Abstract
Objective:
The accurate differentiation of glioma recurrence from pseudoprogression (PSP) after therapy remains a considerable clinical challenge. Several studies have shown that diffusion magnetic resonance imaging (MRI) has potential value in distinguishing these 2 outcomes. The current meta-analysis examined the diagnostic accuracy of diffusion MRI with the apparent diffusion coefficient (ADC) in the differentiation of glioma recurrence from PSP.
Method:
PubMed, Embase, Cochrane Library, and Chinese Biomedical databases were reviewed to identify studies that fulfilled our inclusion/exclusion criteria and were published on or before May 5, 2019. Threshold effects; heterogeneity; pooled sensitivity (SENS), specificity, positive likelihood ratio, and negative likelihood ratio; and diagnostic odds ratio were calculated. The overall diagnostic usefulness of diffusion MRI-derived ADC values was assessed by calculating the area under the curve (AUC) following summary receiver operating characteristic (SROC) analysis.
Results:
Six eligible studies examined a total of 214 patients. Calculation of pooled values indicated the SENS was 0.95 (95% confidence interval [CI] = 0.89–0.98), specificity was 0.83 (95% CI = 0.72–0.91), positive likelihood ratio was 4.82 (95% CI = 2.93–7.93), negative likelihood ratio was 0.08 (95% CI = 0.04–0.17), and diagnostic odds ratio was 59.63 (95% CI = 22.63–157.37). The SROC AUC was 0.9322. Publication bias was not significant, and SENS analysis indicated the results were relatively stable.
Conclusions:
Our meta-analysis indicated that diffusion MRI with quantitative ADC is an effective approach for differentiation of glioma recurrence from PSP, and can be used as an auxiliary tool to diagnose glioma progression.
Keywords: apparent diffusion coefficient, diffusion magnetic resonance imaging, glioma recurrence, meta-analysis, pseudoprogression
1. Introduction
Gliomas are the most common malignant brain tumors affecting adults, accounting for more than half of brain tumors.[1] Surgery, radiotherapy, and chemotherapy are required for patients with high-grade (III/IV) gliomas because these individuals experience high recurrence and poor survival rates. However, these treatments can cause post-treatment reactions, including radiation necrosis and pseudoprogression (PSP).[2–4] PSP is defined as a new or enlarged area of contrast enhancement occurring early after the end of radiotherapy (mostly within 3–4 months) without true tumor growth.[5] Previous studies indicated that the prevalence of PSP in glioma patients after standard treatment (radiotherapy and concurrent chemotherapy with temozolomide is about 20% to 30%.[27,28,39] Conventional contrast-enhanced magnetic resonance imaging (MRI) cannot easily distinguish glioma recurrence and PSP, making treatment selection difficult for these 2 conditions, which are associated with very different outcomes.[6–8] These areas of contrast enhancement in PSP can subside or stabilize without a change in therapy, but patients with recurrent glioma generally must receive repeat surgery or further chemoradiotherapy to improve overall survival.[9] An erroneous diagnosis of glioma recurrence may lead to interruption of standardized first-line therapy and unnecessary surgery.[11] Therefore, the accurate differentiation of glioma recurrence from PSP is essential so that the most effective treatment can be provided.[10]
Currently, numerous advanced MRI technologies have been introduced to resolve the limitations of contrast-enhanced MRI. These include perfusion MRI, diffusion MRI, and magnetic resonance spectroscopy.[12,29] Among these methods, diffusion MRI is a non-invasive, convenient, and easily accessible tool that can distinguish glioma recurrence from PSP. Diffusion MRI detects water diffusion, and uses this information to generate contrast in the resulting images.[13] Two common types of diffusion MRI are diffusion-weighted imaging (DWI), which is based on the Brownian motion of water molecules to generate contrast, and diffusion-tensor imaging, which produces the contrast images provided by DWI and provides additional information about axon anatomy. The apparent diffusion coefficient (ADC), a measure of the extent of water diffusion, is a key parameter of diffusion MRI that is easily collected and has potential use for quantitative analysis during the diagnosis and grading of gliomas.[14]
Several studies have assessed the use of diffusion MRI with the ADC to differentiate recurrent glioma from PSP,[18–23,26,29,30,33] but the resulting ADCs had different sensitivities. The present meta-analysis examined whether there is sufficient evidence to support the use of ADC values derived from diffusion MRI for distinguishing glioma recurrence from PSP.
2. Materials and methods
Ethical approval was unnecessary in this study, because it was a meta-analysis of existing articles, and no individual patient data were handled.
2.1. Search strategy
PubMed, Embase, Cochrane Library, and Chinese Biomedical (CBM) databases were reviewed to identify relevant studies with publication dates on or before May 5, 2019. The search syntax was: (“ADC” or “apparent diffusion coefficient” or “Diffusion MRI” or “diffusion weighted imaging” or “diffusion weighted imaging” or “diffusion tensor imaging” or “ diffusion tensor imaging”) AND (“glioma” or “brain neoplasm”) AND (“pseudoprogression” or “recurrence” or “progression”). References in all identified studies were manually reviewed to identify additional relevant articles, and Endnote X7 was used to import citations and eliminate duplicates.
2.2. Study inclusion criteria
Each included study was a clinical trial that investigated the accuracy of ADC values derived from diffusion MRI for differentiation of glioma recurrence from PSP; provided all necessary diagnostic parameters, including sensitivity (SENS), specificity (SPE), positive likelihood ratio (PLR), negative likelihood ratio, and diagnostic odds ratio (DOR); enrolled at least 10 patients; had no overlap with datasets of other studies; and was written in the English or Chinese language. Publications were excluded if they were abstracts, reviews, case reports, letters, editorials, or animal studies. Two authors (Yang Y and Yue M) independently reviewed all studies based on these criteria, and reached a consensus if there were any disagreements about study inclusion.
2.3. Data extraction
For each included study, patient demographics (number of patients, average age, sex, type of glioma, and type of therapy) and technical details (strength of imaging field; diffusion MRI type; b-value; quantitative parameters; cut-offs; reference standards; and true positive, false positive, false negative, and true negative values) were recorded. The Quality Assessment Tool for Diagnostic Accuracy Studies version 2 (QUADAS-2) was used to assess study quality based on relevant parameters.[15]
2.4. Statistical analysis
SENS, SPE, PLR, negative likelihood ratio (NLR), and DOR were calculated from information in the included studies. A value of 0.5 was substituted for 0 during calculations to prevent problems in odds calculations for studies with SENS or SPEs of 100%.[16,17] The DOR is a measure of the discriminative power of a diagnostic test. In the present study, the DOR is calculated as the ratio of the odds of a positive test result for glioma recurrence to the odds of a positive test result for PSP:
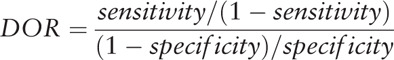 |
The I2 statistic was used to assess study heterogeneity. A random-effects model was used to assess effects across studies if I2 was above 50%; a fixed-effects model was used if I2 was below 50%.[24,25] Threshold effects were assessed by calculating Spearman correlation between SENS and (1 − SPE). The area under the curve (AUC) of the summary receiver-operating characteristic curve (SROC) was calculated. This method is widely used in meta-analysis for comprehensive evaluation of a diagnostic test, and is determined by statistical fits based on SENS and specificity of individual studies.[16]
Meta-Disc v. 1.4 software was used for statistical tests,[17] and Stata 12.0 was used for assessing Deek funnel plot asymmetry.
For SENS analysis, individual studies were removed from the analysis and the results were recalculated.
3. Results
3.1. Study selection and characteristics
We identified 162 articles in PubMed and 83 articles in EMBASE that used MRI to differentiate glioma recurrence from PSP using our search criteria (Fig. 1). After removal of 64 duplicates and 118 ineligible studies, we identified 63 studies that were suitable for full text investigation. Review of the references in these 63 studies indicated no additional studies were relevant. Review of the complete texts indicated that 12 of these 63 studies were potentially eligible. Six of these 12 studies did not provide sufficient data for meta-analysis. The remaining 6 studies[18–23] met our inclusion criteria. These 6 studies examined a total of 214 patients who received concurrent chemoradiation therapy for glioma, and examined glioma recurrence and PSP using diffusion MRI with ADC measurements (Table 1).
Figure 1.
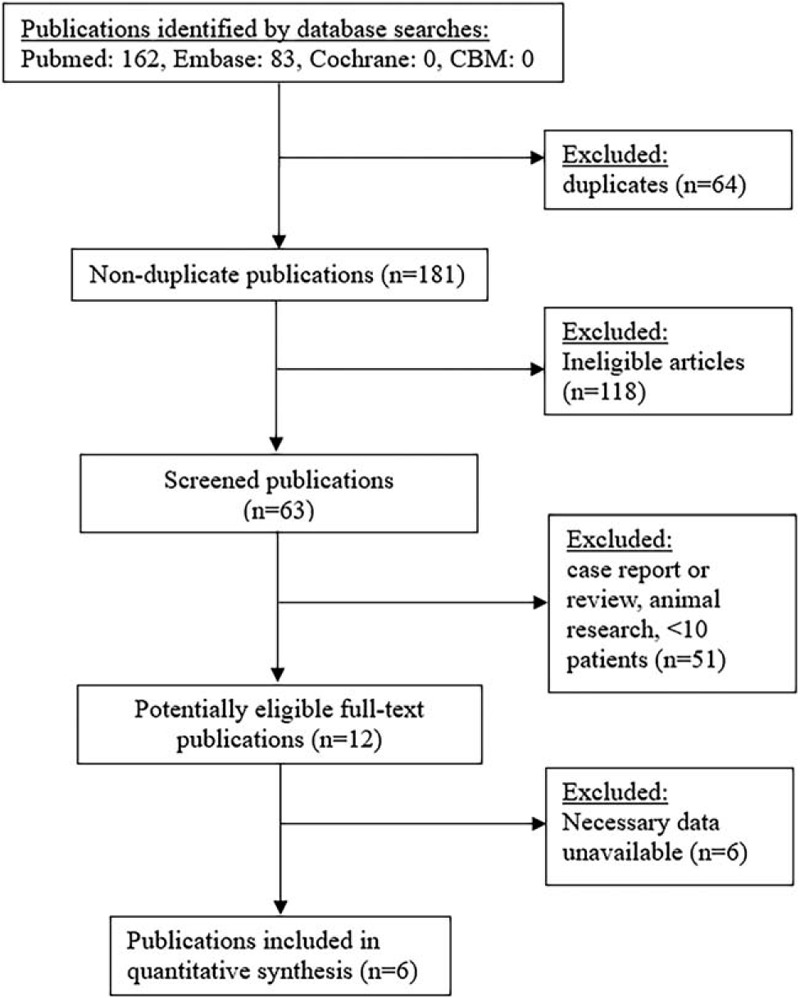
Procedure used for identification and selection of studies.
Table 1.
Characteristics of the 6 studies included in the meta-analysis.
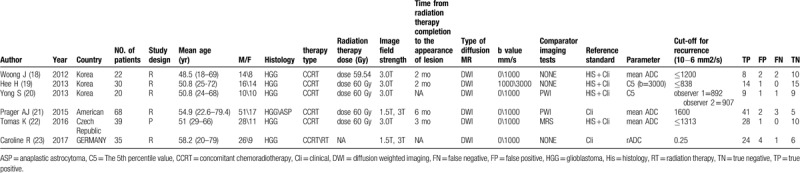
All 6 studies were cohort studies (5 were retrospective and 1 was prospective) and the number of patients ranged from 20 to 68. Four studies used a 3.0-T scanner and 2 studies used a 1.5-T scanner. All 6 studies used DWI. To distinguish PSP and glioma recurrence, 3 studies measured mean ADC values, 2 studies used 5th percentile values, and 1 study calculated relative ADC (rADC). The Prager et al[21] study analyzed a subset of patients who developed worsening lesions 6 months after radiation therapy, and their histopathology results indicated PSP in 8 (15.7%) patients and recurrent tumor in 43 (84.3%) patients.
Figure 2 shows the risk of different types of bias and study applicability for inclusion, based on the QUADAS-2 tool. Overall, these results indicated the quality of each study was satisfactory.
Figure 2.
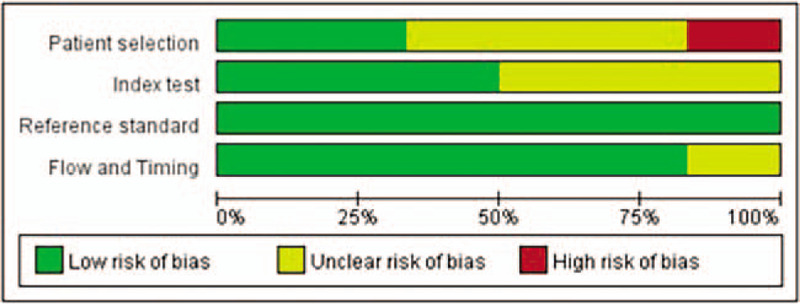
Risk of different types of bias in the 6 included studies (QUADAS-2 results).
3.2. Quantitative synthesis
The Spearman correlation for threshold testing had a value of –0.200 (P = .704), indicating no significant threshold effect. Thus, we plotted the diagnostic parameters (Fig. 3), and calculated the pooled values for SEN (0.95, 95% CI = 0.89–0.98), SPE (0.83, 95% CI = 0.72–0.91), PLR (4.82, 95% CI = 2.93–7.93), and NLR (0.08, 95% CI = 0.04–0.17). The resulting DOR was 59.63 (95% CI = 22.60–157.37). The SROC curve indicated the AUC was 0.9322 (Fig. 4).
Figure 3.
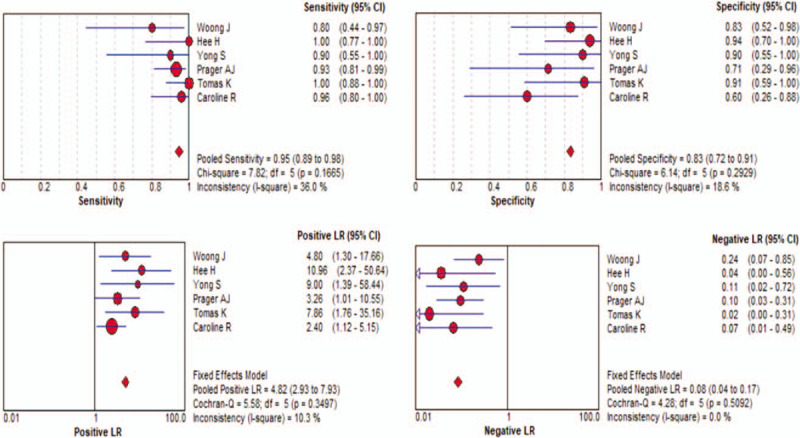
Forest plots of sensitivity (A), specificity (B), positive likelihood ratio (C), and negative likelihood ratio (D) of diffusion MRI with ADC for differentiation of glioma. ADC = apparent diffusion coefficient, MRI = magnetic resonance imaging.
Figure 4.
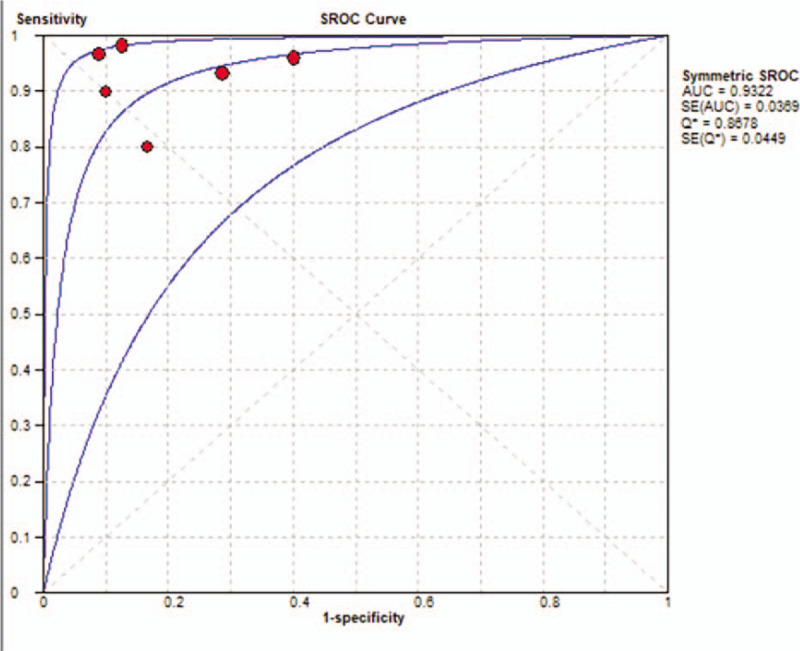
Summary receiver-operating characteristic curve (middle line) and 95% confidence intervals (upper and lower lines) for the 6 included studies.
3.3. Publication bias
Deek funnel plot asymmetry test indicated no significant evidence of publication bias (P = .53), and visual inspection of the funnel plot also indicated no apparent asymmetry of the pooled DOR (Fig. 5).
Figure 5.
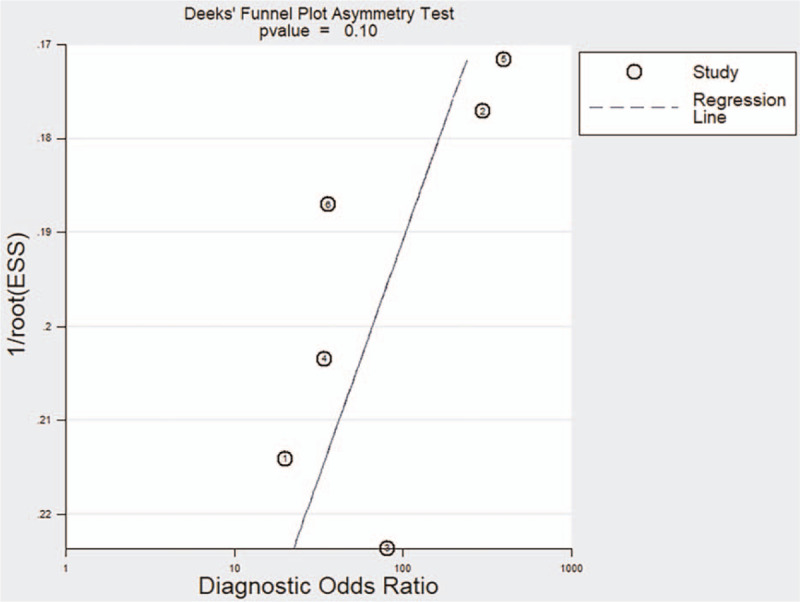
Deek's funnel plot of the relationship of the diagnostic odds ratio with effective sample size for the 6 included studies.
4. Discussion
The differential diagnosis of glioma recurrence and PSP is crucial for appropriate patient management and improving clinical outcome. DWI reflects changes in water diffusivity and cell density.[29] The ADC is a DWI indicator that correlates negatively with tumor cell density and can indicate changes in membrane permeability, macromolecule exchange, volume of extracellular space, and extent of tissue distortion. Ordinarily, the decreased diffusivity is a consequence of enhanced tumor cell proliferation, and a recurrent glioma is therefore indicated by reduced water diffusion and a lower ADC value.[30,31] Other treatment-related changes, such as PSP, are characterized by vasodilation, fibrinoid necrosis, and endothelial cell damage of normal cerebral vessels, and have higher ADC values than those of recurrent glioma.[21] Nevertheless, several previous studies[18–23] have speculated that the newly enhanced portion after concurrent chemoradiation therapy with temozolomide may indicate a wide spectrum of histologic features, ranging from normal brain tissue to necrosis and highly cellular recurrent tumor components. In other words, the individual ADCs within a given enhanced region can vary widely. Thus, an ADC derived from a specific region of interest may underestimate the cellular heterogeneity of the overall enhanced portion, and lead to incorrect or misleading interpretations.
The indeterminacy of quantified ADC values in previous studies that attempted to differentiate glioma recurrence from PSP motivated the present meta-analysis. We assessed the heterogeneity and threshold effects of 6 individual studies, and found that the ADC map had a SEN of 0.95 (95% CI 0.89–0.98) and a SPE of 0.83 (95% CI 0.72–0.91). This suggests this method has high value for distinguishing glioma recurrence from PSP. A PLR above 10 and an NLR below 0.1 are relatively independent clinically significant indexes for evaluating the effectiveness of a diagnostic test. In our meta-analysis, PLR was 4.82 (95% CI 2.93–7.93) and the NLR was 0.08 (95% CI 0.04–0.17), indicating that this method had good accuracy.
The SROC curve is a stable index used to assess the performance of a diagnostic test based on multiple AUC values, without being affected by other changes. An AUC closer to 1 indicates better diagnostic accuracy. Our SROC AUC was 0.9322, indicating that this approach had excellent accuracy in separating glioma recurrence from PSP. We also determined the DOR value (a function of SEN and SPE, in which a higher value indicates better discriminatory accuracy).[32] The summary DOR value for the present meta-analysis was 59.63, supporting the utility of diffusion MRI for distinguishing glioma recurrence from PSP.
Common post-treatment reactions include radiation necrosis and PSP. Zhang et al performed a meta-analysis of 18 studies and 455 patients and reported the SEN was 82%, the SPE was 84%, and the DOR was 23.90 (95% CI: 12.44–45.89) when using the ADC value to differentiate recurrent glioma from radiation necrosis.[37] Their poorer performance could be because the mean interval from completion of radiotherapy to diagnosis of radionecrosis was 11.6 months, so that treatment-related necrosis was more common and more severe because of this long period of time.[38] In contrast, PSP usually occurs within 6 months, and is characterized by less severe edema and necrosis. This may also explain why diffusion MRI with the ADC is valuable in differentiation of glioma recurrence from PSP.
Although our funnel plot and Deek test indicated no evidence of publication bias, there were some factors which could nonetheless lead to bias. As the gold standard for diagnosis of multiple diseases, histopathology remains the reference standard for diagnosis of glioma, and is often considered preferable to clinical and radiologic diagnoses. This could bias the analysis, because lesions thought to be PSP are less likely to be biopsied than those thought to be glioma recurrence. Moreover, the selection of a localized area within an enhanced portion can be subjective, and the resulting sampling bias can affect the results, even when the identical methods are used. Consequently, most studies of the present meta-analysis drew the reference area of interest (ROI) manually on the follow-up contrast-enhanced T1-weighted follow-up image, transferred the ROI to the ADC map, and then recorded the ADC values. Chu et al. suggested that analyzing the ADC of the entire volume of the enhanced portion is more objective, better captures quantitative information about tissue characteristics and heterogeneity of the entire enhanced region, and eliminates the potential of sampling bias.[19] In the present meta-analysis, we abstracted different ADC values, including mean ADC, rADC, and the 5th percentile value. Use of these different indexes could have affected the results, but our SENS analysis suggested that the meta-analysis results were reliable.
Our meta-analysis indicated that the ADC was higher for PSP, and this provided reliable differentiation of glioma recurrence from PSP. Although the mean ADC value is useful for discriminating recurrence from PSP,[18] some factors may affect the SENS of the results.[33,34,35] In particular, Song et al. concluded that the mean ADC value had limited use for differentiation of true progression from PSP because ADC values are higher in cystic and necrotic areas than in solid tumor components, and they thereby affect the accuracy of the final results.[20] They suggested that 5th percentile values were superior for differentiation of true progression from PSP. Chu et al. similarly found that 5th percentile values of cumulative ADC histograms were significantly lower in patients with evidence of glioma recurrence than in those with PSP, and concluded that 5th percentile of the cumulative ADC histogram was best for differentiating true progression from PSP.[19] Caroline et al found that the rADC was 0.25 or lower in patients with recurrence rather than with PSP (P = .005), supporting the presence of more restricted water diffusion in patients with recurrent glioma.[23] However, rADC analyses differ among research groups, potentially complicating this interpretation. Besides, Yamasaki et al. also noted that high b-value diffusion-weighted criteria were ideal for differentiating PSP from glioma recurrence in patients who received bevacizumab.[36] In 1 of the studies in our meta-analysis, Chu et al confirmed this interpretation, explaining that this may be due to a biexponential decay of the diffusion signal in the brain. The diffusion signal intensity is presumed to be dominated by a fast diffusion component at a low b-value and a slow diffusion component at a high-b value.[19]
This study also had some potential limitations. First, the test strength was limited because we only included 6 studies in the analysis, and this prevented calculation of diagnostic values in different patient subgroups. Another limitation is that in spite of the favorable diagnostic value of ADC overall, further research is needed to examine the utility of different ADC parameters, such as min ADC, rADC, mean ADC, and 5th percentile value. In addition, there were also variations in field strength and post-processing software among the 6 studies, and these could potentially influence ADC measurements and introduce additional uncertainty into our findings. Diagnostic accuracy is certainly enhanced by careful interpretation of results and the combined use of other advanced techniques, but overcoming these limitations will require standardization of all aspects of this diagnostic procedure to assure consistent diagnostic accuracy in the diagnosis of glioma recurrence.
5. Conclusions
The results of the present meta-analysis suggest that use of diffusion MRI with quantitative ADC values is an effective approach for differentiation of glioma recurrence from PSP. We suggest use of this method as an auxiliary tool for diagnosis of glioma progression.
Author contributions
DT conceived and designed the study. YY and YM wrote the paper. SMY, WYJ and TTY reviewed and edited the manuscript. All authors read and approved the manuscript.
Footnotes
Abbreviations: ADC = apparent diffusion coefficient, DOR = diagnostic odds ratio, DWI = diffusion weighted imaging, MRI = magnetic resonance imaging, PLR = positive likelihood ratio, PSP = pseudoprogression, SENS = sensitivity, SPE = specificity.
How to cite this article: Yu Y, Ma Y, Sun M, Jiang W, Yuan T, Tong D. Meta-analysis of the diagnostic performance of diffusion magnetic resonance imaging with apparent diffusion coefficient measurements for differentiating glioma recurrence from pseudoprogression. Medicine. 2020;99:23(e20270).
YY and YM are contributed equally to this work.
The authors have no conflicts of interest to disclose.
References
- [1].Gooden berger ML, Jenkinse RB. Genetics of adult glioma. Cancer Genet 2012;201:613–21. [DOI] [PubMed] [Google Scholar]
- [2].Louis DN, H. Ohgaki OD, Wiestler WK, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 2007;114:97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Caulo M, Panara V, Tortora D, et al. Data-driven grading of brain gliomas: a multiparametric MR imaging study. Radiology 2014;272:494–503. [DOI] [PubMed] [Google Scholar]
- [4].Law M, Yang S, Wang H, et al. Glioma grading: sensitivity, specificity, and predictive values of perfusion MR imaging and proton MR spectroscopic imaging compared with conventional MR imaging. Am J Neuroradiol 2003;24:1989–98. [PMC free article] [PubMed] [Google Scholar]
- [5].Thust SC, van den Bent MJ, Smits M. Pseudoprogression of brain tumors J Magn Reson Imaging 2018;48:571–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Brandsma D, Stalpers L, Taal W, et al. Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol 2008;9:453–61. [DOI] [PubMed] [Google Scholar]
- [7].Henson JW, Ulmer S, Harris GJ. Brain tumor imaging in clinical trials. AJNR Am J Neuroradiol 2008;29:419–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sorensen AG, Batchelor TT, Wen PY, et al. Response criteria for glioma. Nat Clin Pract Oncol 2008;5:634–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lu VM1, Jue TR2, McDonald KL2, et al. The survival effect of repeat surgery at glioblastoma recurrence and its trend: a systematic review and meta-analysis. World Neurosurg 2018;115:453–9.e3. [DOI] [PubMed] [Google Scholar]
- [10].Chang L, Su J, Jia X, et al. Treating malignant glioma in Chinese patients: update on temozolomide. Onco Targets Ther 2014;7:235–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wang S, Martinez-Lage M, Sakai Y, et al. Differentiating tumor progression from pseudoprogression in patients with glioblastomas using diffusion tensor imaging and dynamic susceptibility contrast MRI. AJNR Am J Neuroradiol 2016;37:28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Khan MN, Sharma AM, Pitz M, et al. High-grade glioma management and response assessment-recent advances and current challenges. Curr Oncol 2016;23:e383–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bammer R. Basic principles of diffusion-weighted imaging. Eur J Radiol 2003;45:169–84. [DOI] [PubMed] [Google Scholar]
- [14].Arvinda HR, Kesavadas C, Sarma PS, et al. Glioma grading: sensitivity, specificity, positive and negative predictive values of diffusion and perfusion imaging. J Neurooncol 2009;94:87–96. [DOI] [PubMed] [Google Scholar]
- [15].Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529–36. [DOI] [PubMed] [Google Scholar]
- [16].Devillé WL, Buntinx F, Bouter LM, et al. Conducting systematic reviews of diagnostic studies: didactic guidelines. BMC Med Res Methodol 2002;2:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zamora J, Abraira V, Muriel A, et al. Meta-DiSc: a software for meta-analysis of test accuracy data. BMC Med Res Methodol 2006;6:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lee WJ, Choi SH, Park CK. Diffusion-weighted MR imaging for the differentiation of true progression from pseudoprogression following concomitant radiotherapy with temozolomide in patients with newly diagnosed high-grade gliomas. Acad Radiol 2012;19:1353–61. [DOI] [PubMed] [Google Scholar]
- [19].Chu HH, Choi SH, Ryoo I. Differentiation of true progression from pseudoprogression in glioblastoma treated with radiation therapy and concomitant temozolomide: comparison study of standard and high-b-value diffusion-weighted imaging. Radiology 2013;269:831–40. [DOI] [PubMed] [Google Scholar]
- [20].Song YS, Choi SH, Park CK. True progression versus pseudoprogression in the treatment of glioblastomas: a comparison study of normalized cerebral blood volume and apparent diffusion coefficient by histogram analysis. Korean J Radiol 2013;14:662–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Prager AJ, Martinez N, Beal K. Diffusion and perfusion MRI to differentiate treatment-related changes including pseudoprogression from recurrent tumors in high-grade gliomas with histopathologic evidence. AJNR Am J Neuroradiol 2015;36:877–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kazda T, Bulik M, Pospisil P. Advanced MRI increases the diagnostic accuracy of recurrent glioblastoma: Single institution thresholds and validation of MR spectroscopy and diffusion weighted MR imaging. Neuroimage Clin 2016;11:316–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Reimer C, Deike K, Graf M. Differentiation of pseudoprogression and real progression in glioblastoma using ADC parametric response maps. PLoS One 2017;12:e0174620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Leeflang MM, Deeks JJ, Gatsonis C, et al. Cochrane diagnostic test accuracy working group systematic reviews of diagnostic test accuracy. Ann Intern Med 2008;149:889–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Khalifa J, Tensaouti F, Lotterie JA, et al. Do perfusion and diffusion MRI predict glioblastoma relapse sites following chemoradiation? J Neurooncol 2016;130:181–92. [DOI] [PubMed] [Google Scholar]
- [27].Chaskis C, Neyns B, Michotte A, et al. Pseudoprogression after radiotherapy with concurrent temozolomide for high-grade glioma: clinical observations and working recommendations. Surg Neurol 2009;72:423–8. [DOI] [PubMed] [Google Scholar]
- [28].Knudsen-Baas KM, Moen G, Fluge Ø, et al. Pseudoprogression in high-grade glioma. Acta Neurol Scand Suppl 2013;19:31–7. [DOI] [PubMed] [Google Scholar]
- [29].Hyare H, Thust S, Rees J. Advanced MRI techniques in the monitoring of treatment of gliomas. Curr Treat Options Neurol 2017;19:11. [DOI] [PubMed] [Google Scholar]
- [30].Chen ZY, Ma L, Lou X, et al. Diagnostic value of minimum apparent diffusion coefficient values in prediction of neuroepithelial tumor grading. J Magn Reson Imaging 2010;31:1331–8. [DOI] [PubMed] [Google Scholar]
- [31].Fudaba H, Shimomura T, Abe T, et al. Comparison of multiple parameters obtained on 3T pulsed arterial spin-labeling diffusion tensor imaging, and MRS and the Ki-67 labeling index in evaluating glioma grading. Am J Neuroradiol 2014;35:2091–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Glas AS, Lijmer JG, Prins MH, et al. The diagnostic odds ratio: a single indicator of test performance. J Clin Epidemiol 2003;56:1129–35. [DOI] [PubMed] [Google Scholar]
- [33].Yang Z, Zhang Z, Wang X, et al. Intensity-modulated radiotherapy for gliomas: dosimetric effects of changes in gross tumor volume on organs at risk and healthy brain tissue. Onco Targets Ther 2016;9:3545–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Yang I, Huh NG, Smith ZA, et al. Distinguishing glioma recurrence from treatment effect after radiochemotherapy and immunotherapy. Neurosurg Clin N Am 2010;21:181–6. [DOI] [PubMed] [Google Scholar]
- [35].Prager AJ, Martinez N, Beal K, et al. Diffusion and perfusion mri to differentiate treatmentrelated changes including pseudoprogression from recurrent tumors in high-grade gliomas with histopathologic evidence. AJNR Am J Neuroradiol 2015;36:877–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Fumiyuki Y, Kaoru K, Tomokazu A. Advantages of high b-value diffusion-weighted imaging to diagnose pseudo-responses in patients with recurrent glioma after bevacizumab treatment. Eur J Radiol 2012;81:2805–10. [DOI] [PubMed] [Google Scholar]
- [37].Hui Zhang, et al. Diagnostic accuracy of diffusion MRI with quantitative ADC measurements in differentiating glioma recurrence from radiation necrosis. J Neurol Sci 2015;351:65–71. [DOI] [PubMed] [Google Scholar]
- [38].Ruben JD, Dally M, Bailey M, et al. Cerebral radiation necrosis: incidence, outcomes, and risk factors with emphasis on radiation parameters and chemotherapy. Int J Radiat Oncol Biol Phys 2006;65:499–508. [DOI] [PubMed] [Google Scholar]
- [39].Taal W, Brandsma D, De Bruin HG, et al. Incidence of early pseudo-progression in a cohort of malignant glioma patients treated with chemoirradiation with temozolomide. Cancer 2008;113:405–10. [DOI] [PubMed] [Google Scholar]


