcomes in West Nile virus neuroinvasive infection - Result of a multinational ID-IRI study. J Clin Virol. 2020;122:104213. [DOI] [PubMed] [Google Scholar]
- 9.Ferrarini I, Rigo A, Gandini A, Vinante F. West Nile virus encephalitis in haematological setting: report of two cases and a brief review of the literature. Mediterr J Hematol Infect Dis. 2019;11:e2019033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rizzo C, Napoli C, Venturi G, et al. West Nile virus transmission: results from the integrated surveillance system in Italy, 2008 to 2015. Euro Surveill. 2016;21:30340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Montgomery RR, Murray KO. Risk factors for West Nile virus infection and disease in populations and individuals. Expert Rev Anti Infect Ther. 2015;13:317–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar D, Drebot MA, Wong SJ, et al. A seroprevalence study of west nile virus infection in solid organ transplant recipients. Am J Transplant. 2004;4:1883–1888. [DOI] [PubMed] [Google Scholar]
- 13.Morjaria S, Arguello E, Taur Y, et al. West Nile virus central nervous system infection in patients treated with rituximab: implications for diagnosis and prognosis, with a review of literature. Open Forum Infect Dis. 2015;2:ofv136. [DOI] [PMC free article] [PubMed] [Google Scholar]