Abstract
Liver steatosis could affect the accuracy of FibroScan in patients with chronic hepatitis B (CHB) and nonalcoholic fatty liver disease (NAFLD). This study aimed to assess the accuracy and cut-off values of FibroScan for diagnosing liver fibrosis and cirrhosis in patients with concomitant CHB and NAFLD.
A total of 116 patients with concomitant CHB and NAFLD who underwent FibroScan test and liver biopsy were retrospectively enrolled. Liver fibrosis was staged according to the METAVIR scoring system. Calculations of the areas under receiver-operating characteristic curves (AUROC) were performed and compared for the staging of liver fibrosis.
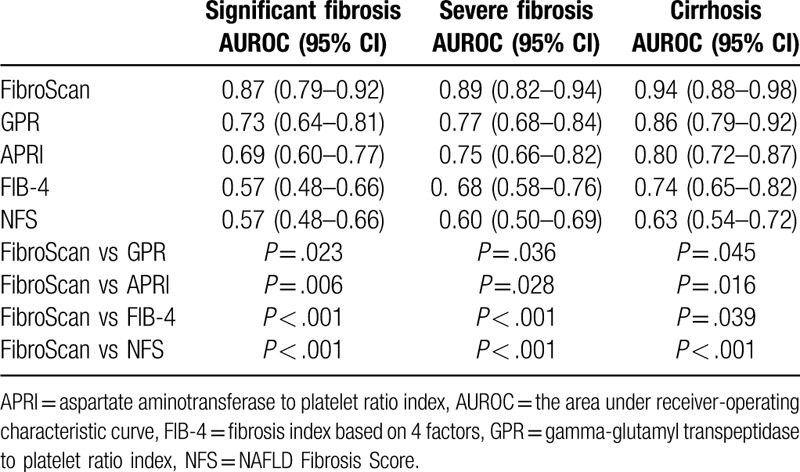
The AUROCs for FibroScan, gamma-glutamyl transpeptidase to platelet ratio (GPR), aspartate aminotransferase to platelet ratio index (APRI), fibrosis index based on 4 factors (FIB-4), and NAFLD Fibrosis Score (NFS) were 0.87, 0.73, 0.69, 0.57, and 0.57 for the diagnosis of significant liver fibrosis (METAVIR ≥ F2); 0.89, 0.77, 0.75, 0.68, and 0.60 for severe liver fibrosis (METAVIR ≥ F3); and 0.94, 0.86, 0.80, 0.74, and 0.63 for cirrhosis (F4), respectively. The cutoff values of FibroScan for staging liver fibrosis with sensitivity at least 90% were: 8.0 kPa for significant liver fibrosis, and 10.5 kPa for cirrhosis. The cutoff values of FibroScan for staging liver fibrosis with specificity at least 90% were: 10.8 kPa for significant liver fibrosis, and 17.8 kPa for cirrhosis.
FibroScan provides high value for the diagnosis of liver fibrosis and cirrhosis in patients with concomitant CHB and NAFLD.
Keywords: chronic hepatitis B, FibroScan, liver fibrosis, liver stiffness measurement, nonalcoholic fatty liver disease
1. Introduction
Chronic hepatitis B virus (HBV) infection is one of the leading causes of cirrhosis and hepatocellular carcinoma (HCC) in China.[1] Nonalcoholic fatty liver disease (NAFLD) is also a common disease that affects 20% to 40% of the general population.[2] In recent years, the number of patients with concomitant CHB and NAFLD is increasing gradually. A study performed by Bondini et al[3] reported that the prevalence of NAFLD was 20% in patients with CHB. The mortality of patients with concomitant CHB and NAFLD is related to the development of liver fibrosis and cirrhosis, which can progress to HCC, liver function de-compensation, and liver failure.[4] Therefore, it is very necessary to distinguish liver fibrosis and cirrhosis for optimization of therapy, evaluation of prognosis, and prevention of disease progression in patients with concomitant CHB and NAFLD.
Liver biopsy is considered the criterion standard for assessment of liver fibrosis and cirrhosis. However, it is not routinely performed due to its invasiveness, cost, and potential complications.[5] In recent years, new noninvasive techniques have been developed to assess the degree of liver fibrosis. Of these techniques, FibroScan has been the most widely used. Numerous studies have confirmed the efficiency of FibroScan in the diagnosis of liver fibrosis and cirrhosis in patients with CHB.[6–8] However, the concomitant existence of NAFLD raises issues and challenges for the clinical applications of FibroScan in patients with CHB. Because major components of NAFLD such as obesity,[9] liver steatosis,[10] and liver inflammation may affect the liver stiffness measurement (LSM) values evaluated by FibroScan. The increased fat in patients with NAFLD might lead to a poorer transmission of the ultrasound when FibroScan measurements were performed, leading to unreliable FibroScan results.[11]
FibroScan is a reliable tool for the diagnosis of liver fibrosis and cirrhosis in patients with CHB, patients with hepatitis C, and patients with NAFLD.[12] However, the use of FibroScan has not mentioned for patients with concomitant CHB and NAFLD in current guidelines.[1,2,13] For patients with concomitant CHB and NAFLD, it is necessary to question whether FibroScan would be more or less effective for the diagnosis of liver fibrosis. Therefore, this study aimed to evaluate the accuracy of FibroScan for the staging of liver fibrosis in patients with concomitant CHB and NAFLD.
2. Patients and methods
2.1. Patients
We retrospectively enrolled 184 consecutive patients with concomitant CHB and NAFLD from Shanghai Public Health Clinical Center, a tertiary hospital in Shanghai, China, between January 2013 and January 2019. CHB was diagnosed as the persistent positivity of serum HBsAg and/or HBV DNA for >6 months.[14] NAFLD was diagnosed as at least 5% biopsy-proven hepatic steatosis without significant alcohol consumption. No significant alcohol consumption was defined as alcohol consumption less than 20 g/day and history of drinking <5 years. The inclusion criteria were: serum HBsAg and/or HBV DNA positivity for >6 months; biopsy-proven NAFLD; underwent routine laboratory testing, FibroScan, and liver biopsy. The exclusion criteria were: alcohol consumption >20 g/day for >5 years (n = 20), previous or current antiviral therapy (n = 5), hepatitis C virus (HCV), hepatitis D virus (HDV), or human immunodeficiency virus (HIV) co-infection (n = 9), combined with autoimmune liver disease (n = 1), inappropriate biopsy samples (n = 5), failure of FibroScan measurement (n = 10), and unreliable LSM values (n = 18). Finally, 116 patients with concomitant CHB and NAFLD were included in this study.
This study was approved by the ethics board of Shanghai Public Health Clinical Center. The informed consent for FibroScan (non-medicare test in China) and liver biopsy (invasive test) had been obtained as part of routine clinical practices. The medical data could be used for further studies and were also obtained as part of the written informed consent.
2.2. Liver histological examination
Because of its invasiveness and potential complications, liver biopsy was suggested when the noninvasive tests could not provide enough information for the causes and/or severity of liver injury and fibrosis. In this retrospective study, liver biopsy was recommended by physicians based on the comprehensive evaluation of age, alanine aminotransferase (ALT) levels, HBV DNA levels, the course of liver disease, family history of cirrhosis and HCC, and the LSM values of FibroScan tests with full respect for the wishes of the patients.
Liver biopsy was performed within 1 week after enrollment. Liver biopsies were fixed in formalin, embedded in paraffin, and a minimum of 15 mm of liver tissue with at least 6 portal tracts was considered suitable for histopathological analysis.[15] The double examination for liver biopsy specimens routinely was done in clinical practice in our hospital. Therefore, all liver biopsy specimens were routinely analyzed by 2 pathologists, and were further reviewed by a third senior pathologist if discrepant readings occurred. Liver fibrosis was staged according to the METAVIR scoring system[16]: F0, absence of fibrosis; F1, portal fibrosis without septa; F2, portal fibrosis with rare septa; F3, numerous septa without cirrhosis; F4, cirrhosis. Liver steatosis was staged according to the NASH Clinical Research Network scoring system[17]: S0, <5%; S1, 5% to 33%; S2, 34% to 66%; and S3, >67%. Significant liver fibrosis, severe liver fibrosis, and cirrhosis were defined as METAVIR fibrosis score ≥ F2, ≥ F3, and F4, respectively.
2.3. Liver stiffness measurement
In clinical practice, the FibroScan tests were firstly recommended to evaluate liver fibrosis and cirrhosis in patients with CHB and NAFLD because of its noninvasive nature and relatively high diagnostic performance. Transient elastography examinations were performed by trained operators according to the manufacturers’ recommendations using the standard probe (M probe) 1 to 3 days before liver biopsy.[18] The LSM values were considered reliable when 10 valid examinations were obtained with the maximum number of attempts set at 20. The FibroScan evaluation was considered as unreliable when IQR/LSM was >0.30 in patients with LSM ≥7.1 kPa.[19]
2.4. Noninvasive serum fibrosis models calculation
Fasting blood samples were obtained, and routine laboratory tests were performed 1 to 3 days before liver biopsy. The gamma-glutamyl transpeptidase (GGT) to platelet ratio (GPR), aspartate aminotransferase (AST) to platelet ratio index (APRI), and fibrosis index based on 4 factors (FIB-4) have been widely adopted for evaluation of liver fibrosis in patients with CHB.[15] The NAFLD Fibrosis Score (NFS) has been proposed to evaluate liver fibrosis in patients with NAFLD.[15] Therefore, GPR, APRI, FIB-4, and NFS were selected as serum fibrosis models to compare with FibroScan.
-
(1)
GPR = (GGT [IU/L]/ULN of GGT)/platelet count (109 cells/L) × 100.[20]
-
(2)
APRI = (AST [IU/L]/ULN of AST)/platelet count (109 cells/L) × 100.[21]
-
(3)
FIB-4 = (age [years] × AST [IU/L])/(platelet count [109 cells/L] × (ALT [IU/L]1/2).[22]
-
(4)
NFS = (−1.675 + 0.037 × age [years] + 0.094 × BMI [kg/m2] + 1.13 × impaired fasting glucose (IFG)/diabetes (yes = 1, no = 0) + 0.99 × AST/ALT ratio −0.013 × platelet count (109/L)–0.66 × albumin [g/dL]).[15] IFG was diagnosed when a participant did not have diabetes, but had a fasting blood glucose of 5.6 to <7.0 mmol/L.
2.5. Statistical analysis
The normality test was performed for continuous variables using the Kolmogorov-Smirnov test. Normal distribution variables, non-normal distribution continuous variables, and categorical variables were shown as means ± standard deviations, medians and interquartile ranges (IQRs), and counts (percentage), respectively. The diagnostic performances were assessed by correlating noninvasive tests results and liver biopsy results using the Spearman correlation coefficient, and building the receiver-operating characteristics (ROC) curves. Areas under the ROC curves (AUROCs) of noninvasive tests were calculated for the diagnosis of significant liver fibrosis, severe liver fibrosis, and cirrhosis. The AUROC values were compared using the Delong test.[23] Two sets of cutoff values were calculated respectively: sensitivity ≥90%, specificity ≥90%. All significance tests were 2-tailed, and P ≥ .05 was considered no significant difference between 2 groups/methods. Statistical analysis was performed using SPSS 15.0 (SPSS Inc, Chicago, IL) and MedCalc 16.1 (MedCalc Software bvba, Ostend, Belgium).
3. Results
3.1. Patient characteristics
Patient characteristics are shown in Table 1. In this study, 66.4% were male, 79.3% were HBeAg-positive, and median age of enrolled patients was 36 years. The median HBV DNA, ALT, AST, GGT, body mass index (BMI), and LSM values were 7.5 log10 copies/mL (IQR 6.9–7.7), 51 IU/L (IQR 34–78), 29 IU/L (IQR 23–40), 25 IU/L (IQR 15–72), 25.5 kg/m2 (IQR 22.4–28.4), and 8.7 kPa (IQR 5.4–12.8), respectively.
Table 1.
Baseline characteristics of the study population.

The percentages of patients >40 years, 30 to 40 years, and <30 years were 27.6%, 41.4%, and 31.0%, respectively. The percentages of patients with HBV DNA >6 log10 copies/mL, and 3 to 6 log10 copies/mL, were 86.2% and 13.8%, respectively. Among the 116 enrolled patients, 37 (31.9%) had normal ALT levels, 52 (44.8%) had mildly elevated ALT levels (1–2 upper limit of normal [ULN]), and 27 (23.3%) had significantly elevated ALT levels (>2 ULN). The ULN of ALT is 40 IU/L in this study.
3.2. Liver histologic results
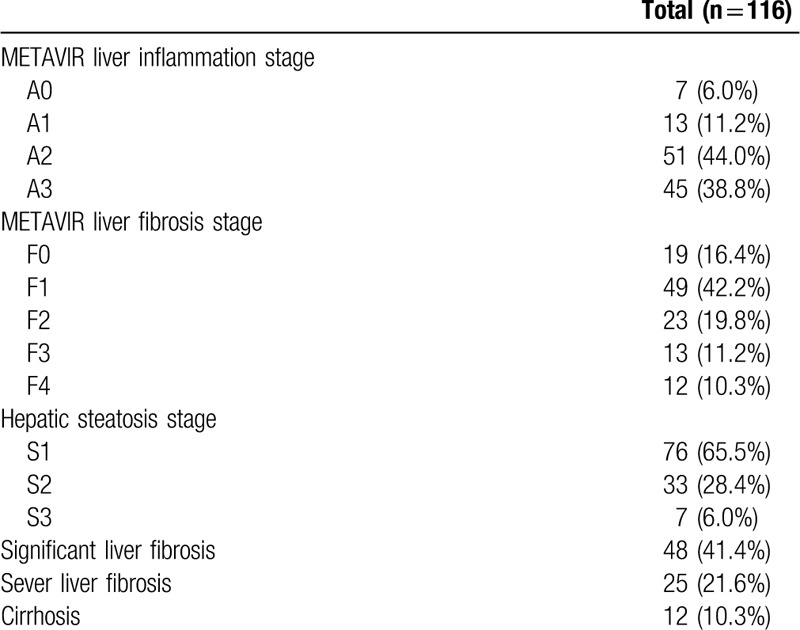
The liver histological results of enrolled patients are shown in Table 2. The liver inflammation stages were as follows: A0 = 7 (6.0%); A1 = 13 (11.2%); A2 = 51 (44.0%); and A3 = 45 (38.8%). The liver fibrosis stages were as follows: F0 = 19 (16.4%); F1 = 49 (42.2%); F2 = 23 (19.8%); F3 = 13 (11.2%); and F4 = 12 (10.3%). The hepatic steatosis stages were as follows: S1 = 76 (65.5%); S2 = 33 (28.4%); and S3 = 7 (6.0%). Of 116 patients with concomitant CHB and NAFLD, 48 (41.4%), 25 (21.6%), and 12 (10.3%) were classified as having significant liver fibrosis, severe liver fibrosis, and cirrhosis, respectively.
Table 2.
Liver histological results of the study population.
3.3. Correlations between noninvasive fibrosis tests and histological fibrosis stages
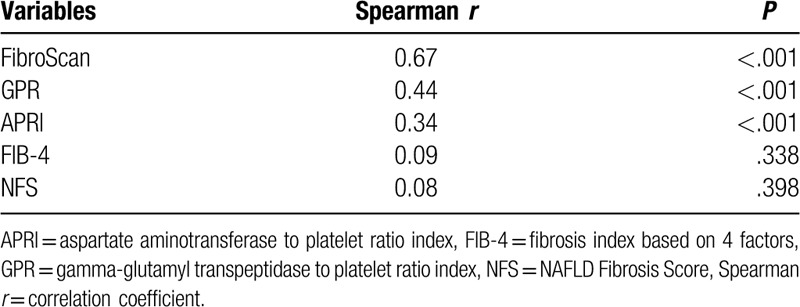
Correlations between noninvasive fibrosis tests and histological fibrosis stages are shown in Table 3. FibroScan (r = 0.67, P < .001), GPR (r = 0.44, P < .001), and APRI (r = 0.34, P < .001) demonstrated a correlation with liver histological fibrosis stages. The correlation between FibroScan tests and liver histological fibrosis stages was significantly superior to that between serum fibrosis models and liver histological fibrosis stages.
Table 3.
Correlations between noninvasive fibrosis tests and liver histological fibrosis stages.
3.4. Pairwise comparison for diagnostic performances of noninvasive fibrosis tests
ROC curves of noninvasive fibrosis tests for the diagnosis of significant liver fibrosis, severe liver fibrosis, and cirrhosis are shown in Figure 1. Pairwise comparisons of AUROC values were presented in Table 4. For the diagnosis of significant liver fibrosis, FibroScan had a significantly better diagnostic performance than GPR, APRI, FIB-4, and NFS. Similarly, for severe liver fibrosis, FibroScan had a significantly better diagnostic performance than GPR, APRI, FIB-4, and NFS (AUROC of 0.89, 0.77, 0.75, 0.68, and 0.60 for FibroScan, GPR, APRI, FIB-4, and NFS, respectively; all P < .05). For cirrhosis, FibroScan also had a significantly better diagnostic performance than GPR, APRI, FIB-4, and NFS.
Figure 1.
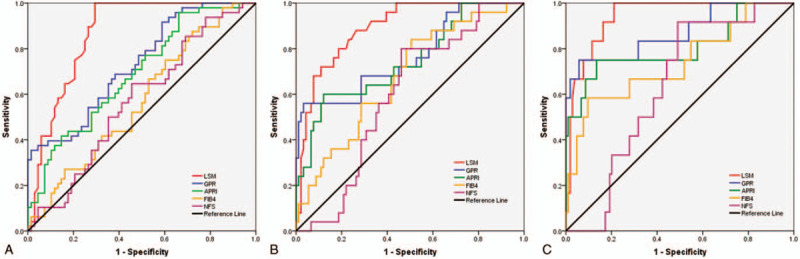
ROC curves of noninvasive fibrosis tests for the diagnosis of significant liver fibrosis (A), severe liver fibrosis (B), and cirrhosis (C). APRI = aspartate transaminase to platelet ratio, FIB-4 = fibrosis index based on 4 factors, GPR = gamma-glutamyl transpeptidase to platelet ratio, LSM = liver stillness measurement, NFS = NAFLD Fibrosis Score, ROC = receiver-operating characteristic curve.
Table 4.
The AUROCs of noninvasive fibrosis tests.
3.5. Diagnostic thresholds of FibroScan
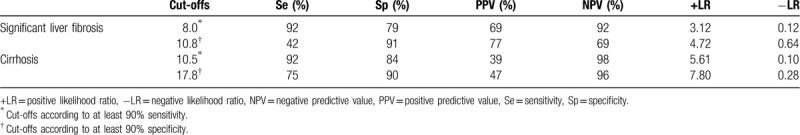
The diagnostic thresholds of FibroScan are shown in Table 5. The cutoff values of FibroScan for ruling out diseases with at least 90% sensitivity were: 8.0 kPa for significant liver fibrosis (the sensitivity, specificity, PPV, and NPV was 92%, 79%, 69%, and 92%, respectively), and 10.5 kPa for cirrhosis (the sensitivity, specificity, PPV, and NPV was 92%, 84%, 39%, and 98%, respectively). The cutoff values for ruling in diseases with a specificity of at least 90% were: 10.8 kPa for significant liver fibrosis (the sensitivity, specificity, PPV, and NPV was 42%, 91%, 77%, and 69%, respectively), and 17.8 kPa for cirrhosis (the sensitivity, specificity, PPV, and NPV was 75%, 90%, 47%, and 96%, respectively).
Table 5.
Diagnostic thresholds of FibroScan.
4. Discussion
FibroScan is a noninvasive test to estimate liver fibrosis and cirrhosis in patients with CHB,[24] however the diagnostic performance is affected by several factors including ALT flares,[25] BMI,[9] and hepatic steatosis.[10] A study of 170 patients with CHB demonstrated that hepatic steatosis was independently related to the severity of liver histological fibrosis.[26] Petta et al[27] found that patients with liver steatosis had higher LSM values measured by FibroScan, which led to overestimations of the severity of liver fibrosis. So far, for diagnosis of liver fibrosis in patients with CHB, whether FibroScan would be interfered by the co-occurrence of NAFLD is not clear. The diagnostic performances and corresponding cutoff values of FibroScan for liver fibrosis and cirrhosis is unclear in patients with concomitant CHB and NAFLD.
In this retrospective cohort of 116 patients with concomitant CHB and NAFLD, FibroScan performed well for the diagnosis of different stages of liver fibrosis. The AUROC of FibroScan was 0.87 for the diagnosis of significant liver fibrosis, 0.89 for severe liver fibrosis, and 0.94 for cirrhosis, suggesting that FibroScan enabled accurate evaluation of liver fibrosis in patients with coexisting CHB and NAFLD. The reliability of FibroScan for the detection of fibrosis in NAFLD and chronic viral hepatitis had been evaluated in other studies.[28,29] Gaia et al[28] confirmed that FibroScan can be considered a valid support to detect fibrosis in chronic liver disease related to HCV but it should be interpreted cautiously in CHB and NAFLD patients, where host or disease-related factors may modify its accuracy.
The performance of FibroScan in the assessment of liver fibrosis in patients with hepatitis C has been examined in numerous studies.[30,31] In comparison, relatively few studies are dedicated to FibroScan in subjects with HBV. Cardoso et al found that in HBV patients, FibroScan measurement accurately predicts the absence or presence of significant fibrosis, advanced fibrosis or cirrhosis, and shows similar performances as compared to HCV patients (P = .975, P = .820, P = .740, respectively).[32] Marcellin et al[24] also found that FibroScan appears to be reliable for detection of significant fibrosis or cirrhosis in patients with hepatitis B and cutoff values are only slightly different from those observed in patients with hepatitis C. According to the WHO guidelines on the treatment of patients with CHB, the cutoff values for FibroScan were 7 to 8.5 kPa for the diagnosis of significant liver fibrosis and 11 to 14 kPa for the diagnosis of cirrhosis, respectively.[33] In this study, the cutoff values of FibroScan for ruling in disease were 10.8 kPa for significant liver fibrosis and 17.8 kPa for cirrhosis, respectively.
The strength of this study is that we not only demonstrated the good performance of FibroScan for the diagnosis of liver fibrosis in patients with coexisting CHB and NAFLD, but also determined the specific cutoff values of FibroScan to identify significant liver fibrosis and cirrhosis in patients with concomitant CHB and NAFLD. Indeed, the major challenge using FibroScan in patients with NAFLD is the lower measurement success rate in obese patients. Once the measurement is successful, the LSM values could evaluate accurately liver fibrosis and cirrhosis in patients with concomitant CHB and NAFLD.
According to guidelines,[1,2] immune tolerant phase is characterized by the presence of serum HBeAg, very high levels of HBV DNA and ALT persistently within the normal range; immune clearance phase is characterized by the presence of serum HBeAg, high levels of HBV DNA, and elevated ALT; inactive carrier phase is characterized by the presence of serum antibodies to HBeAg (anti-HBe), undetectable or low (<2000 IU/mL) HBV DNA levels and normal ALT; reactivation of HBeAg-negative phase is characterized by the lack of serum HBeAg, moderate to high levels of serum HBV DNA and elevated ALT values. In this study, subjects with HBeAg positive, HBV DNA >105 copies/mL, and ALT ≤40 IU/mL were classified as immune tolerant phase; subjects with HBeAg positive, HBV DNA >105 copies/mL, and ALT >40 IU/mL were classified as immune clearance phase; subjects with anti-HBe positive, HBV DNA <104 copies/mL, and ALT ≤40 IU/mL were classified as inactive carrier phase; subjects with HBeAg negative, HBV DNA >103 copies/mL, and ALT >40 IU/mL were classified as reactivation of HBeAg-negative phase. Based on the grouping criterion, in this study, 31 (26.7%), 61 (52.6%), 3 (2.6%), and 21 (18.1%) were classified as having immune tolerance phase, immune clearance phase, inactive carrier phase, and reactivation of HBeAg-negative phase, respectively. The enrollment of skewed patient groups could be explained as follows. According to the clinical guidelines on the treatment of CHB, patients with HBV DNA >20,000 IU/mL and ALT >2 ULN can start treatment even without a liver biopsy.[1] Therefore, a considerable proportion of the patients with high ALT and HBV DNA levels who might be in immune clearance phase or reactivation of HBeAg-negative phase were not enrolled because they started antiviral therapy without liver biopsy tests. Some patients with HBeAg-negative, undetectable or low (<2000 IU/mL) HBV DNA levels and normal ALT levels who were in inactive carrier phase, usually had no indication for liver biopsy, and were not enrolled because they had no liver biopsy tests.[1]
According to the WHO guidelines on the treatment of patients with CHB, the limitations with FibroScan include the following situation: it uses a single cut-off and therefore reported sensitivities and specificities of FibroScan may be overestimated across fibrosis stages.[33] Therefore, in this study, FibroScan uses 2 cutoff points for the diagnosis of specific fibrosis stages, as the use of a single cut-off would result in suboptimal sensitivity and specificity.[33] A high cut-off with high specificity is used to diagnose persons with a particular stage of fibrosis, and a low cut-off with high sensitivity to rule out the presence of a particular stage of fibrosis.[33] Adolescent et al[34] also found that a dual cut-off algorithm allowed for correctly classifying both significant fibrosis and cirrhosis in the majority of the patients with CHB, independent of ALT values, thus reducing the need for liver biopsy investigations. Although the WHO guidelines recommend APRI as the preferred noninvasive test to assess the presence of cirrhosis in resource-limited settings,[33] in this study, the GPR showed better accuracy for the diagnosis of significant liver fibrosis, severe liver fibrosis, and cirrhosis. The results were in agreement with previous studies performed by Lemoine et al,[20] Li et al,[35] and Cai et al,[36] in which GPR not only yielded good AUROCs for predicting significant fibrosis and cirrhosis but also showed better performance compared with APRI.
In this study, the cutoff values of FibroScan for the diagnosis of significant hepatic fibrosis were higher than other reports in CHB.[24,32] The possible reasons were as follows. First, this study enrolled many patients in immune tolerance stage of CHB (26.7%), who were considered to be at low risk of liver fibrosis and cirrhosis. The enrollment of skewed patient groups might lead to the difference in prevalence of fibrosis and cirrhosis, and then lead to the different cutoff values of FibroScan in the studied populations, known as the spectrum bias.[37,38] Second, the existence of NAFLD might increase the liver inflammation levels, and affected the cutoff values of FibroScan. One limitation of FibroScan is that the LSM values increase with hepatic necroinflammatory levels regardless of the fibrosis stage. Verveer et al demonstrated that hepatic inflammation assessed by hepatic necroinflammatory index (Ishak) increased LSM values regardless of fibrosis stage (P < .001).[39]
This study has several limitations. First, all enrolled patients in this study came from a tertiary hospital for the management of chronic liver disease, and it is uncertain whether the results would be influenced by a selection bias. Second, this study is a retrospective, single-center study, and the sample size is small. Large-scale, multicenter, prospective cohort studies are needed for further evaluation of the clinical use of FibroScan in patients with concomitant CHB and NAFLD.
In conclusion, this study confirmed that FibroScan is a valuable diagnostic tool for liver fibrosis and cirrhosis in patients with coexisting CHB and NAFLD. The cutoff values of FibroScan for ruling in disease were: 10.8 kPa for significant liver fibrosis, and 17.8 kPa for cirrhosis, respectively. It is important to note that most patients in this study were in immune tolerance phase and immune clearance phase of CHB; therefore, the cutoff values of FibroScan should be interpreted with caution and further validated in other clinical phases of CHB in a cohort with large sample size.
Author contributions
Data curation: Qiang Li, Chenlu Huang, Wei Xu, Qiankun Hu, Liang Chen.
Formal analysis: Qiang Li, Chenlu Huang, Wei Xu, Qiankun Hu, Liang Chen.
Funding acquisition: Liang Chen.
Investigation: Qiang Li, Chenlu Huang, Liang Chen.
Methodology: Qiang Li, Chenlu Huang, Wei Xu.
Project administration: Liang Chen.
Resources: Chenlu Huang, Wei Xu.
Software: Qiang Li, Chenlu Huang, Wei Xu.
Validation: Qiang Li, Liang Chen.
Visualization: Chenlu Huang, Wei Xu.
Writing – original draft: Qiang Li.
Writing – review & editing: Liang Chen.
Footnotes
Abbreviations: ALT = alanine aminotransferase, APRI = aspartate aminotransferase to platelet ratio index, AST = aspartate aminotransferase, AUROC = area under the receiver operating characteristic curve, BMI = body mass index, CHB = chronic hepatitis B, FIB-4 = fibrosis index based on 4 factors, GGT = gamma-glutamyl transpeptidase, GPR = gamma-glutamyl transpeptidase to platelet ratio, HBV = hepatitis B virus, HCC = hepatocellular carcinoma, HCV = hepatitis C virus, HDV = hepatitis D virus, HIV = human immunodeficiency virus, LSM = liver stiffness measurement, NAFLD = nonalcoholic fatty liver disease, NFS = NAFLD fibrosis score, NPV = negative predictive value, PPV = positive predictive value, ROC curve = receiver-operating characteristic curve.
How to cite this article: Li Q, Huang C, Xu W, Hu Q, Chen L. Accuracy of FibroScan in analysis of liver fibrosis in patients with concomitant chronic Hepatitis B and nonalcoholic fatty liver disease. Medicine. 2020;99:23(e20616).
QL, CH, and WX contributed equally in this study.
The authors report no conflicts of interest.
Financial Support: This study was supported by grant No.SHDC12015129 from Shanghai Shen Kang Hospital Development Center, grant NO.17411969700 from Shanghai Association for Science and Technology, and grant NO.19YF1441200 from Shanghai Sailing Plan Program.
Role of the Sponsor: The funding organizations are public institutions and had no role in the design and conduct of the study; collection, management, and analysis of the data; or preparation, review, and approval of the manuscript.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Terrault NA, Lok A, McMahon BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018;67:1560–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol 2017;67:370–98. [DOI] [PubMed] [Google Scholar]
- [3].Bondini S, Kallman J, Wheeler A, et al. Impact of non-alcoholic fatty liver disease on chronic hepatitis B. Liver Int 2007;27:607–11. [DOI] [PubMed] [Google Scholar]
- [4].Cadranel JF, Rufat P, Degos F. Practices of liver biopsy in France: results of a prospective nationwide survey. For the Group of Epidemiology of the French Association for the Study of the Liver (AFEF). Hepatology 2000;32:477–81. [DOI] [PubMed] [Google Scholar]
- [5].Grant A, Neuberger J. Guidelines on the use of liver biopsy in clinical practice. British Society of Gastroenterology. Gut 1999;45: suppl 4: V1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Dong H, Xu C, Zhou W, et al. The combination of 5 serum markers compared to FibroScan to predict significant liver fibrosis in patients with chronic hepatitis B virus. Clin Chim Acta 2018;483:145–50. [DOI] [PubMed] [Google Scholar]
- [7].Chon YE, Choi EH, Song KJ, et al. Performance of transient elastography for the staging of liver fibrosis in patients with chronic hepatitis B: a meta-analysis. PLoS One 2012;7:e44930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Friedrich-Rust M, Ong MF, Martens S, et al. Performance of transient elastography for the staging of liver fibrosis: a meta-analysis. Gastroenterology 2008;134:960–74. [DOI] [PubMed] [Google Scholar]
- [9].Petta S, Di Marco V, Camma C, et al. Reliability of liver stiffness measurement in non-alcoholic fatty liver disease: the effects of body mass index. Aliment Pharmacol Ther 2011;33:1350–60. [DOI] [PubMed] [Google Scholar]
- [10].Macaluso FS, Maida M, Camma C, et al. Steatosis affects the performance of liver stiffness measurement for fibrosis assessment in patients with genotype 1 chronic hepatitis C. J Hepatol 2014;61:523–9. [DOI] [PubMed] [Google Scholar]
- [11].Castera L, Foucher J, Bernard PH, et al. Pitfalls of liver stiffness measurement: a 5-year prospective study of 13,369 examinations. Hepatology 2010;51:828–35. [DOI] [PubMed] [Google Scholar]
- [12].Tsochatzis EA, Gurusamy KS, Ntaoula S, et al. Elastography for the diagnosis of severity of fibrosis in chronic liver disease: a meta-analysis of diagnostic accuracy. J Hepatol 2011;54:650–9. [DOI] [PubMed] [Google Scholar]
- [13].Kemp W, Levy M, Weltman M, et al. Australian Liver Association (ALA) expert consensus recommendations for the use of transient elastography in chronic viral hepatitis. J Gastroenterol Hepatol 2015;30:453–62. [DOI] [PubMed] [Google Scholar]
- [14].Sarin SK, Kumar M, Lau GK, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int 2016;10:1–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].EASL-ALEH Clinical Practice Guidelines: Non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol 2015;63:237–64. [DOI] [PubMed] [Google Scholar]
- [16].Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology 1996;24:289–93. [DOI] [PubMed] [Google Scholar]
- [17].Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005;41:1313–21. [DOI] [PubMed] [Google Scholar]
- [18].De Ledinghen V, Wong VW, Vergniol J, et al. Diagnosis of liver fibrosis and cirrhosis using liver stiffness measurement: comparison between M and XL probe of FibroScan(R). J Hepatol 2012;56:833–9. [DOI] [PubMed] [Google Scholar]
- [19].Boursier J, Zarski JP, de Ledinghen V, et al. Determination of reliability criteria for liver stiffness evaluation by transient elastography. Hepatology 2013;57:1182–91. [DOI] [PubMed] [Google Scholar]
- [20].Lemoine M, Thursz M, Mallet V, et al. Diagnostic accuracy of the gamma-glutamyl transpeptidase to platelet ratio (GPR) using transient elastography as a reference. Gut 2017;66:195–6. [DOI] [PubMed] [Google Scholar]
- [21].Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003;38:518–26. [DOI] [PubMed] [Google Scholar]
- [22].Vallet-Pichard A, Mallet V, Nalpas B, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and fibrotest. Hepatology 2007;46:32–6. [DOI] [PubMed] [Google Scholar]
- [23].DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44:837–45. [PubMed] [Google Scholar]
- [24].Marcellin P, Ziol M, Bedossa P, et al. Non-invasive assessment of liver fibrosis by stiffness measurement in patients with chronic hepatitis B. Liver Int 2009;29:242–7. [DOI] [PubMed] [Google Scholar]
- [25].Arena U, Vizzutti F, Corti G, et al. Acute viral hepatitis increases liver stiffness values measured by transient elastography. Hepatology 2008;47:380–4. [DOI] [PubMed] [Google Scholar]
- [26].Petta S, Camma C, Di Marco V, et al. Hepatic steatosis and insulin resistance are associated with severe fibrosis in patients with chronic hepatitis caused by HBV or HCV infection. Liver Int 2011;31:507–15. [DOI] [PubMed] [Google Scholar]
- [27].Petta S, Maida M, Macaluso FS, et al. The severity of steatosis influences liver stiffness measurement in patients with nonalcoholic fatty liver disease. Hepatology 2015;62:1101–10. [DOI] [PubMed] [Google Scholar]
- [28].Gaia S, Carenzi S, Barilli AL, et al. Reliability of transient elastography for the detection of fibrosis in non-alcoholic fatty liver disease and chronic viral hepatitis. J Hepatol 2011;54:64–71. [DOI] [PubMed] [Google Scholar]
- [29].Wong GL, Wong VW, Chan HL. Is transient elastography inaccurate in chronic hepatitis B and non-alcoholic fatty liver disease? J Hepatol 2011;55:497–8. [DOI] [PubMed] [Google Scholar]
- [30].Degos F, Perez P, Roche B, et al. Diagnostic accuracy of FibroScan and comparison to liver fibrosis biomarkers in chronic viral hepatitis: a multicenter prospective study (the FIBROSTIC study). J Hepatol 2010;53:1013–21. [DOI] [PubMed] [Google Scholar]
- [31].Castera L, Vergniol J, Foucher J, et al. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology 2005;128:343–50. [DOI] [PubMed] [Google Scholar]
- [32].Cardoso AC, Carvalho RJ, Stern C, et al. Direct comparison of diagnostic performance of transient elastography in patients with chronic hepatitis B and chronic hepatitis C. Liver Int 2012;32:612–21. [DOI] [PubMed] [Google Scholar]
- [33].Guidelines for the Prevention. Care and Treatment of Persons with Chronic Hepatitis B Infection[M]. Geneva: World Health Organization; 2015. [PubMed] [Google Scholar]
- [34].Vigano M, Paggi S, Lampertico P, et al. Dual cut-off transient elastography to assess liver fibrosis in chronic hepatitis B: a cohort study with internal validation. Aliment Pharmacol Ther 2011;34:353–62. [DOI] [PubMed] [Google Scholar]
- [35].Li Q, Li W, Huang Y, et al. The gamma-glutamyl transpeptidase-to-platelet ratio predicts liver fibrosis and cirrhosis in HBeAg-positive chronic HBV infection patients with high HBV DNA and normal or mildly elevated alanine transaminase levels in China. J Viral Hepat 2016;23:912–9. [DOI] [PubMed] [Google Scholar]
- [36].Cai YJ, Dong JJ, Wang XD, et al. A diagnostic algorithm for assessment of liver fibrosis by liver stiffness measurement in patients with chronic hepatitis B. J Viral Hepat 2017;24:1005–15. [DOI] [PubMed] [Google Scholar]
- [37].Ransohoff DF, Feinstein AR. Problems of spectrum and bias in evaluating the efficacy of diagnostic tests. N Engl J Med 1978;299:926–30. [DOI] [PubMed] [Google Scholar]
- [38].Poynard T, Halfon P, Castera L, et al. Standardization of ROC curve areas for diagnostic evaluation of liver fibrosis markers based on prevalences of fibrosis stages. Clin Chem 2007;53:1615–22. [DOI] [PubMed] [Google Scholar]
- [39].Verveer C, Zondervan PE, Ten KF, et al. Evaluation of transient elastography for fibrosis assessment compared with large biopsies in chronic hepatitis B and C. Liver Int 2012;32:622–8. [DOI] [PubMed] [Google Scholar]


