Abstract
The mortality of pregnant women with pulmonary arterial hypertension (PAH) remains high. The aim of this study was to evaluate and analyze perinatal and postpartum outcomes in patients with PAH.
A total of 79 pregnant patients with PAH who underwent abortion or parturition were reviewed retrospectively. Preoperative characteristics, anesthesia method, intensive care management, PAH-specific therapy, and maternal and neonatal outcomes were analyzed in this case series study.
This study was a retrospective analysis of 79 pregnant women with PAH. We collected data on maternal, obstetrical, and neonatal outcomes. The mean age of the parturient women with mild and severe PAH was 26.6 ± 5.7 and 26.0 ± 4.9 years, respectively, and the mean systolic pulmonary arterial pressure of the 2 groups was 43.8 ± 4.2 mmHg and 76.7 ± 15.6 mmHg, respectively. Of the 79 patients, 43 (54.4%) had severe PAH and 36 (45.6%) had mild PAH. The gestational weeks were significantly shorter and the rate of fetal death was higher in the severe PAH group than in the mild PAH group (36.0 vs 37.3 weeks and 6/24 vs 1/30, respectively; P < .05). Fifty-seven patients received PAH-specific therapy during pregnancy, including sildenafil, iloprost, and treprostinil. Overall, 22 PAH patients underwent therapeutic abortion and 57 continued their pregnancy. A total of 9 women, all of whom had severe PAH, died within 3 months of labor, giving a mortality rate of 15.8% (9/57). Of the 57 parturients, 21 (35.6%) gave birth prematurely and 36 (64.4%) delivered at term. Overall, 55 (96.5%) patients delivered by cesarean section and 2 (3.5%) delivered vaginally. There were 7 fetal deaths - 6 in the severe PAH group and one in the mild PAH group (6/24 vs 1/30).
Although the mortality rate of this group of women with PAH was lower than that previously reported, patients with PAH should still be advised against pregnancy.
Keywords: China, pregnancy outcomes, pulmonary arterial hypertension
1. Introduction
Pulmonary arterial hypertension (PAH), defined as a mean pulmonary arterial pressure (mPAP) ≥25 mmHg at rest, is a rare and serious clinical syndrome characterized by pulmonary vascular remodeling and increased PAP.[1,2] Right heart catheterization (RHC) is the gold standard diagnostic tool for PAH but is an invasive procedure, so transthoracic echocardiography is currently used for screening.[3] Due to treatment algorithms available for PAH, from general measures to novel drugs and interventional strategies, the survival are improved, but none of them are actually curative and the long-term prognosis is still poor.[4]
PAH affects the pulmonary vasculature and the heart, and pregnancy imposes a major burden on the cardiovascular system. In a normal pregnancy, plasma volume increases by approximately 50% and cardiac output increases by 35% due to increases in stroke volume and heart rate. The pulmonary vascular remodeling seen in PAH patients limits their ability to handle this increased blood volume and cardiac output, ultimately resulting in right ventricular failure. This right ventricular dysfunction and elevation in pulmonary vascular resistance leads to a reduction in left ventricular filling, thereby decreasing cardiac output. This results in hypotension and eventually leads to death. Therefore, pregnancy in patients with PAH is a serious clinical situation and is very difficult to handle. Recently, Duarte et al showed that developments in the treatment of PAH and the implementation of multidisciplinary care have improved maternal survival in the United States.[5] Despite these improvements, the consequences of PAH are exacerbated by the physiologic changes that occur during pregnancy. Therefore, maternal mortality remains high, especially in patients with PAH associated with Eisenmenger syndrome (ES).[6,7] In an early report of maternal outcomes associated with PAH in pregnancy, 9 of 16 patients (56%) died during or shortly after childbirth.[8] Other case series described episodes of sudden hemodynamic instability associated with maternal and fetal mortality rates of 11% to 50%.[9–11] In developing countries like China, advanced PAH-specific therapies are unavailable. Some PAH-targeted drugs such as sildenafil, bosentan, opsumit, and riociguat can be obtained, but they are unaffordable and are not covered by social health insurance. The high cost gives the family a heavy economic burden; therefore, they may not have good compliance. Some patients are often seen for the first time in the second or third trimester when they seek medical care for worsening symptoms. Moreover, most reports of PAH in pregnancy are from developed countries, and the impact of PAH on maternal and fetal outcomes has not been well studied in China.
To better understand the clinical course of parturients with PAH in China, the authors performed a retrospective chart review of 79 consecutive pregnant PAH patients admitted to the First and Second Xiangya Hospital, Central South University, between 2004 and 2016. The primary aim of this study was to describe the clinical characteristics, multidisciplinary management strategies with PAH-specific therapy, and maternal and fetal outcomes of PAH patients from China.
2. Materials and methods
This was a retrospective study. Approval was obtained from the Institutional Review Board of the First and Second Xiangya Hospital, Central South University. The medical records of pregnant women with PAH who were admitted between 2004 and 2016 to the First and Second Xiangya Hospital, a regional tertiary referral center, were analyzed.
2.1. Subjects
We reviewed the medical records of 79 pregnant patients with PAH treated between November 2004 and June 2016 at the First and Second Xiangya Hospital, Central South University. Pregnant patients with a confirmed diagnosis of PAH who either underwent pregnancy termination or were managed to parturition were included in this study. PAH is defined as an increase in mPAP ≥25 mmHg at rest as assessed by RHC. However, only 11 (13.9%) patients had undergone RHC before pregnancy. PAP was monitored via echocardiography in most of the patients during pregnancy. Therefore, in this study, we used echocardiography to measure PAP. PAH is suggested when an echocardiography-derived estimate of systolic pulmonary artery pressure (SPAP) exceeds 36 mmHg. In this study, PAH was divided into mild (50 mmHg ≥ SPAP >36 mmHg) and severe (SPAP >50 mmHg) based on SPAP during pregnancy.[12] ES was defined as PAH in the presence of bidirectional or reversal of systemic – pulmonary right to left) shunt as in ventricular or atrial septal defect (VSD/ASD) or patent ductus arteriosus (PDA). World Health Organization Functional Class (WHO-FC) was used to evaluate cardiac status. Clinical visits were performed monthly until 3 to 12 months postpartum. Data regarding demographics, gestational age at presentation, parity, SPAP during pregnancy on echocardiography, WHO-FC, PAH-specific therapy, mode of delivery (cesarean section or vaginal delivery), time of delivery (gestational weeks), anesthetic agent administered, and maternal and fetal outcomes were collected.
2.2. Data collection
Data were analyzed using SPSS 17.0 software (SPSS Inc., Chicago, IL). Continuous variables were expressed as mean ± standard deviation or median with range. The Student's t test was performed for analysis of normally distributed data between mild and severe group, otherwise the Wilcoxon test was used. Continuous variables were compared between the four clinical groups of congenital heart disease (CHD)-PAH using analysis of variance. Proportions were compared using the Chi-square test and Fisher exact test. Statistical significance was set at P < .05.
3. Results
A total of 79 pregnant women with PAH were included in this study and their baseline characteristics, management, and outcomes are shown in Table 1. Of these patients, 43 (54.4%) had severe PAH and 36 (45.6%) had mild PAH. Their age ranged from 26 to 34 years (mean 26.2 ± 5.2), gestational weeks were ranged from 16 to 38 weeks. The gestational week in the severe PAH group was significantly shorter than the mild PAH group (36.0 vs 37.3 weeks, P < .05). Overall, 74 patients had CHD, 4 had systemic lupus erythematosus (SLE), and 1 had idiopathic PAH (IPAH). The number of patients with each PAH subtype is summarized in Table 2. Five patients had undergone previous cardiac surgery. Of the 79 patients, 57 (72.15%) elected to continue their pregnancies and 22 (27.85%) chose to undergo induced abortion. Overall, 9 women, all of whom had severe PAH, died within 3 months of childbirth, resulting in a mortality rate of 15.8% (9/57).
Table 1.
Baseline characteristics, management and outcome of patients between PAH.
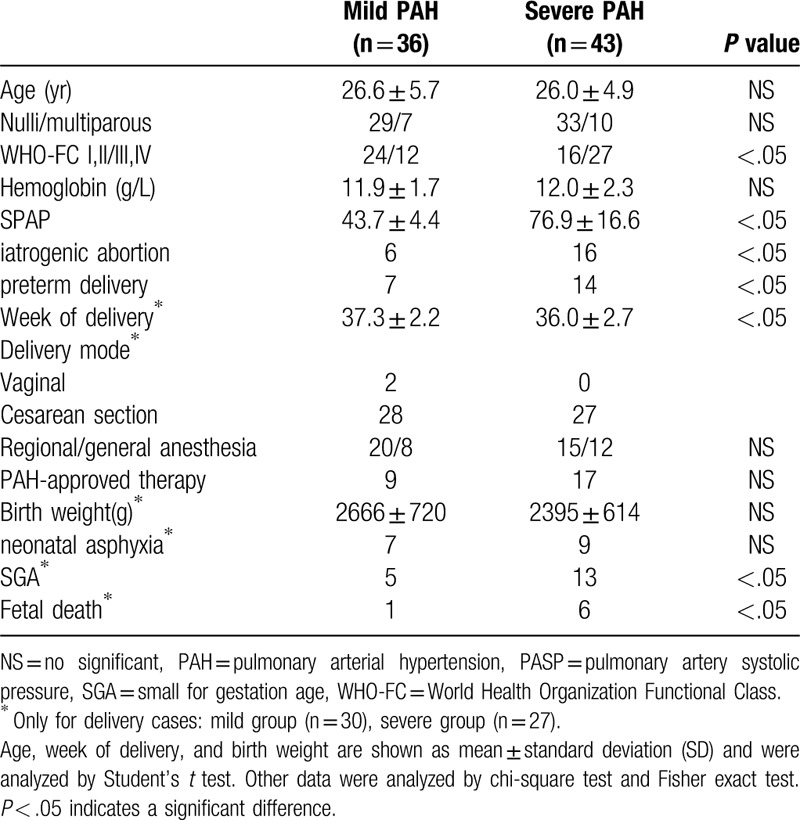
Table 2.
Underlying diseases in women with PAH.

3.1. Echocardiographic data
Echocardiographic data from the mild and severe PAH groups are summarized in Table 3. There were no significant differences in ejection fraction, fractional shortening, or cardiac output between the severe and mild PAH patients. Right atrial diameter, right ventricular diameter, and the number of pulmonary artery dimension larger than aorta dimension were found to be significantly in patients with severe PAH compared to those with mild PAH (Table 3).
Table 3.
Echocardiographic data in patients with PAH.

3.2. Management of patients
Of the 57 parturients had delivered, all the patients were treated with PAH-specific therapy (sildenafil, iloprost, and treprostinil) after initial diagnosis and before parturition or pregnancy termination. They received sildenafil (37.5–75 mg/day orally) alone or combined with subcutaneously/intravenously administered treprostinil (2–10 ng/kg/min). Preoperatively, almost all patients received anticoagulation therapy to prevent thrombosis and diuretics/digoxin for heart failure management. Pulmonary artery catheters were placed in all patients before surgery. PAP, systemic vascular resistance index, and cardiac output were monitored intraoperatively and postoperatively. When the mPAP/mean systemic arterial pressure ratio increased intraoperatively, treprostinil was used to prevent PAH crisis. Pulmonary artery catheters were removed postpartum when the patient's condition became relatively stable almost within 5 days. Most patients (32 with CHD and 3 with SLE) received epidural anesthesia for delivery, while the rest (19 with CHD and 1 with IPAH) underwent general anesthesia. We decided to use general anesthesia because it allows for adequate control of pain and early initiation of thromboprophylaxis. The results showed that either epidural or general anesthesia has attributed to the successful outcomes. A total of 23 patients (21 with CHD and 2 with SLE) experienced intraoperative hypotension (systemic blood pressure <90/60 mmHg) and were treated with dopamine, dobutamine, norepinephrine to maintain hemodynamic stability. In addition, oxytocin was infused slowly for uterine hemostasis after placental extraction in 11 patients with CHD. Treprostinil was initiated at 3 ng/kg/min and titrated to 15 to 22.5 ng/kg/min to control pulmonary hypertension (Table 4).
Table 4.
Management of Patients.

3.3. Perinatal outcomes
Fifty-seven parturients had delivered, 21 (35.6%) gave birth prematurely and 36 (64.4%) delivered at term. Overall, 55 (96.5%) patients delivered by caesarean section and 2 (3.5%) delivered vaginally. Three patients died during the perinatal period and were diagnosed with PDA, ASD, and VSD, respectively. All 3 patients had severe PAH when they came to our hospital. One had previously been diagnosed with VSD with PAH when she was 4 years old and underwent VSD repair surgery at the time of diagnosis. She had resistant PAH after the surgery and was advised to avoid pregnancy. However, she decided to continue the pregnancy and did not receive any PAH-targeted therapy. She was referred to our hospital at 32 weeks of gestation and delivered via cesarean section 1 week later. The other 2 patients had not been diagnosed with CHD or PAH before pregnancy, and PAH and ES were newly diagnosed during their pregnancy. They were referred to our hospital at 33 weeks and 34 weeks of gestation, respectively. One delivered via cesarean section after 1 week, and the other after 2 days. All 3 patients were immediately transferred to the intensive care unit after delivery, and all died due to refractory heart failure shortly after (days 3, 2, 5) (Table 2).
The week of delivery was found to be significantly longer in patients with mild PAH than in those with severe PAH (37.3 ± 2.2 weeks vs 36.0 ± 2.7 weeks, P < .05). Overall, 7 patients with mild PAH and 14 patients with severe PAH delivered prematurely (P < .05). Patients with severe PAH had a significantly higher incidence of therapeutic abortion (16 vs 6, P < .05). There was a higher incidence of small-for-gestational-age (SGA) babies in the severe PAH group (P < .05). There were 7 fetal deaths—6 in the severe PAH group and 1 in the mild PAH group (6/24 vs 1/30, P < .05). Of the fetuses who died, 5 died in utero at less than 24 weeks of gestation and the other 2 were stillborn at 34 weeks of gestation. The number of neonatal asphyxia in mild PAH group and severe PAH group was 7 and 9, respectively, had no significant difference (Table 2).
3.4. Pregnancy outcomes of patients with CHD-PAH
Of the 74 patients with CHD-PAH, 17 (22.9%) had ES, 44 (59.5%) had PAH associated with systemic-to-pulmonary shunts, 8 (10.8%) had small defects, and 5 (6.8%) had corrected defects. The baseline characteristics, management, and outcomes of the patients according to CHD-PAH subtype are shown in Table 4. Patients with ES had a higher SPAP than those with other CHD-PAH subtypes. Moreover, the patients with ES delivered earlier. The birth weight of neonates in the ES group was lighter than that in the other subgroups. A total of 26 (35.1%) CHD-PAH patients received PAH-specific therapy. Three patients died—2 with ES and 1 with corrected VSD. There were six fetal deaths - three in the ES subgroup and three in the systemic-to-pulmonary shunts subgroup (Table 5).
Table 5.
Baseline characteristics, management and perinatal outcome of 74 pregnant women with CHD-PAH.

3.5. Pregnancy outcomes of patients with IPAH
Only 1 pregnant woman with IPAH was included in our study; she had no history of cardiopulmonary disease and was referred to our hospital at 30 weeks of gestation. In addition to clinical presentation, echocardiography showed a dilated right ventricle and increased PAP (estimated PASP of 70 mmHg). The patient underwent a cesarean section under general anesthesia a week later. The newborn diagnosed with neonatal asphyxia at weight of 1185 g, was transferred to the neonatal unit. Inhaled iloprost was used during pregnancy and labor. She was discharged 5 days postpartum in a stable condition. She attended monthly follow-up visits, and her treatment included sildenafil and warfarin. She died at 13 months post-delivery because of severe refractory heart failure. The infant survived and being healthy.
3.6. Postnatal follow-up information
Overall, 61 of the 76 discharged patients (80.2%) were followed up for more than 3 months or until death. The remaining 15 (19.8%) patients were out of contact. Of the 61 followed-up patients, 31 had severe PAH. Overall, 8 patients died after discharge—6 within 3 months of delivery (median time to death: 20 days postpartum, range: 10–90 days) and 2 over 1 year after delivery (13 and 21 months postpartum). All 8 patients had severe PAH during pregnancy. Severe right heart failure and pulmonary hypertension crisis were the causes of maternal death. There were 15 therapeutic abortions and 4 fetal deaths in the 61 cases. All live-born infants (42 infants) in our follow-up study survived. Of the 42 infants, 2 were born with CHD and underwent successful surgery and the other 40 were healthy.
4. Discussion
The physiologic changes that occur during pregnancy and the postpartum period are typically well tolerated in healthy women, but are usually disastrous in women with PAH. PAH is a severe progressive disorder that predominantly affects women of childbearing age and results in high maternal and fetal mortality rates. During pregnancy, plasma volume and cardiac output increase by approximately 50% at 32 to 34 weeks of gestation. During labor, cardiac output may increase by an additional 30% to 50%, resulting in an overall increase of 80%.[13] Katsuragi et al reported that in patients with severe PAH, PAP increased throughout pregnancy from 53.5 mmHg before pregnancy to 72.8 mmHg at 31 ± 3 weeks of gestation.[14] Pregnancy is contraindicated in women with PAH.[15] As such, current guidelines strongly recommend that women with PAH who are of childbearing potential use effective contraception to avoid pregnancy. In the event of pregnancy, early termination is recommended.[16] However, an increasing number of women with PAH are expressing their desire to be a mother. The wishes of these women cannot be ignored and they should be made aware of the options that are currently available as well as the risks associated with pregnancy. These women are advised to terminate the pregnancy even though termination itself is also associated with high maternal risk. However, some women do not accept termination and insist on continuing with their pregnancy.
Some reports have found that pregnancy termination in women with PAH is associated with a low risk of maternal complications,[17,18] and our study reached the same conclusions. In our retrospective study, 22 (27.8%) patients with PAH underwent therapeutic abortion, none of whom experienced maternal complications. A total of 26 (32.3%) patients received PAH-specific therapy several weeks prior to delivery (diuretic, sildenafil, and treprostinil). According to a systematic review of maternal outcomes from 1997 to 2007, the maternal mortality rate of those with IPAH, CHD-PAH, and other PAH subtypes was 17%, 28%, and 33%, respectively.[6] Recently, Zhang et al. reviewed 17 consecutive pregnant IPAH patients and found that maternal death up to 1 week after delivery occurred in 3 patients (17.6%),[19] with another 4 out of 11 patients who presented for follow-up (36.4%) dying within 3 days of delivery.[7] In our study, the maternal mortality rate within 3 months of labor was 15.8%. The lower maternal mortality rate noted in this study may be related to the fact that patients were referred to our experienced institution and received multidisciplinary care. For women with PAH, if they are pregnant, it is important to establish continuous close monitors by obstetricians, cardiologists, and PAH specialists to create a management strategy. Moreover, making a scheduled date to prepare for complicated delivery is also critical. At the First and Second Xianya Hospitals of Central South University, a multidisciplinary team has been created for the diagnosis and treatment of PAH, which consists of obstetricians, cardiac PAH physicians, heart surgeons, pediatricians, and anesthesiologists. The best treatment strategy for PAH patients have been come to agreement among us. Compared to previous reports from other centers, the outcomes were improved in these 2 hospitals.
In this study, most patients visited our hospital for medical care during the last trimester. The reason for this is that most patients were rural residents in Central China who had limited access to education and were in poor physical health. Due to a lack of medical knowledge, most rural patients did not attend routine check-ups with cardiologists. Most of them were insufficiently conscious of the increased risks, and there was lack of pre-conception counseling nor regular care with the cardiologist and obstetrician during gestation. Although early pregnancy termination is recommended for patients with PAH, none of them chose to discontinue their pregnancy in their first trimester. After 32 weeks of gestation, the hemodynamic burden of pregnancy peaks, and it was at this stage that most patients came to the hospital looking for medical care. In this study, pregnant women with ES had the highest SPAP and worst perinatal outcomes among the four subgroups of patients with CHD-PAH. This could be because the increased right-to-left shunting and exaggerated pre-existing hypoxemia in patients with ES promoted further pulmonary vasoconstriction and a rise in PAP. Therefore, women with ES should avoid pregnancy or undergo early termination.
All of the maternal deaths in this study occurred after delivery. Following the delivery of the neonate, several factors lead to hemodynamic instability in PAH patients, because of increased preload from additional blood return from the placenta and uterus or aorta-caval decompression.[20] Therefore, perinatal care is important to improve patient outcomes. In this study, 2 late maternal deaths occurred more than 1 year post-delivery, there may be little association of their death with pregnancy. In addition, some studies have shown that PAH-approved drugs such as sildenafil and prostacyclin analogues improve the outcomes of both pregnant women and fetuses.[21–23] In our study, 32.3% of pregnant women treated with iloprost, sildenafil, and treprostinil during pregnancy, but we could not evaluate the efficacy and safety of these drugs due to the absence of a control group. However, compared to developed countries,[24] the use of PAH-approved drugs is insufficient. Obstetricians and PAH physicians should have a better understanding of PAH-approved drugs and provide special counseling for pregnant patients with PAH. In addition, the Chinese government should implement policies to decrease the price of PAH-approved drugs and improve insurance coverage.
The decision regarding the time and manner of delivery depends on the balance between maternal risk and fetal health. PAH may deteriorate throughout pregnancy, and labor onset is unpredictable. Therefore, delivery is usually scheduled as early as fetal maturation permits. With respect to the manner of delivery, cesarean section avoids prolonged labor and allows for the careful preparation of anesthesia, optimization of hemodynamics, and development of contingency plans. Frequent use of planned cesarean section has been described.[6,25] In this study, most patients (96.5%) delivered by cesarean section. However, a best evidence topic reported that vaginal birth is safe in patients with adult CHD of all severities and that a higher cesarean rate does not translate into improved outcomes.[26] The suitable delivery mode of gravidas with PAH remains to be confirmed by more series.
4.1. Study limitations
The limitations of this study relate to its retrospective nature. This study evaluated all pregnant patients with PAH, thus bias is limited. Another limitation concerns the lack of control regarding PAH therapy administration. Further clinical information may be obtained from long-term follow-up of patients enrolled in multicenter registries. In addition, in this study, we did not monitor the changes in hemodynamics as pregnancy advanced using RHC. PAH is defined as an mPAP ≥25mmHg at rest as assessed by RHC. However, we used echocardiography to monitor SPAP during pregnancy because RHC is invasive for both the mother and fetus. This Doppler-derived pressure estimation may be inaccurate in individual patients, and SPAP may commonly be overestimated by >10 mmHg.[27,28] Furthermore, during the follow-up period, 15 patients were out of contact. Therefore, we do not know the final results of those patients, and the results will be affected.
The final limitation is that we were unable to obtain hemodynamic data to confirm ES in 17 patients who were included based on clinical diagnosis because of the retrospective nature of our analysis. The inclusion of these patients had the potential to alter the survival of the total population.
5. Conclusions
Pregnancies with PAH is serious and rare condition. Although the maternal mortality rate noted in this study was lower that than reported in previous studies, the severity of maternal complications and high rates of prematurity and SGA remain a concern. In line with current guidelines, women with PAH should be counseled against pregnancy or advised to undergo early termination if pregnant. If they decide to continue their pregnancy, antenatal and postpartum care should be provided by an experienced multidisciplinary team. Lastly, PAH specialists should be actively involved in the postpartum care of these patients for months after hospital discharge.
Author contributions
Contribution to authorship: Dr Shi and Dr Luo is the guarantor of the manuscript and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Author contributions:
Dr Shi and Dr Su: contributed to study design, data acquisition, data interpretation and statistical analysis of the data, drafting of the submitted manuscript, and approval of the final manuscript.
Dr Li: contributed to study design, data interpretation and statistical analysis of the data, drafting of the submitted manuscript, and approval of the final manuscript.
Dr Luo: contributed to data interpretation, statistical analysis of the data, and approval of the final Manuscript.
Conceptualization: Huafang Shi.
Funding acquisition: Jiang Li.
Investigation: Jun Luo, Huafang Shi, Wei Su.
Methodology: Wei Su.
Project administration: Jun Luo.
Supervision: Li Xu, Jiang Li.
Writing – original draft: Huafang Shi, Li Xu.
Writing – review & editing: Jiang Li.
Footnotes
Abbreviations: ASD = atrial septal defect, CHD = congenital heart disease, CHD-PAH = pulmonary arterial hypertension associated with congenital heart disease, PAH = pulmonary arterial hypertension, PAP = pulmonary artery pressure, PASP = pulmonary artery systolic pressure, PDA = patent ductus arteriosus, RHC = right heart catheterization, SLE = systemic lupus erythematosus, SPAP = systolic pulmonary artery pressure, VSD = ventricular septal defect, WHO-FC = World Health Organization Functional Class.
How to cite this article: Luo J, Shi H, Xu L, Su W, Li J. Pregnancy outcomes in patients with pulmonary arterial hypertension: a retrospective study. Medicine. 2020;99:23(e20285).
This work was supported by National Natural Science Foundation of China (Project 81870233 and 81600249) and Hunan Provincial Natural Science Foundation of China (Project 2017JJ3455).
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request. All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
- [1].Hoeper MM, Bogaard HJ, Condliffe R, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol 2013;62:42–50. [DOI] [PubMed] [Google Scholar]
- [2].McGoon MD, Benza RL, Escribano-Subias P, et al. pulmonary arterial hypertension: epidemiology and registries. Turk Kardiyoloji Dernegi Arsivi 2014;42:67–77. [PubMed] [Google Scholar]
- [3].Bossone E, D’Andrea A, Fau - D’Alto M, et al. Echocardiography in pulmonary arterial hypertension: From diagnosis to prognosis. J Am Soc Echocardiogr 2013;26:1–4. [DOI] [PubMed] [Google Scholar]
- [4].Kylhammar D, Persson L, Hesselstrand R, et al. Prognosis and response to first-line single and combination therapy in pulmonary arterial hypertension. Scand Cardiovasc J 2014;48:223–33. [DOI] [PubMed] [Google Scholar]
- [5].Duarte AG, Thomas S, Safdar Z, et al. Management of pulmonary arterial hypertension during pregnancy: a retrospective, multicenter experience. Chest 2013;143:1330–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bedard E, Dimopoulos K, Fau - Gatzoulis MA, et al. Has there been any progress made on pregnancy outcomes among women with pulmonary arterial hypertension? Eur Heart J 2009;30:256–65. [DOI] [PubMed] [Google Scholar]
- [7].Duan R, Xu X, Wang X, et al. Pregnancy outcome in women with eisenmenger's syndrome: a case series from west china. BMC Pregnancy Childbirth 2016;16:356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].McCaffrey RM, Dunn LJ. Primary pulmonary hypertension in pregnancy. Obstet Gynecol Surv 1964;19:567–91. [DOI] [PubMed] [Google Scholar]
- [9].Weiss BM, Zemp L, Fau - Seifert B, et al. Outcome of pulmonary vascular disease in pregnancy: a systematic overview from 1978 through 1996. JACC 1998;31:1650–7. [DOI] [PubMed] [Google Scholar]
- [10].Bonnin M, Mercier FJ, Sitbon O, et al. Severe pulmonary hypertension during pregnancy: mode of delivery and anesthetic management of 15 consecutive cases. Anesthesiology 2005;102:1133–7. [DOI] [PubMed] [Google Scholar]
- [11].Ladouceur M, Benoit L, Radojevic J, et al. Pregnancy outcomes in patients with pulmonary arterial hypertension associated with congenital heart disease. Heart 2017;103:287–92. [DOI] [PubMed] [Google Scholar]
- [12].Galie N, Torbicki A, Barst R, et al. Guidelines on diagnosis and treatment of pulmonary arterial hypertension. The task force on diagnosis and treatment of pulmonary arterial hypertension of the European society of cardiology. Eur Heart J 2004;25:2243–78. [DOI] [PubMed] [Google Scholar]
- [13].Savu O, Jurcut R, Giusca S, et al. Morphological and functional adaptation of the maternal heart during pregnancy. Circ Cardiovasc Imaging 2012;5:289–97. [DOI] [PubMed] [Google Scholar]
- [14].Katsuragi S, Yamanaka K, Neki R, et al. Maternal outcome in pregnancy complicated with pulmonary arterial hypertension. Circ J 2012;76:2249–54. [DOI] [PubMed] [Google Scholar]
- [15].Galie N, Corris PA, Frost A, et al. Updated treatment algorithm of pulmonary arterial hypertension. JACC 2013;62:60–72. [DOI] [PubMed] [Google Scholar]
- [16].Galie N, Humbert M, Vachiery Jl Fau - Gibbs S, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: The joint task force for the diagnosis and treatment of pulmonary hypertension of the european society of cardiology (ESC) and the european respiratory society (ERS): Endorsed by: Association for European Paediatric and congenital cardiology (AEPC), international society for heart and lung transplantation (ISHLT). Eur Respir J 2015;46:409–16. [DOI] [PubMed] [Google Scholar]
- [17].Myles PS. Anaesthetic management for laparoscopic sterilisation and termination of pregnancy in a patient with severe primary pulmonary hypertension. Anaesth Intens Care 1994;22:465–9. [DOI] [PubMed] [Google Scholar]
- [18].Bowers C, Devine Pa Fau - Chervenak FA, Chervenak FA. Dilation and evacuation during the second trimester of pregnancy in a woman with primary pulmonary hypertension. A case report. J Reprod Med 1988;33:787–8. [PubMed] [Google Scholar]
- [19].Zhang J, Lu J, Zhou X, et al. Perioperative management of pregnant women with idiopathic pulmonary arterial hypertension: An observational case series study from china. J Cardiothorac Vasc Anesth 2018;32:2547–59. [DOI] [PubMed] [Google Scholar]
- [20].European Society of G, Association for European Paediatric C, German Society for Gender M, Vera Regitz-Zagrosek, Carina Blomstrom Lundqvist, Claudio Borghi, et al. ESC guidelines on the management of cardiovascular diseases during pregnancy: The task force on the management of cardiovascular diseases during pregnancy of the European society of cardiology (ESC). Eur Heart J 2011;32:3147–97. [DOI] [PubMed] [Google Scholar]
- [21].Sun X, Wang K, Wang W, et al. Clinical study on sildenafil in treatment of pregnant women with pulmonary arterial hypertension. Zhong Hua Fu Chan Ke Za Zhi 2014;49:414–8. [PubMed] [Google Scholar]
- [22].Goland S, Tsai F, Fau - Habib M, et al. Favorable outcome of pregnancy with an elective use of epoprostenol and sildenafil in women with severe pulmonary hypertension. Cardiology 2010;115:205–8. [DOI] [PubMed] [Google Scholar]
- [23].Elliot CA, Stewart P, Fau - Webster VJ, et al. The use of iloprost in early pregnancy in patients with pulmonary arterial hypertension. Eur Respir J 2005;26:168–73. [DOI] [PubMed] [Google Scholar]
- [24].Jiang X, Jing ZC. Epidemiology of pulmonary arterial hypertension. Curr Hypertens Rep 2013;15:638–49. [DOI] [PubMed] [Google Scholar]
- [25].Chakravarty EF, Khanna D, Chung L. Pregnancy outcomes in systemic sclerosis, primary pulmonary hypertension, and sickle cell disease. Obstet Gynecol 2008;111:927–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Asfour V, Murphy Mo Fau - Attia R, Attia R. Is vaginal delivery or caesarean section the safer mode of delivery in patients with adult congenital heart disease? Interact Cardiovasc Thorac Surg 2013;17:144–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Fisher MR, Forfia Pr Fau - Chamera E, Chamera E, et al. Accuracy of Doppler echocardiography in the hemodynamic assessment of pulmonary hypertension. Am J Resp Crit Care Med 2009;179:615–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Galiè NHM, Humbert M, Torbicki A, et al. Guideline for the diagnosis and treatment of pulmonary hypertension: The task force for the diagnosis and treatment of pulmonary hypertension of the European society of cardiology (ESC) and the European respiratory society (ERS), endorsed by the international society of heart and lung transplantation (ISHLT). Eur Heat J 2009;30:2493–537. [DOI] [PubMed] [Google Scholar]


