Supplemental Digital Content is available in the text
Keywords: congenital heart disease, global, incidence, mortality, temporal trend
Abstract
Congenital heart disease (CHD) is the most commonly diagnosed congenital disorder in newborns. The incidence and mortality of CHD vary worldwide. A detailed understanding of the global, regional, and national distribution of CHD is critical for CHD prevention.
We collected the incidence and mortality data of CHD from the Global Burden of Disease study 2017 database. Average annual percentage change was applied to quantify the temporal trends of CHD incidence and mortality at the global, regional, and national level, 1990–2017. A sociodemographic index (SDI) was created for each location based on income per capita, educational attainment, and fertility.
The incidence of CHD was relatively high in developing countries located in Africa and Asia, while low in most developed countries. Between 1990 and 2017, the CHD incidence rate remained stable at the global level, whereas increased in certain developed countries, such as Germany and France. The age-standardized mortality rate of CHD declined substantially over the last 3 decades, regardless of sex, age, and SDI region. The decline was more prominent in developed countries. We also detected a significant positive correlation between CHD incidence and CHD mortality in both 1990 and 2017, by SDI.
The incidence of CHD remained stable over the last 3 decades, suggesting little improvement in CHD prevention strategies and highlighting the importance of etiological studies. The mortality of CHD decreased worldwide, albeit the greatly geographical heterogeneity. Developing countries located in Africa and Asia deserve more attention and priority in the global CHD prevention program.
1. Introduction
Congenital heart disease (CHD) is 1 of the most frequently diagnosed congenital disorders afflicting approximately 0.8% to 1.2% of live births worldwide.[1,2] Generally, CHD is defined as a structural abnormality of the heart and (or) great vessels that is present at birth.[3] Although numerous etiologic investigations have been conducted, only approximately 15% of cases of CHD can be attributable to a known cause.[4] Moreover, the incidence and mortality of CHD are substantially heterogeneous across the world.[5,6] Limited knowledge for the etiologies of CHD and the high heterogeneity in CHD epidemics constitutes the major obstacles for CHD prevention and early screening.
Before 1950 s, severe CHD was an almost universally fatal disease. Fortunately, the introduction of cardiopulmonary bypass transformed it into a manageable disorder.[7] Although advances in cardiovascular medicine and surgery over the past decades have decreased mortality drastically and enabled most patients to reach adulthood, CHD remains the leading cause of mortality from birth defects and imposes a heavy disease burden worldwide.[1,5] Understanding the epidemiology of CHD incidence along with mortality is of great interest for epidemiologists and policymakers and of great importance for knowing the disease burden of CHD. Furthermore, differences in incidence rates and time trends among different regions may also lead to better insight into its etiology.[2,8]
In this study, we comprehensively analyzed the epidemiologic characteristics of CHD at the global, regional, and national level. We aim to further describe the landscape of CHD worldwide, while also assisting in the precise estimate for CHD burden and the establishment of more tailored preventive strategies.
2. Methods
We collected the incidence and mortality data of CHD from the Global Burden of Disease (GBD) study 2017 online database (available at http://ghdx.healthdata.org/gbd-results-tool).[9] The data were stratified by sex, age, region (5 regions according to the socio-demographical index [SDI]), and nation (a total of 195 countries or territories). We also retrieved the incidence data of CHD by its subtypes (Fig. 1). In the GBD database, CHD was subdivided as the follows: a) single ventricle and single ventricle pathway defects; b) severe congenital heart defects excluding single ventricle and single ventricle pathway defects; c) critical malformations of great vessels, congenital valvular heart disease and patent ductus arteriosis; d) ventricular septal defect and atrial septal defect; and e) other congenital cardiovascular anomalies.[10] List of International Classification of Diseases version 10 codes mapped to each subtype in GBD 2017 database was showed in supplemental material (Table S1). Because the data used in this study can be downloaded from open access databases, ethics approval and informed consent were not required.
Figure 1.
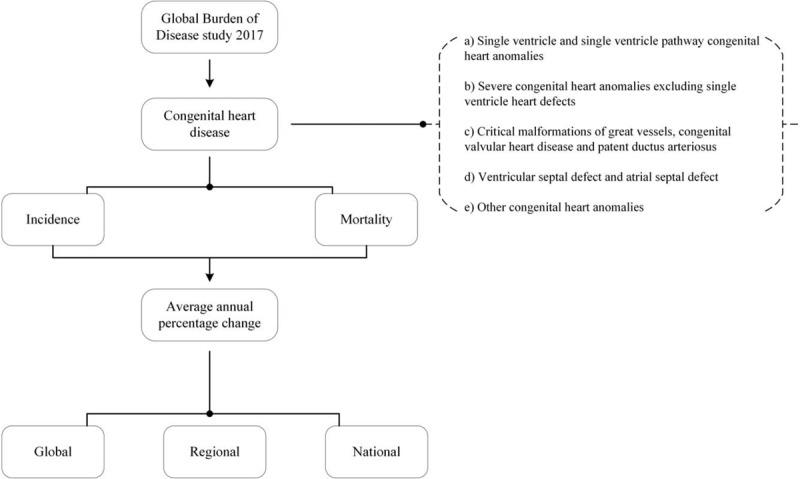
Flow diagram of the study design.
SDI, as an indicator of national sociodemographic development, is a composite measure of the incomes per capita, educational attainment, and total fertility rates.[5] It is expressed on a scale of 0 to 1, with higher values indicating higher levels of socioeconomic status. Countries or territories are categorized into the following 5 groups based on SDI values in the GBD 2017: high SDI (≥0.8), high-middle SDI (<0.8 to ≥0.6), middle SDI (<0.6 to ≥0.4), low-middle SDI (<0.4 to ≥0.2), and low SDI (<0.2).
We used the World 2000 standard population to standardize the CHD mortality rate. The temporal trends of the incidence and age-standardized mortality rate (ASMR) of CHD were quantified using the average annual percentage change (AAPC).[11] Briefly, a regression line was fitted to the natural logarithm of the rates, that is, y = α + βx + ε, where y = ln(rate) and x = calendar year. The AAPC was calculated as 100 × (exp(β)-1), and its 95% confidence interval (CI) was also obtained from the linear regression model.[12] The rate was deemed to be increase if the AAPC estimation and the lower boundary of its 95%CI are both >0. On the contrary, the rate was considered as decreasing if the AAPC and the upper boundary of its 95%CI are both <0. Otherwise, the rate was deemed to be stable during the study period. All analyses were implemented using R software (version 3.5.1). All tests were 2-sided. A P value < .05 was considered statistically significant.
3. Results
3.1. The incidence rate and its temporal trend of CHD
In 2017, the incidence rate of CHD was 17.9/1000 worldwide, with 19.1/1000 for male and 16.6/1000 for female (Table 1; Fig. 2A). Ventricular septal defect and atrial septal defect were the most common subtype of CHD with an incidence of 5.29/1000 and accounted for about 29.6% of all cases of CHD (Table 2). Overall, global incidence rate of CHD remained stable from 1990 to 2017 (AAPC = 0.05, 95%CI: -0.01, 0.12) (Table 1; Fig. 2A). The stable trend was also observed in both male and female.
Table 1.
The incident case and incidence rate of congenital heart disease between 1990 and 2017.
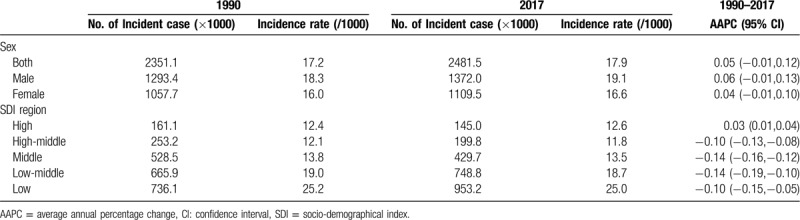
Figure 2.
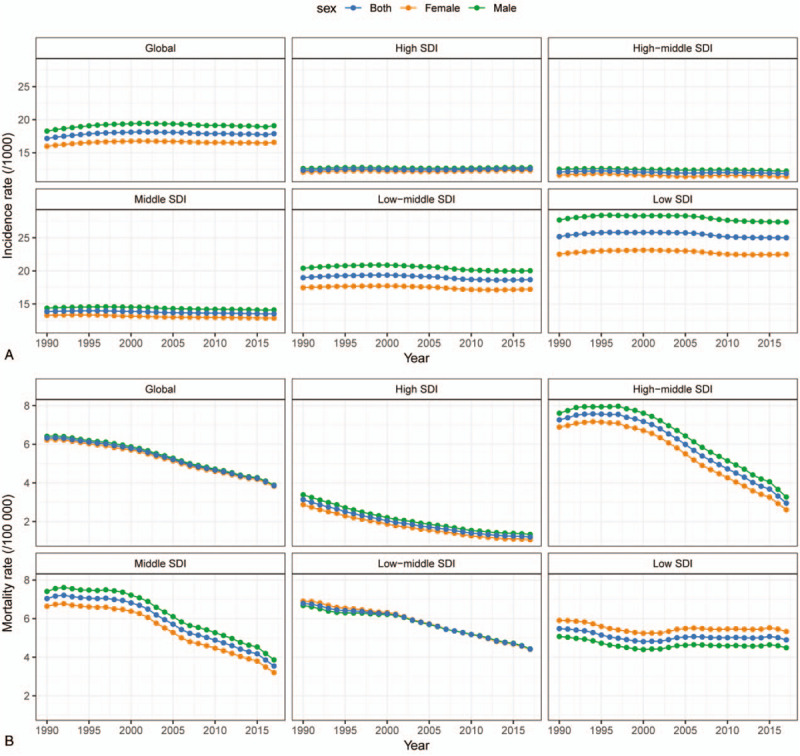
(A, B) The incidence and mortality rate of congenital heart disease between 1990 and 2017, by sex and socio-demographical index (SDI) region.
Table 2.
The incident case and incidence rate of congenital heart disease between 1990 and 2017, by subtypes.
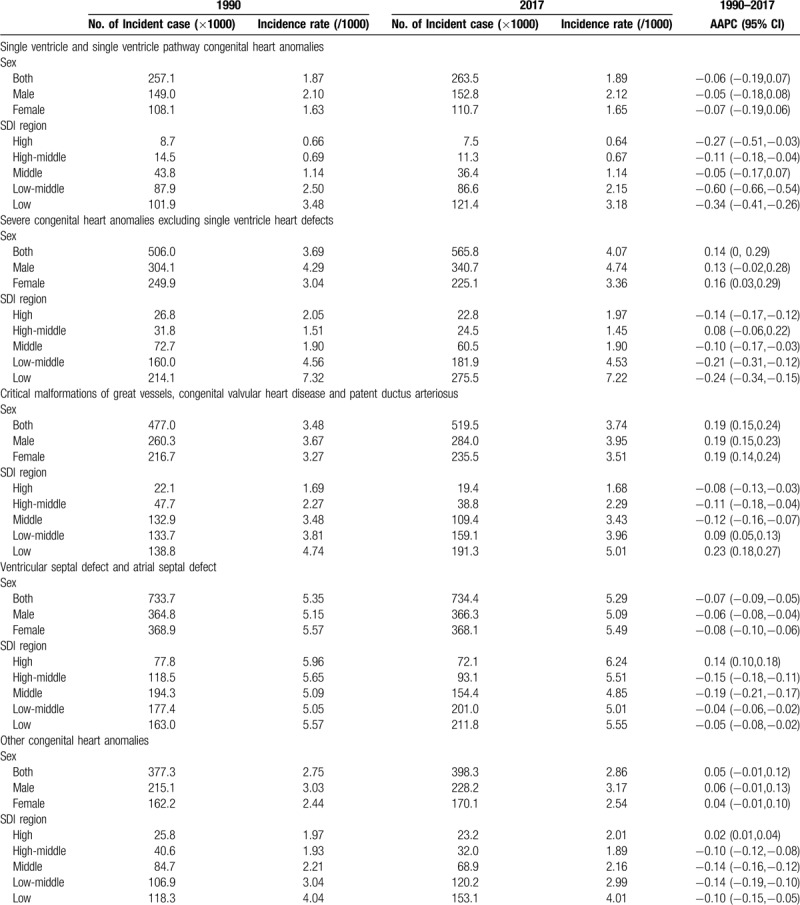
However, trends in incidence rates differed by CHD subtype and SDI region. Between 1990 and 2017, the incidence rate of CHD experienced a decline in all SDI regions except for high-SDI region, in which the incidence rate increased from 12.4/1000 to 12.6/1000 (AAPC = 0.03, 95%CI: 0.01, 0.04) (Table 1; Fig. 2A). The increase of CHD incidence in high-SDI region during this period was largely due to an increase in ventricular septal defect and atrial septal defect (AAPC = 0.14, 95%CI: 0.10, 0.18) (Table 2). Regarding CHD subtype, the incidence rate of single ventricle and single ventricle pathway congenital heart anomalies was declined in all 5 SDI regions, and a decreasing trend in severe congenital heart anomalies excluding single ventricle heart defects incidence was found in all SDI regions with the exception of high-middle-SDI region, in which the incidence rate remained stable (AAPC = 0.08, 95%CI: -0.06, 0.22) (Table 2).
At the national level, the highest CHD incidence rates were mostly observed in developing countries located in Africa and Asia, such as Central African Republic (33.8/1000), Somalia (31.9/1000), and Burundi (30.6/1000) (Fig. 3a). The lowest incidence rates were mainly found in developed countries, such as Qatar (6.2/1000), Portugal (6.7/1000), and France (8.6/1000). Additionally, half of the top 10 countries with the lowest CHD incidence were located in Central or South America, including Costa Rica, Panama, Colombia, Venezuela, and Bermuda.
Figure 3.
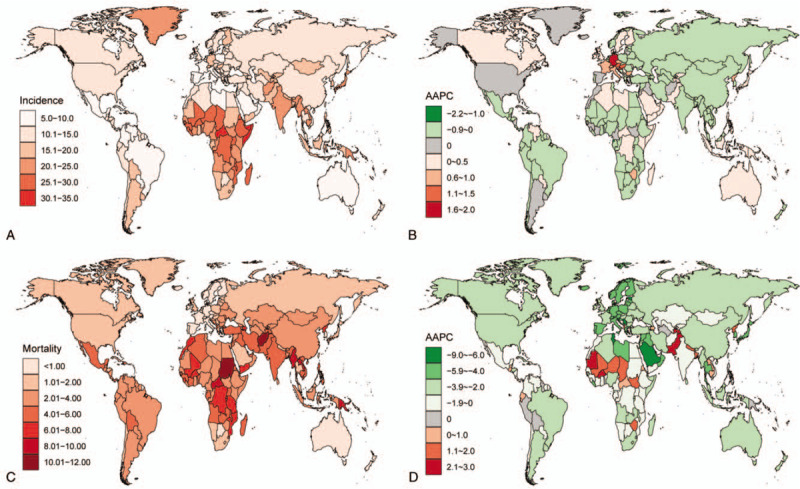
(A) The incidence rate of congenital heart disease (CHD) by location, in 2017. Rate is per 1000 population. (B) The average annual percentage change (AAPC) of CHD incidence rate by location, between 1990 and 2017. (C) The mortality rate of CHD by location, in 2017. Rate is per 100,000 population. (D) The AAPC of CHD mortality rate by location, between 1990 and 2017.
Between 1990 and 2017, a total of 68 countries had a decreased, 96 countries had an increased and 31 countries had a stable CHD incidence rate (Fig. 3B). Countries with the greatest increases were largely located in Western Europe, such as Germany (AAPC = 1.91, 95%CI: 1.75, 2.07), Austria (AAPC = 1.23, 95%CI: 0.91, 1.55), and France (AAPC = 0.80, 95%CI: 0.68, 0.91). The most significant declines were primarily seen in countries in Africa, such as Equatorial Guinea (AAPC = -2.18, 95%CI: -2.49, -1.87), Mozambique (AAPC = -0.78, 95%CI: -0.87, -0.69), and Ethiopia (AAPC = -0.61, 95%CI: -0.76, -0.46).
3.2. The mortality rate and its temporal trend of CHD
Worldwide, the ASMR of CHD declined about 38.1%, from 6.3 per 100,000 population in 1990 to 3.9 per 100,000 population in 2017 (Table 3; Fig. 2B). The ASMR was slightly higher among male than female. Of note, global death due to CHD primarily occurred in children under 5 years, although the ASMR in this age group had decreased by 1.92% (95%CI: 1.76%, 2.08%) per year from 1990 to 2017.
Table 3.
The death number and mortality rate of congenital heart disease between 1990 and 2017.
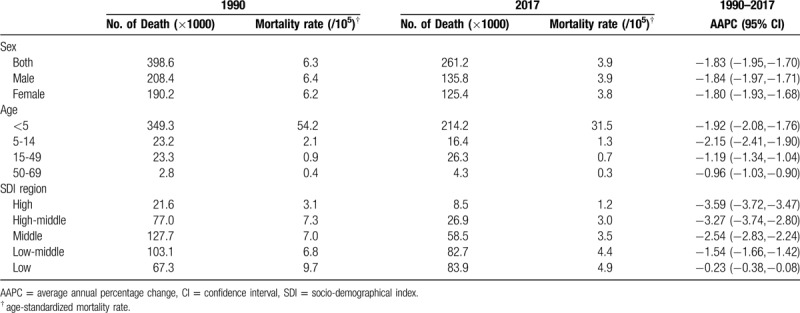
Significant differences of CHD ASMR between SDI level were found in 2017, with the highest ASMR in low-SDI region (4.9/100,000), followed by low-middle-SDI region (4.4/100,000), middle-SDI region (3.5/100,000), high-middle-SDI region (3.0/100,000), and high-SDI region (1.2/100,000) (Table 3; Fig. 2B). At all levels of SDI and for all age groups, the ASMR of CHD exhibited a sharp drop between 1990 and 2017. Indeed, the degree of decrease was positively correlated with SDI level. The most pronounced decrease was seen in high-SDI region (AAPC = -3.59, 95%CI: -3.72, -3.47).
We also recorded a high degree of heterogeneity at the national level (Fig. 3C). The highest CHD ASMRs were mostly observed in developing countries located in Africa and Asia, such as Sudan (18.3/100,000) and Afghanistan (17.0/100,000) in 2017. On the contrary, in certain developed countries located in Europe and North America, the ASMRs of CHD were below 1.0/100,000. And the top 10 countries with the lowest ASMR were all in Europe.
Between 1990 and 2017, a total of 27 countries had an increase, 158 countries had a decrease, and 10 countries had a stable CHD ASMR (Fig. 3D). The greatest increases were detected in developing countries, such as Dominica (AAPC = 2.87, 95%CI: 2.54, 3.20) and Pakistan (AAPC = 2.47, 95%CI: 2.24, 2.70). More than 80% of countries experienced a decrease in ASMR over the last 3 decades. The decline was more prominent in developed countries (Fig. 3D). The fastest decline was found in Serbia (AAPC = -8.06, 95%CI: -8.77, -7.34), followed by Maldives and Saudi Arabia.
3.3. The association between CHD incidence and CHD mortality
We detected a significant positive correlation between CHD incidence and CHD mortality in both 1990 and 2017, by SDI (Fig. 4). Generally, countries with higher CHD incidence occurred more CHD deaths. This phenomenon was more evident in 1990 and in developing countries. In 2017, we found a disequilibrium between CHD incidence and CHD mortality, especially in most developed countries. In these countries, the CHD mortality rates were at a low level, although the CHD incidence rates might be at a medium level. For example, countries with high SDI were lower in CHD mortality than countries with high-middle SDI, albeit the CHD incidences were similar.
Figure 4.
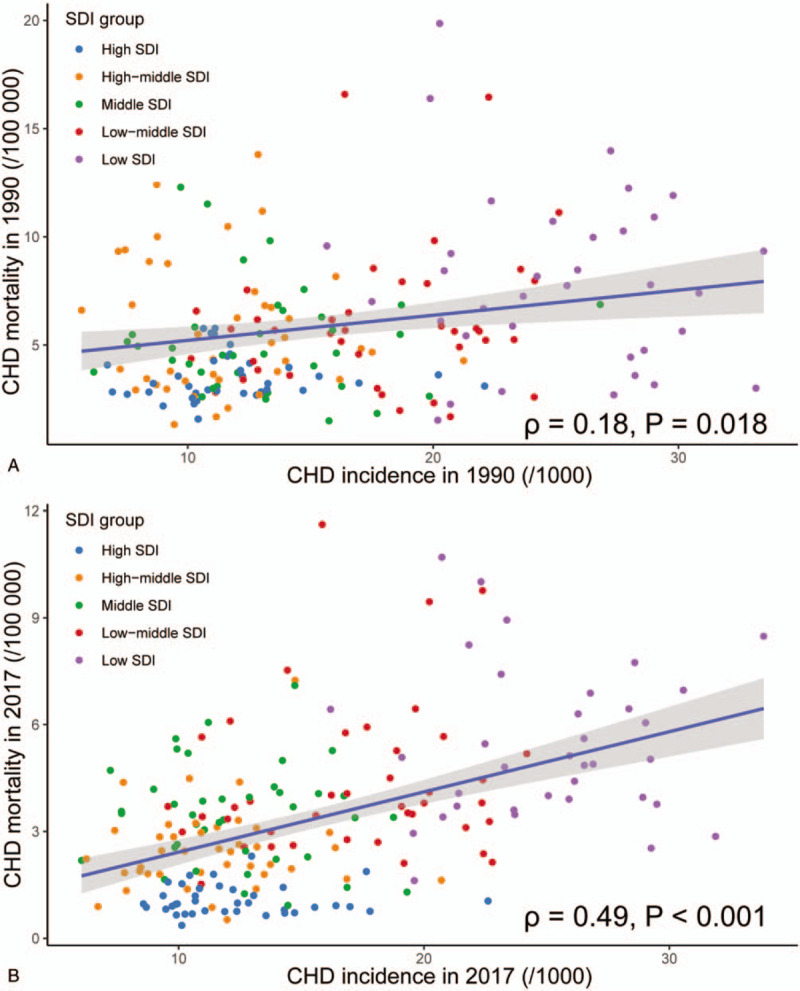
The association between congenital heart disease (CHD) incidence and CHD mortality. The CHD mortality rates were positively related to the CHD incidence rate in both 1990 and 2017, by socio-demographical index (SDI) region.
4. Discussion
CHD is 1 of the most common birth defects, and there has been an increase in the prevalence of CHD over the past decade with improvement in CHD screening and pediatric care.[1,2,13] The incidence of CHD is considered to vary significantly with the definition, population, and research methods.[2,4,5] In our study, we found that the CHD incidence remained stable in both males and females from 1990 through 2017 at the global level. This result might suggest that there is little improvement in CHD prevention over the last 3 decades and highlighted the importance of etiological studies. Although the underlying causes of CHD are still obscure, most cases of CHD are multifactorial and caused by both genetic and environmental factors.[4,14] Epidemiologic studies strongly suggest genetic factors as the predominant cause.[15] For example, aneuploidies were the first identified genetic causes of CHD. Also, copy number variations (CNVs) have been proposed as risk factors for CHD.[14] Only about 2% of all cases of CHD can be attributed to known environmental factors.[16,17] Maternal diabetes mellitus and phenylketonuria are well-known risk factors for CHD.[18–21] Other proposed risk factors include maternal obesity, rubella infection, alcohol consumption, febrile illnesses, drug abuse, e.g. thalidomide and retinoic acid, and exposure to organic solvents.[22–26] With these issues in mind, the prevention of CHD still has a long way to go, and the early screening for those at high risk is therefore critical for the reduction in CHD burden.[27] Screening strategies to detect CHD include antenatal ultrasound, physical examination of the newborn, and pulse oximetry.[27] Of note, the implementation of screening may drive an increase in the incidence of CHD.[2] In our study, we found an unexpected mild increase in CHD incidence in high-SDI region, such as Germany, France, the UK, and Australia. This increase was primarily due to a rose in ventricular septal defect and atrial septal defect subtype, and was most likely a consequence of the screening program for newborns in this region.[3,28] On the other hand, prenatal screening may lead to a higher termination rate of pregnancies with severe CHD.[29] In our study, a decreasing trend of incidence rates in single ventricle and single ventricle pathway congenital heart anomalies subtype and severe congenital heart anomalies excluding single ventricle heart defects subtype was found in most SDI regions. The driving force underlying the decrease may be an increase in termination rate following a prenatal diagnosis of severe CHD.
CHD is the leading cause of infant death due to congenital defects. Several studies have shown a dramatic reduction in mortality and morbidity over the past century due to improvements in surgery, anesthesia and pediatric care.[30–32] In our research, we found the mortality of CHD experienced a significant decline between 1990 and 2017 at the global level, regardless of sex, age, and SDI region. However, the mortality was substantially heterogeneous across the world, primarily due to the disequilibrium in economics and medical facilities. We found the ASMR of CHD in low-SDI region (4.9/100,000) was more than 4 times that of high-SDI region in 2017 (1.2/100,000). The highest CHD mortality rates were in developing countries located in Africa and Asia. Moreover, an increasing trend in CHD mortality was mainly observed in countries with non-advanced economy, such as North Korea and Pakistan. In contrast, CHD mortality in developed countries experienced a dramatic decrease during the same period as the previous studies reported.[33,34] This inequality suggested that more attention and priority should be placed on these less developed regions to alleviate the burden of CHD worldwide. Additionally, more than 80% of CHD related deaths occurred in children aged under 5 years, which emphasized the importance of prevention and early screening for CHD again. Since the fairly low detection rate of the current screening tools for isolated congenital heart defects, the development of more advanced and stable screening methods deserves more priority in future studies.
The limitations of our study should be noted. First, we used the data from GBD dataset, which might subject to the miscoding. We are unable to estimate the degree of overreporting and underreporting in our data. However, the accurate estimation of the CHD burden is hard due to the scarcity in validated data sources, particularly in developing countries. The GBD dataset provides a unique opportunity for us to learn the global epidemiology of CHD. Second, as we described in the methods section, CHD comprises several sub-cause categories. In the current study, we cannot tease out all categories and then analyzed them individually due to the integrated data in the GBD database. As a result, a more precise and targeted estimation for CHD is needed.
In conclusion, in the current study, we comprehensively described the global distribution of CHD incidence and mortality and analyzed their temporal trends from 1990 to 2017. We found that the CHD incidence was relatively high in developing countries located in Africa and Asia, while low in most developed countries. The CHD incidence remained stable at the global level, whereas increased in particular developed countries. For CHD mortality, the relatively high rate was found in countries with higher CHD incidence. We also found a ubiquitous decrease in CHD mortality worldwide, regardless of sex, age, and region. The most pronounced decline was found in developed countries. Our results further describe the global landscape of CHD and are of importance for the understanding the CHD epidemiology worldwide.
Acknowledgments
We would like to thank the GBD Collaborative Network for the use of the data in the GBD study 2017 online database.
Author contributions
Conceptualization: Weiliang Wu, Jinxian He, Xiaobo Shao.
Data curation: Weiliang Wu,Jinxian He, Xiaobo Shao.
Methodology: Weiliang Wu,Jinxian He.
Software: Jinxian He.
Writing – original draft: Weiliang Wu, Xiaobo Shao.
Writing – review & editing: Weiliang Wu, Xiaobo Shao.
Supplementary Material
Footnotes
Abbreviations: AAPC = average annual percentage change, ASMR = age-standardized mortality rate, CHD = congenital heart disease, GBD = global burden of disease, SDI = socio-demographical index.
How to cite this article: Wu W, He J, Shao X. Incidence and mortality trend of congenital heart disease at the global, regional, and national level, 1990–2017. Medicine. 2020;99:23(e20593).
The data used to support the findings of this study can be downloaded from the Global Burden of Disease (GBD) study 2017 online database (available at http://ghdx.healthdata.org/gbd-results-tool).
The authors have no conflicts of interest to disclose.
Supplemental Digital content is available for this article.
The datasets generated during and/or analyzed during the current study are publicly available.
References
- [1].Bouma BJ, Mulder BJ. Changing landscape of congenital heart disease. Circ Res 2017;120:908–22. [DOI] [PubMed] [Google Scholar]
- [2].van der Linde D, Konings EE, Slager MA, et al. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol 2011;58:2241–7. [DOI] [PubMed] [Google Scholar]
- [3].Liu Y, Chen S, Zuhlke L, et al. Global birth prevalence of congenital heart defects 1970-2017: updated systematic review and meta-analysis of 260 studies. Int J Epidemiol 2019;48:455–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].van der Bom T, Zomer AC, Zwinderman AH, et al. The changing epidemiology of congenital heart disease. Nat Rev Cardiol 2011;8:50–60. [DOI] [PubMed] [Google Scholar]
- [5].Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet (London, England) 2018;392:1736–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Go AS, Mozaffarian D, Roger VL, et al. Executive summary: heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation 2014;129:399–410. [DOI] [PubMed] [Google Scholar]
- [7].Blalock A, Taussig HB. Landmark article May 19, 1945: The surgical treatment of malformations of the heart in which there is pulmonary stenosis or pulmonary atresia. By Alfred Blalock and Helen B. Taussig. JAMA 1984;251:2123–38. [DOI] [PubMed] [Google Scholar]
- [8].Leirgul E, Fomina T, Brodwall K, et al. Birth prevalence of congenital heart defects in Norway 1994-2009--a nationwide study. Am Heart J 2014;168:956–64. [DOI] [PubMed] [Google Scholar]
- [9].Global Burden of Disease Collaborative Network. Global Burden of Disease Study 2017 (GBD 2017) Results. Seattle, United States: Institute for Health Metrics and Evaluation (IHME), 2018. Available at: http://ghdx.healthdata.org/gbd-results-tool. [Google Scholar]
- [10].Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet (London, England) 2018;392:1789–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Jain NB, Ayers GD, Peterson EN, et al. Traumatic spinal cord injury in the United States, 1993-2012. JAMA 2015;313:2236–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gao S, Yang WS, Bray F, et al. Declining rates of hepatocellular carcinoma in urban Shanghai: incidence trends in 1976-2005. Eur J Epidemiol 2012;27:39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Marelli AJ, Ionescu-Ittu R, Mackie AS, et al. Lifetime prevalence of congenital heart disease in the general population from 2000 to 2010. Circulation 2014;130:749–56. [DOI] [PubMed] [Google Scholar]
- [14].Zaidi S, Brueckner M. Genetics and genomics of congenital heart disease. Circ Res 2017;120:923–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gelb BD, Chung WK. Complex genetics and the etiology of human congenital heart disease. Cold Spring Harb Perspect Med 2014;4:a013953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kuciene R, Dulskiene V. Selected environmental risk factors and congenital heart defects. Medicina (Kaunas, Lithuania) 2008;44:827–32. [PubMed] [Google Scholar]
- [17].Simeone RM, Tinker SC, Gilboa SM, et al. Proportion of selected congenital heart defects attributable to recognized risk factors. Ann Epidemiol 2016;26:838–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Correa A. Pregestational diabetes mellitus and congenital heart defects. Circulation 2016;133:2219–21. [DOI] [PubMed] [Google Scholar]
- [19].Engineer A, Saiyin T, Lu X, et al. Sapropterin treatment prevents congenital heart defects induced by pregestational diabetes mellitus in mice. J Am Heart Assoc 2018;7:e009624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Levy HL. Congenital heart disease in maternal PKU. Mol Genet Metab 2012;107:648–9. [DOI] [PubMed] [Google Scholar]
- [21].Rouse B, Matalon R, Koch R, et al. Maternal phenylketonuria syndrome: congenital heart defects, microcephaly, and developmental outcomes. J Pediatr 2000;136:57–61. [DOI] [PubMed] [Google Scholar]
- [22].Botto LD, Panichello JD, Browne ML, et al. Congenital heart defects after maternal fever. Am J Obstet Gynecol 2014;210:359.e351–11. [DOI] [PubMed] [Google Scholar]
- [23].Gilboa SM, Desrosiers TA, Lawson C, et al. Association between maternal occupational exposure to organic solvents and congenital heart defects, National Birth Defects Prevention Study, 1997-2002. Occup Environ Med 2012;69:628–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lazar M, Perelygina L, Martines R, et al. Immunolocalization and distribution of rubella antigen in fatal congenital rubella syndrome. EBioMedicine 2016;3:86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Persson M, Razaz N, Edstedt Bonamy AK, et al. Maternal overweight and obesity and risk of congenital heart defects. J Am Coll Cardiol 2019;73:44–53. [DOI] [PubMed] [Google Scholar]
- [26].Yang J, Qiu H, Qu P, et al. Prenatal alcohol exposure and congenital heart defects: a meta-analysis. PloS One 2015;10:e0130681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Thangaratinam S, Brown K, Zamora J, et al. Pulse oximetry screening for critical congenital heart defects in asymptomatic newborn babies: a systematic review and meta-analysis. Lancet (London, England) 2012;379:2459–64. [DOI] [PubMed] [Google Scholar]
- [28].Cox AT, Boos CJ, Sharma S. The Hearts of Heroes: the epidemiology of cardiac disease in the UK Armed Forces. J R Army Med Corps 2015;161:169–72. [DOI] [PubMed] [Google Scholar]
- [29].Jicinska H, Vlasin P, Jicinsky M, et al. Does first-trimester screening modify the natural history of congenital heart disease? Analysis of outcome of regional cardiac screening at 2 different time periods. Circulation 2017;135:1045–55. [DOI] [PubMed] [Google Scholar]
- [30].Boneva RS, Botto LD, Moore CA, et al. Mortality associated with congenital heart defects in the United States: trends and racial disparities, 1979-1997. Circulation 2001;103:2376–81. [DOI] [PubMed] [Google Scholar]
- [31].Erikssen G, Liestol K, Seem E, et al. Achievements in congenital heart defect surgery: a prospective, 40-year study of 7038 patients. Circulation 2015;131:337–46. [DOI] [PubMed] [Google Scholar]
- [32].Moons P, Bovijn L, Budts W, et al. Temporal trends in survival to adulthood among patients born with congenital heart disease from 1970 to 1992 in Belgium. Circulation 2010;122:2264–72. [DOI] [PubMed] [Google Scholar]
- [33].Jortveit J, Oyen N, Leirgul E, et al. Trends in mortality of congenital heart defects. Congenit Heart Dis 2016;11:160–8. [DOI] [PubMed] [Google Scholar]
- [34].Mandalenakis Z, Rosengren A, Skoglund K, et al. Survivorship in children and young adults with congenital heart disease in Sweden. JAMA Intern Med 2017;177:224–30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


