Abstract
Colon ischemia (CI) is the most common ischemic disorder of the gastrointestinal tract. Although some markers of CI, such as procalcitonin and alkaline phosphatase, have been reported, few specific serum markers have been identified. We investigated whether serum stromal cell-derived factor-1 (SDF-1) is a specific marker of CI and clarified the relationship between serum SDF-1 level and CI according to a history of combined chronic cardiovascular disease (CVD).
We measured SDF-1 level and other serological markers in 84 patients (control, n = 20; CI without chronic CVD, n = 21; chronic CVD without CI, n = 20; CI with chronic CVD, n = 23).
Patients with CI were older than those without CI. There were more women in the CI groups than those without CI. At admission, SDF-1 level was significantly higher in patients having CI with chronic CVD (P < .001) than in other groups. SDF-1 level was significantly higher at admission than at discharge in patients having CI with chronic CVD (P < .001) but not in patients having CI without chronic CVD. SDF-1 level did not differ according to symptoms, involved sites, or duration of hospitalization. At a cutoff value of 0.5 pg/mL for the SDF-1 level in patients having CI with chronic CVD, the sensitivity and specificity for SDF-1 were 91.3% and 95%, respectively. The area-under-the-curve (AUC) value was 0.95. In the logistic regression analysis, an elevation of the SDF-1 level to >0.5 pg/mL was a significant indicator of CI with chronic CVD [odds ratio (OR), 114.914; 95% confidence interval, 10.51 to >999.999; P < .001].
SDF-1 could be a useful early biomarker for the diagnosis of CI in patients with chronic CVD.
Keywords: biomarker, cardiovascular disease, colon ischemia, serum, stromal cell-derived factor-1
1. Introduction
Colon ischemia (CI) is the most common ischemic disorder of the gastrointestinal tract.[1–3] CI is caused by hypoperfusion of the gastrointestinal tract and accounts for 1 in 2000 of acute hospitalizations.[1,4,5] However, CI is likely to be underdiagnosed because of it lacks a specific diagnostic marker.[2] Clinically, CI appears as a spectrum of injury from transient ischemia, which has a good prognosis, to acute fulminant ischemia, which can progress to necrosis and death.[6,7] Most CI transiently involves the mucosa and submucosa and can be managed conservatively with a good prognosis.[7,8] The most commonly involved parts of the colon are the splenic flexure and rectosigmoid junction; i.e., the so-called “watershed” areas.[5,8–10]
The methods used in diagnosing CI, such as colonoscopy, are invasive. Invasive colonoscopy is regarded as the standard diagnostic method for CI, despite its risks of bowel perforation and aggravation of the ischemia.[2,5–8,12] Invasive colonoscopy cannot be performed in elderly patients having multiple comorbidities, such as cardiovascular or respiratory disease, and it may be difficult to accurately diagnose CI in these patients.[13]
As noninvasive methods for diagnosing CI, such as abdominal computed tomography (CT) scanning and serological markers are available.[14,15] Abdominal multidetector computed tomography (MDCT) scanning combined with angiography may be sufficient for diagnosing CI and estimating the CI grade.[7,8,14–16] Several biological markers have been evaluated, including the levels of alkaline phosphatase (ALP), amylase, creatine phosphokinase (CPK), lactate dehydrogenase (LDH), D-dimer, D-lactate and procalcitonin.[7,14,17,18] However, these markers are nonspecific and have not been shown to have a consistent sensitivity and specificity according to the different cutoff levels.[8,17,19,20] Because the principal modality for the diagnosis of CI is colonoscopy, we intended to find a new specific serum marker.
Stromal cell-derived factor-1 (SDF-1) regulates many aspects of stem cell function including stem cell trafficking and development.[21] SDF-1 is widely expressed in many tissues during development, often in parallel with the expression of the C-X-C chemokine receptor type 4 (CXCR4).[22] It is unclear whether SDF-1 expression is upregulated in hypoxic conditions, such as ischemic heart disease.[23] However, in endothelial cells, hypoxia can induce activation of the hypoxia-inducible transcription factor-1 (HIF-1), which binds to specific binding sites in the SDF-1 promotor.[24] In acute myocardial infarction, SDF-1 expression is rapidly upregulated and SDF-1 promotes myocardial repair.[25] SDF-1 is also an important marker of ischemia in other organs.[26,27]
Currently, there are insufficient data to determine whether serum SDF-1 level is a specific marker that can be used for the diagnosis of CI. Since cardiovascular disease (CVD) is known to be closely related to CI, we sought to evaluate whether serum SDF-1 is a unique diagnostic marker of CI according to the presence or absence of chronic CVD.
2. Methods
2.1. Participants
We enrolled 100 participants in this study between January 2010 and January 2018. Patients who were admitted via the emergency room in the Uijeongbu St. Mary's Hospital for CI were eligible for this study. The control group included individuals who underwent a general health examination without any symptoms or signs of CI such as hematochezia, abdominal pain, diarrhea, nausea, or vomiting. We excluded 16 patients according to the exclusion criteria. Out of the 84 remaining participants, 27 were men and 57 were women.
The participants were divided into 4 groups as follows: control group (controls, n = 20); CI with no history of CVD group (CI no CVD, n = 21); chronic CVD with no history of CI group (CVD no CI, n = 20); and chronic CVD with CI group (CI plus CVD, n = 23). Chronic CVD included the nonacute forms of coronary artery disease and hypertensive heart disease.[28] The inclusion criteria for CI were based on the combination of the following clinical, radiological, endoscopic, and pathological findings: (1) sudden onset of abdominal pain, diarrhea, lower gastrointestinal bleeding, and peritoneal signs; (2) colonoscopically diagnosed erythema, edema, submucosal hemorrhage, ulcer, exudate, and mucosal granularity; (3) evidence of pathological edema, lymphocyte and neutrophilic granulocyte infiltration, epithelial erosion, ulceration, necrosis, and gangrene; and (4) CT findings such as segmental wall thickening, with or without pericolic streaking.[29–31]
The exclusion criteria were as follows: acute forms of CVD as revealed by checking CPK level, pseudomembranous colitis, inflammatory bowel disease, CI secondary to vascular surgery, and thromboembolism of the mesenteric artery and/or colorectal cancer. This study was approved by the Institutional Review Board of the Catholic University of Korea and was registered as a clinical trial (registration number UC10SISI0091), and all participants provided their written consent to participate in this study.
2.2. Laboratory analysis
Venous blood was collected at the time of admission and discharge in the CI no CVD group and the CI plus CVD group. The blood was centrifuged at 10,000 rpm and stored at –80°C until the SDF-1 level was measured. Serum SDF-l level was measured using an antibody sandwich ELISA (R & D Systems, Minneapolis, MN) with a detection range of 62 to 50,000 pg/mL.
ALP, amylase, CPK, and LDH levels were also measured using an automated chemical analyzer (747; Hitachi, Tokyo, Japan), currently used for analyzing patient samples in the Clinical Laboratory of the Catholic University of Korea Uijeongbu St. Mary's Hospital.
2.3. Statistical analysis
Numerical data were expressed as mean ± standard deviation (SD) or median (minimum – maximum), where appropriate. Nominal data were presented as “Number (%).” The mean differences between subgroups were analyzed using the 1-way analysis of variance (ANOVA) test, and differences in proportions were analyzed using the Chi-square test or Fisher exact test. Given the non-normal distribution of SDF-1 levels and the small sample sizes of the subgroups, we performed a nonparametric analysis.
The differences in medians between subgroups were analyzed using the Wilcoxon rank-sum test or Kruskal–Wallis test. The differences in medians across times for the same patients were analyzed using the Wilcoxon signed-rank test. Receiver-operating characteristic (ROC) curve analysis was applied to evaluate the diagnostic potential of serum SDF-1 level. The ability of SDF-1 level to predict CI with chronic CVD was analyzed using multiple logistic regression after adjusting for age using the Firth logistic regression method. The data are presented as odds ratio (OR) and 95% confidence interval. A P value < .05 was considered statistically significant. All statistical analyses were conducted using SAS version 9.3 (SAS Institute Inc., Cary, NC)
3. Results
3.1. Characteristics of the participants
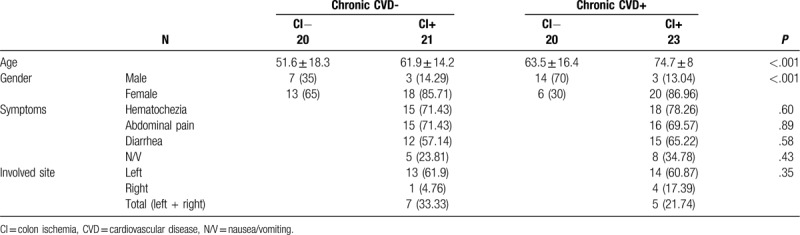
The characteristics of the participants such as age, sex, underlying disease, presenting symptoms, and involved site are summarized in Table 1. The participants were significantly older in the CI no CVD group, CVD no CI group, and CI plus CVD group than in the control group (P < .001). The overall ratio of men to women was 1:2.11 (27 men and 57 women), and there were more women than men in the control, CI no CVD, and CI plus CVD groups (P < .001). The segments involved in CI were as follows: left colon in 27 patients, right colon in 5 patients, and whole colon in 12 patients (Table 1).
Table 1.
Characteristics of the controls and the patients with colon ischemia.
3.2. Serum SDF-1 level
The serum SDF-1 level measured in the patients showed wide variation from 0.1 to > 454.94 pg/mL. At admission, the median serum SDF-1 level was significantly higher in the CI plus CVD group (102.65 pg/mL; range, 0.1–454.94) than in the control group (0.1 pg/mL; range, 0.1–37.65), CI no CVD group (0.1 pg/mL; range, 0.1–134.07), and CVD no CI group (0.1 pg/mL; range, 0.1–0.5) (Table 2) (P < .01). In the CI plus CVD group, the median serum SDF-1 level (102.65 pg/mL; range, 0.1–454.94) was significantly higher at admission than at discharge (0.1 pg/mL; range, 0.1–414.76) (P < .001). In contrast, the SDF-1 level at admission and discharge did not differ significantly in the CI no CVD group (P = .63) (Table 2). The SDF-1 level did not differ significantly according to the duration of hospitalization (less or more than 10 days). The symptoms of CI were not significantly associated with the serum SDF-1 level (Table 3). Although not significant, the median serum SDF-1 level was higher in patients with an ischemic right colon (median, 98.9 pg/mL; range, 0.1–111.46) than in those with an ischemic left colon (median, 65.54 pg/mL; range, 0.1–454.94) or total colon (median, 32.785 pg/mL; range, 0.1–348.99) (P = .90).
Table 2.
Differences in serum SDF-1 level between admission and discharge.

Table 3.
Comparison of SDF-1 level by symptoms and involved sites.

3.3. Serum levels of ALP, amylase, CPK, and LDH
The serum levels of other putative biomarkers of CI (LDH, CPK, amylase, and ALP) are summarized in Table 4. Their levels did not differ significantly between the 4 groups.
Table 4.
Comparison of other markers of colon ischemia with cardiovascular disease.

3.4. Ability of SDF-1 level to predict CI with chronic CVD
The discriminative diagnostic parameters at admission to distinguish the CI plus CVD group from the CVD no CI group were calculated using ROC curve analysis (Fig. 1). The area under the curve (AUC) in the current study was 0.95. At a cutoff level of 0.5 pg/mL for distinguishing the CI plus CVD group at the time of admission from the CVD no CI group, the sensitivity and specificity were calculated as 91.3% and 95%, respectively.
Figure 1.
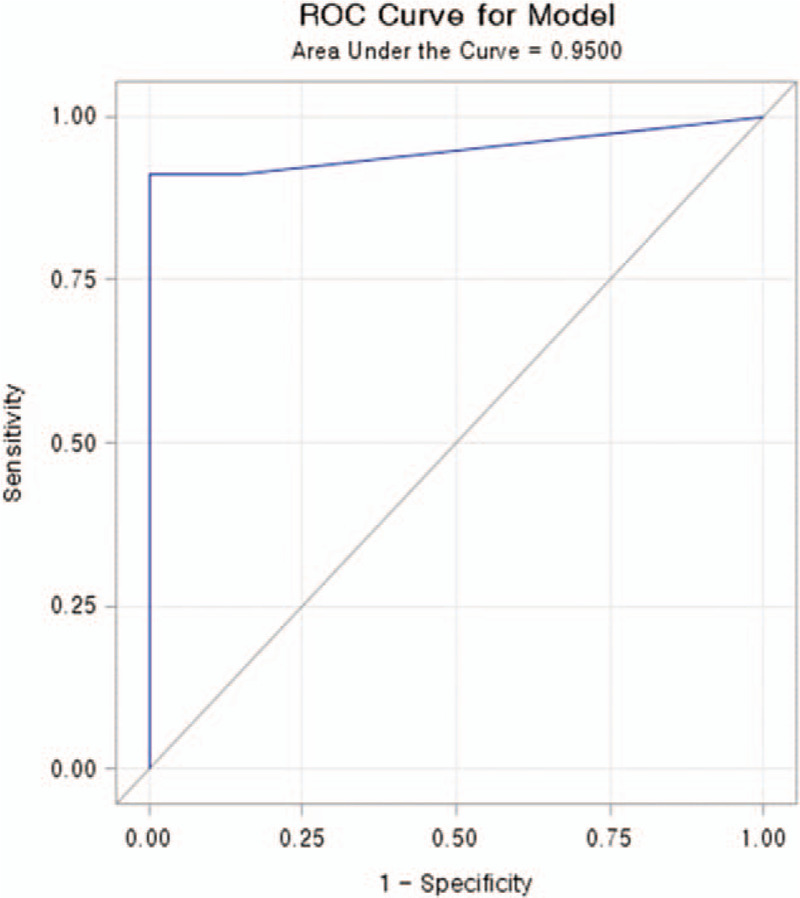
Receiver operating characteristic (ROC) curve analysis of SDF-l in colon ischemia with chronic cardiovascular disease. The cut-off value of SDF-l in colon ischemia with cardiovascular disease was 0.5 pg/mL with a sensitivity of 91.3% and specificity of 95%. The area under the curve (AUC) was 0.95. P < .001.
3.5. Age-adjusted OR according to SDF-1 level
At a cutoff of 0.5 pg/mL for the serum SDF-1 level, the OR was 114.914 for discriminating the CI plus CVD group from the CVD no CI group (Table 5).
Table 5.
Age-adjusted multiple logistic regression analysis of SDF-1 according to the presence of CVD with or without colon ischemia.

4. Discussion
CI is a disease requiring rapid and early diagnosis in order to exclude the potentially disastrous progression to conditions needing exploratory surgery.[11] There are 2 diagnostic methods used in conjunction with clinical symptoms and signs: invasive colonoscopy as the standard diagnostic tool, and noninvasive methods such as laboratory tests and radiologic examination.[5,8,9] However, colonoscopy for the diagnosis of CI has several risks, including bowel perforation and aggravation of the ischemia caused by hyperinflation of air during the procedure. Radiological abdominal CT scanning and angiography are specific and informative, especially for discriminating between occlusive and nonocclusive CI.[7,8,15,16]
To date, several serological markers, such as procalcitonin and ALP, have been used for the diagnosis of CI despite their low sensitivity and specificity, and there have been few attempts to evaluate CI according to its pathogenic mechanisms and association with chronic CVD.[14,18–20] Therefore, it is important to find a new specific serological markers that can be used in the early diagnosis and treatment for CI. We investigated whether SDF-1 is an early serological marker of CI because several studies had examined the relationship between SDF-1 level and ischemic disorders in other organs such as the kidney and heart.[26,27] We measured serum SDF-1 level in patients having CI with and without chronic CVD. We found that serum SDF-1 level was markedly elevated in patients having CI with chronic CVD compared to controls. We also found wide variations in the serum SDF-1 level in all participants regardless of their clinical findings and involved sites. Among the patients in the CI plus CVD group, the serum SDF-1 level at discharge decreased significantly to 65% of the level at the time of admission (P = .02).
The SDF-1 level did not differ significantly between the CI no CVD and controls. Interestingly, SDF-1 level was elevated at admission in 5 of 21 patients with CI no CVD, but this level decreased to that in the CI plus CVD group at discharge (data not shown). These findings suggest that the pathogenesis of CI may differ between patients having CI with and without chronic CVD, and that other unknown mechanisms may be related to CI in patients without chronic CVD. In other words, SDF-1 may not be a valid serological marker of CI in the absence of chronic CVD. Future studies are needed to identify the conditions associated with the development of CI without chronic CVD, especially in the subgroup of patients with high SDF-1 level. In addition, future studies should focus on the sequential changes during the entire CI disease course and the time to reach the nadir SDF-1 level after discharge. We found that the serum SDF-1 levels did not differ significantly according to the duration of hospitalization (more or less than 10 days (P > .05). This finding suggests that the duration of hospitalization is not related to SDF-1 level, which may reflect the short half-life of SDF-1.[22]
CI occurs predominantly in older people and in women, and is closely associated with CVD.[5,9] In this study, the patients’ characteristics were similar to those reported previously. Other biological markers for the diagnosis of CI have been reported, including the levels of ALP, amylase, CPK, LDH, D-dimer, D-lactate, and procalcitonin.[7,17–19] However, all of these markers are nonspecific or have shown inconsistent results with different cutoff levels.[7,17–19] In this study, LDH, CPK, amylase, and ALP levels did not differ significantly between patients with CI at admission and healthy controls (P > .05). Reliable biochemical markers for identifying CI have not been identified. Block et al[19] reported that CPK and ALP levels did not differ significantly, but LDH level was significantly elevated in patients with ischemic intestinal disease. However, its specificity was low, and the total LDH activity seems to be a marker of tissue damage in a wide spectrum of conditions. Therefore, LDH cannot be considered as a specific marker of intestinal ischemia.
We used ROC curve analysis to identify the SDF-1 level that distinguished between the CI plus CVD group and the CVD no CI group at the time of admission (Fig. 1). At a cutoff level of 0.5 pg/mL for distinguishing these 2 groups, the sensitivity was 91.3% and the specificity was 95%. The age-adjusted OR at a serum SDF-1 cutoff level of 0.5 pg/mL was 114.914 (Table 5). The right colon appears to be more severely affected by CI than the left colon because the mesenteric vascular anatomy of the right colon has little natural collateral blood flow to accommodate any deprivation of blood supply.[32–35] The clinical course of CI of the right colon may be more unfavorable, which may lead to a greater likelihood of surgery and mortality compared with CI of the left colon.[36] However, our data do not indicate whether the SDF-1 level is a predictor of the involved site and the severity of CI because our study did not include severe cases of CI, and only a small number of patients whose CI involved the right colon were included. Additional studies are needed to determine the association between SDF-1 level and CI, and the ability of SDF-1 level to distinguish CI from other severe forms of intestinal vascular disease, such as mesenteric ischemia or infarction.
Our study has limitations, mainly because of the small sample size, lack of severe forms of CI, and few patients with CI of the right colon. SDF-1 level may be elevated in other forms of ischemic disease, including that of the heart, kidney, and other types of colitis such as infectious colitis and inflammatory bowel diseases.
In conclusion, serum SDF-1 level may be a useful serological marker for the diagnosis of CI in patients with chronic CVD.
Acknowledgments
We thank Woo Ji Hiun for expert technical assistance with SDF-1 immunoassays. The results presented in this paper have not been published previously.
We thank to Yong Gyu Park, the professor of department of biostatics, College of Medicine, The Catholic University of Korea, Seoul, Republic of Korea, for statistical analysis.
Author contributions
Conceptualization: Hiun Suk Chae.
Data curation: Hae Kyung Lee, Yeongsic Kim.
Formal analysis: Sang Woo Kim, Hyung Keun Kim.
Funding acquisition: Yonggoo Kim.
Investigation: Hyunjung Kim, Hyun Ho Choi, Sang Woo Kim, Hyung Keun Kim.
Methodology: Hyunjung Kim, Hyun Ho Choi.
Supervision: Hiun Suk Chae.
Writing – original draft: Ka Young Kim.
Writing – review & editing: Hae Kyung Lee, Sang Woo Kim.
Footnotes
Abbreviations: ALP = alkaline phosphatase, AUC = area-under-the-curve, CI = colon ischemia, SDF-1 = serum stromal cell-derived factor-1, CPK = creatine phosphokinase, CT = computed tomography, CVD = cardiovascular disease, CXCR-4 = C-X-C chemokine receptor type 4, ELISA = enzyme-linked immunosorbent assay, HIF-1 =hypoxia-inducible factor-1, LDH = lactate dehydrogenase, OR = odds ratio, ROC curve = receiver operating characteristic curve, SD = standard deviation.
How to cite this article: Kim KY, Lee HK, Kim H, Kim Y, Kim Y, Choi HH, Kim SW, Kim HK, Chae HS. Stromal cell-derived factor-1 as a serologic biomarker for the diagnosis of colon ischemia with chronic cardiovascular disease. Medicine. 2020;99:23(e20539).
This research was supported by the Samkwang Medical Center.
This research was supported by the National Research Foundation of Korea (grant number NRF-2017R1D1A1A 02019310).
No potential conflict of interest relevant to this article was reported.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Brandt LJ, Boley SJ. Colonic ischemia. Surg Clin North Am 1992;72:203–29. [DOI] [PubMed] [Google Scholar]
- [2].Flynn AD, Valentine JF. Update on the diagnosis and management of colon ischemia. Curr Treat Options Gastroenterol 2016;14:128–39. [DOI] [PubMed] [Google Scholar]
- [3].Korotinski S, Katz A, Malnick SD. Chronic ischaemic bowel diseases in the aged: go with the flow. Age Ageing 2005;34:10–6. [DOI] [PubMed] [Google Scholar]
- [4].Liberski SM, Koch KL, Atnip RG, et al. Ischemic gastroparesis: resolution after revascularization. Gastroenterology 1990;99:252–7. [DOI] [PubMed] [Google Scholar]
- [5].Zou X, Cao J, Yao Y, et al. Endoscopic findings and clinicopathologic characteristics of ischemic colitis: a report of 85 cases. Dig Dis Sci 2009;54:2009–15. [DOI] [PubMed] [Google Scholar]
- [6].Khor TS, Lauwers GY, Odze RD, et al. Mass-forming” variant of ischemic colitis is a distinct entity with predilection for the proximal colon. Am J Surg Pathol 2015;39:1275–81. [DOI] [PubMed] [Google Scholar]
- [7].Green BT, Tendler DA. Ischemic colitis: a clinical review. South Med J 2005;98:217–22. [DOI] [PubMed] [Google Scholar]
- [8].Elder K, Lashner BA, Al Solaiman F. Clinical approach to colonic ischemia. Cleve Clin J Med 2009;76:401–9. [DOI] [PubMed] [Google Scholar]
- [9].Habu Y, Tahashi Y, Kiyota K, et al. Reevaluation of clinical features of ischemic colitis. Analysis of 68 consecutive cases diagnosed by early colonoscopy. Scand J Gastroenterol 1996;31:881–6. [DOI] [PubMed] [Google Scholar]
- [10].Nowicki PT. Ischemia and necrotizing enterocolitis: where, when, and how. Semin Pediatr Surg 2005;14:152–8. [DOI] [PubMed] [Google Scholar]
- [11].Sotiriadis J, Brandt LJ, Behin DS, et al. Ischemic colitis has a worse prognosis when isolated to the right side of the colon. Am J Gastroenterol 2007;102:2247–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zuckerman GR, Prakash C, Merriman RB, et al. The colon single-stripe sign and its relationship to ischemic colitis. Am J Gastroenterol 2003;98:2018–22. [DOI] [PubMed] [Google Scholar]
- [13].Antolovic D, Koch M, Hinz U, et al. Ischemic colitis: analysis of risk factors for postoperative mortality. Langenbecks Arch Surg 2008;393:507–12. [DOI] [PubMed] [Google Scholar]
- [14].Sun MY, Maykel JA. Ischemic colitis. Clin Colon Rectal Surg 2007;20:5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Graziella DG, Gianluca G, Roberta R, et al. MDCT in acute ischaemic left colitis: a pictorial essay. La radologia medica 2019;124:103–8. [DOI] [PubMed] [Google Scholar]
- [16].Daniella B, Francesca I, Maria AM, et al. MDCT in ischaemic colitis: how to define the aetiology and acute, subacute and chronic phase of damage in the emergency setting. Br J Radiol 2016;89:20150821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Evennett NJ, Petrov MS, Mittal A, et al. Systematic review and pooled estimates for the diagnostic accuracy of serological markers for intestinal ischemia. World J Surg 2009;33:1374–83. [DOI] [PubMed] [Google Scholar]
- [18].Cosse C, Sabbagh C, Browet F, et al. Serum value of procalcitonin as a marker of intestinal damages: type, extension, and prognosis. Surg Endosc 2015;29:3132–9. [DOI] [PubMed] [Google Scholar]
- [19].Block T, Nilsson TK, Björck M, et al. Diagnostic accuracy of plasma biomarkers for intestinal ischaemia. Scand J Clin Lab Invest 2008;68:242–8. [DOI] [PubMed] [Google Scholar]
- [20].Lammers KM, Innocenti G, Venturi A, et al. The effect of transient intestinal ischemia on inflammatory parameters. Int J Colorectal Dis 2003;18:78–85. [DOI] [PubMed] [Google Scholar]
- [21].Nagasawa T, Hirota S, Tachibana K, et al. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature 1996;382:635–8. [DOI] [PubMed] [Google Scholar]
- [22].McGrath KE, Koniski AD, Maltby KM, et al. Embryonic expression and function of the chemokine SDF-1 and its receptor, CXCR4. Dev Biol 1999;213:442–56. [DOI] [PubMed] [Google Scholar]
- [23].Bromage DI, Davidson SM, Yellon DM. Stromal derived factor 1(: a chemokine that delivers a two-pronged defence of the myocardium. Pharmacol Ther 2014;143:305–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ceradini DJ, Kulkarni AR, Callaghan MJ, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med 2004;10:858–64. [DOI] [PubMed] [Google Scholar]
- [25].Ziegler M, Elvers M, Baumer Y, et al. The bispecific SDF1-GPVI fusion protein preserves myocardial function after transient ischemia in mice. Circulation 2012;125:685–96. [DOI] [PubMed] [Google Scholar]
- [26].Penn MS, Pastore J, Miller T, et al. SDF-1 in myocardial repair. Gene Ther 2012;19:583–7. [DOI] [PubMed] [Google Scholar]
- [27].Stokman G, Stroo I, Claessen N, et al. SDF-1 provides morphological and functional protection against renal ischaemia/reperfusion injury. Nephrol Dial Transplant 2010;25:3852–9. [DOI] [PubMed] [Google Scholar]
- [28].McGill HC, Jr, McMahan CA, Gidding SS. Preventing heart disease in the 21st century: implications of the Pathobiological Determinants of Atherosclerosis in Youth (PDAY) study. Circulation 2008;117:1216–27. [DOI] [PubMed] [Google Scholar]
- [29].Chung JW, Cheon JH, Park JJ, et al. Development and validation of a novel prognostic scoring model for ischemic colitis. Dis Colon Rectum 2010;53:1287–94. [DOI] [PubMed] [Google Scholar]
- [30].Scowcroft CW, Sanowski RA, Kozarek RA. Colonoscopy in ischemic colitis. Gastrointest Endosc 1981;27:156–61. [DOI] [PubMed] [Google Scholar]
- [31].Sreenarasimhaiah J. Diagnosis and management of ischemic colitis. Curr Gastroenterol Rep 2005;7:421–6. [DOI] [PubMed] [Google Scholar]
- [32].Flynn TC, Rowlands BJ, Gilliland M, et al. Hypotension-induced post-traumatic necrosis of the right colon. Am J Surg 1983;146:715–8. [DOI] [PubMed] [Google Scholar]
- [33].Musa BU. Intestinal infarction without mesenteric vascular occlusion. A report of 31 cases. Ann Intern Med 1965;63:783–92. [DOI] [PubMed] [Google Scholar]
- [34].Sakai L, Keltner R, Kaminski D. Spontaneous and shock-associated ischemic colitis. Am J Surg 1980;140:755–60. [DOI] [PubMed] [Google Scholar]
- [35].Stellos K, Ruf M, Sopova K, et al. Plasma levels of stromal cell-derived factor-1 in patients with coronary artery disease: effect of clinical presentation and cardiovascular risk factors. Atherosclerosis 2011;219:913–6. [DOI] [PubMed] [Google Scholar]
- [36].Landreneau RJ, Fry WJ. The right colon as a target organ of nonocclusive mesenteric ischemia. Case report and review of the literature. Arch Surg 1990;125:591–4. [DOI] [PubMed] [Google Scholar]


