Abstract
Background:
Incarcerated groin hernia (IGH) is a common surgical emergency. However, there are few accurate and applicable predictors for differentiating patients with strangulated groin hernia from those with IGH. In this study, we aimed to identify the independent risk factors for bowel resection in patients with IGH.
Methods:
We retrospectively collected 323 patients who underwent emergency hernia repair surgery for IGH between January 2010 and October 2019. The patients were categorized into those who received bowel resection and those who did not require bowel resection. The receiver-operating characteristic curve was used to identify the best cutoff values for continuous variables. Following this, univariate and multivariate analyses were performed to identify potential risk factors for bowel resection in these patients.
Results:
Univariate analysis identified 6 variables that were significantly associated with bowel resection among patients with IGH. On multivariate analysis, neutrophil-to-lymphocyte ratio (NLR) (odds ratio [OR] = 3.362, 95% confidence interval [CI] 1.705–6.628, P = .000) and bowel obstruction (OR = 3.191, 95% CI 1.873–5.437, P = 0.000) were identified as independent risk factors for bowel resection among patients with IGH.
Conclusion:
In this study, an elevated NLR and those with bowel obstruction are associated with an increased risk of bowel resection among patients with IGH. Based on our findings, surgeons should prioritize prompt emergency surgical repair for patients who present with elevated NLR and bowel obstruction concurrent with IGH.
Keywords: bowel resection, incarcerated groin hernia, risk factors
1. Introduction
Groin hernia repair is a common procedure in general surgery; although groin hernias should be electively repaired to avoid complications, delayed diagnosis or repair of groin hernias often result in incarceration, defined as irreducibility of the hernia.[1] Incarcerated groin hernias (IGH) are one of the most frequent causes of acute abdomen, and approximately 5% to 15% patients with IGH require emergency surgery because of bowel incarceration or bowel strangulation, defined by objective evidence of ischemia.[2,3] Moreover, due to the difficulties in clinical diagnosis, approximately 15% to 36% patients with IGH develop strangulated groin hernia (SGH),[4–9] requiring prompt emergency hernioplasty with bowel resection, which has a higher morbidity (21%–39%) and mortality (4%–5%) rate than those reported for elective hernioplasty surgery.[5,8,10,11] Early evaluation of bowel resection risk and timely surgery are critical for the outcomes in IGH patients.
Several risk factors have been reported for bowel resection, for example, female sex, older age, delayed hospitalization, severe comorbidities, femoral hernia, signs and symptoms of peritonitis, bowel obstruction, white blood cell count, neutrophilic leukocyte count, and poor American Society of Anesthesiologists physical status classification.[6,8,11–13] However, previous research has determined these risk factors with limited sample size and without multivariate regression analysis, and thus may include confounding factors. This study aimed to conduct univariate and multivariate analyses to identify risk factors for bowel resection in 323 patients with IGH at a single institution, to provide further evidence for the clinical management of IGH.
2. Methods
2.1. Patient selection and data collection
We retrospectively collected data regarding 323 patients who underwent emergency hernia surgery for IGH from January 2010 to October 2019 at the West China Hospital in Chengdu, China. All data were collected from medical records of patient admitted during the study period and the following information was extracted: patient registration number, sex, age, duration of incarceration, admission season, hernia type, presence of bowel obstruction symptoms, history of previous abdominal surgery, presence of recurrent hernia, presence of concomitant disease, CT scan findings, bowel resection (yes/no) during hernioplasty, and preoperative hematological inflammatory markers such as white blood cell count, neutrophilic leukocyte count, neutrophil-to-lymphocyte ratio (NLR). This study was conducted in accordance with the standards of the Helsinki Declaration and current ethical guidelines and was approved by the Biomedical Ethics Committee of West China Hospital of Sichuan University. All patients were waived of informed consents due to the retrospective nature of this study.
The diagnosis of bowel obstruction was established when CT showed multiple stepwise air-fluid levels and dilated bowel loops. Duration of incarceration referred to the period from when the mass became irreducible to the time of hospitalization. Season of admission was defined as autumn and winter from September 23rd of 1 year to March 21st of the next year and spring and summer as the period from March 22nd to September 22nd. Concomitant diseases included chronic obstructive pulmonary disease, history of multiple pregnancies, liver cirrhosis or liver cancer, and benign prostatic hyperplasia.
2.2. Statistical analysis
ROC curve analysis was used to determine the best cutoff value for continuous variables such as age, duration of incarceration, pre-operative white blood cell count, preoperative neutrophilic leukocyte count, and preoperative NLR. The optimal cutoff value was selected according to the maximum value of Youden index.[14] The optimal cutoff value for NLR was calculated to be 6.5. The cutoff values for age, duration of incarceration, preoperative white blood cell count, and preoperative neutrophilic leukocyte count were 57 years, 13 hours, 8.5 × 103 cells/mm3, and 7 × 103/mm3, respectively.
χ2 test was used to compare categorical variables. Continuous data are presented as mean ± standard deviation (SD) or median (range). Comparisons of normal and non-normal continuous variables were conducted by Student t test and Mann–Whitney U test, respectively. Univariate and multivariate logistic regression analyses were used to explore risk factors for bowel resection in patients with IGH. All statistical analyses were performed using SPSS version 24.0 (SPSS, Inc, Chicago, IL). A 2-tailed P value of <.05 was considered significant.
3. Results
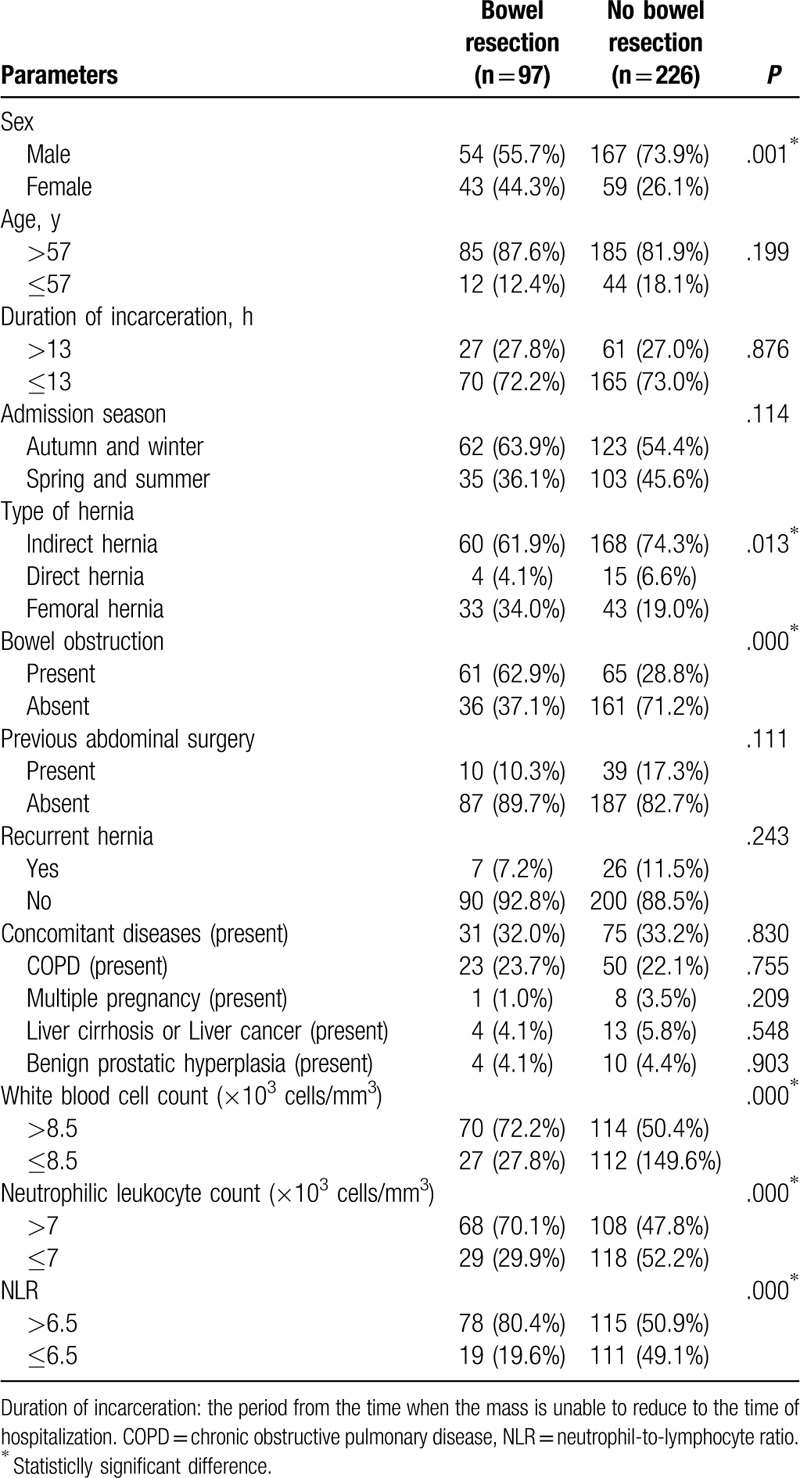
A total of 323 patients with IGH were included in this study (221 males [68.4%] and 102 females [31.6%]), with a median age of 73 (14–98) years. The patients were categorized into 2 groups: those who received bowel resection and those who did not require bowel resection during hernioplasty, as determined by clinical judgment of bowel ischemia during the operation. In total, 97 patients (30.0%) underwent bowel resection surgery and 226 (70.0%) did not. We used traditional non-mesh tissue repair techniques for hernioplasty such as Bassini or Shouldice procedure for inguinal hernia and McVay procedure for femoral hernia in patients with and without necrosis of hernia content, as demonstrated in Figure 1.[15,16] Abdominal CT scanning was done in all patients and showed the following findings:126 cases with multiple stepwise air-fluid levels and dilated bowel loops, 123 patients with thickening of bowel wall, 50 patients with ascites, and 8 patients with free intraperitoneal air. The proportion of patients undergoing bowel resection was 24.4% (54/221) in males and 42.2% (43/102) in females, both of which were statistically significant according to univariable analysis (P = .001). In addition, we found significant differences between the 2 groups of patients regarding rates of bowel obstruction, preoperative white blood cell count, preoperative neutrophilic leukocyte count, and preoperative NLR (P < .05)(Table 1).
Figure 1.
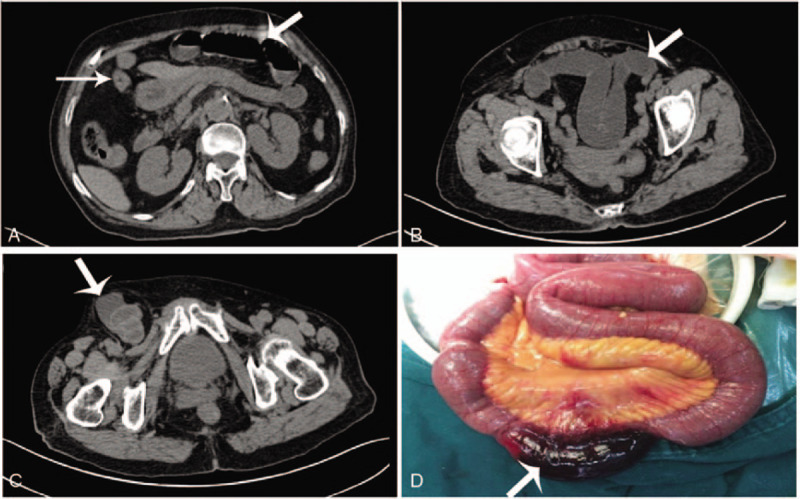
Description of emergency surgery of IGH. This was an 84-year-old female patient whose complaint was “groin pain, vomiting, and abdominal distension sustained for 14 hours” and diagnosed as incarcerated femoral hernia with bowel obstruction. (A–C) Horizontal view of abdominal CT scan. (D) Surgical exploration through the right inguinal incision. The white arrow in A and B shows the patients had bowel obstruction. The white arrow in C indicates the strangulated femoral hernia in the right-side groin area. The white arrow in D indicates the necrotic incarcerated small bowel. IGH = incarcerated groin hernia.
Table 1.
Univariate analysis of the factors (categorical variables) affecting bowel resection in incarcerated groin hernia.
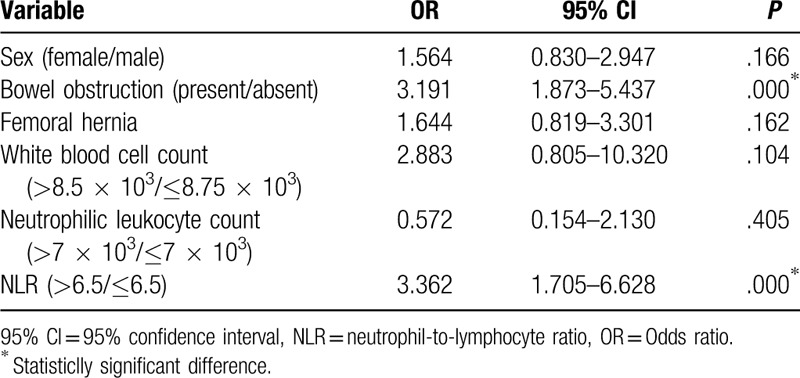
A comparison of the continuous variables between the 2 groups showed that duration of incarceration, preoperative white blood cell count, preoperative neutrophilic leukocyte count, and preoperative NLR were all significantly higher in the patients with bowel resection than in patients without bowel resection (P < .05) (Table 2). The multivariate logistic regression analysis showed that NLR (odds ratio [OR] = 3.362, 95% confidence interval [CI] 1.705–6.628, P = .000) and bowel obstruction (OR = 3.191, 95% CI 1.873–5.437, P = .000) were independent risk factors for bowel resection in patients with IGH (Table 3).
Table 2.
Univariate analysis of the factors (continuous variables) affecting bowel resection in incarcerated groin hernia.
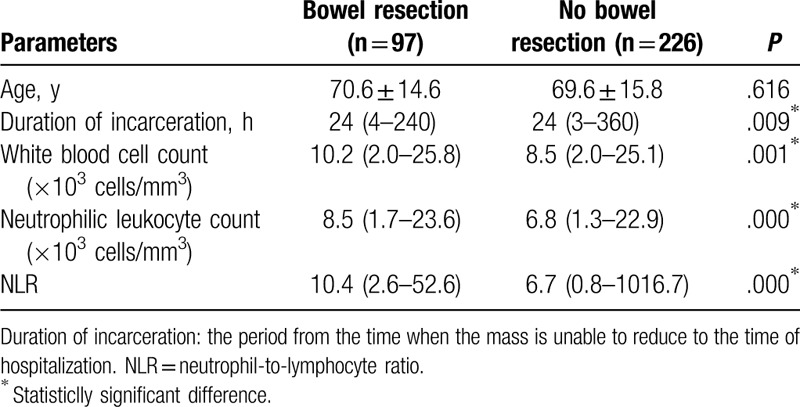
Table 3.
Binary logistic regression analysis of independent risk factors for bowel resection in incarcerated groin hernia.
4. Discussion
For patients with IGH, if bowel necrosis is confirmed or highly suspected intraoperatively, bowel resection should be performed immediately[8]; the need for bowel resection significantly affects patient prognosis, with increased hospital stay, postoperative complications, and mortality.[4,8,17] Therefore, it is of great clinical value to recognize risk factors which may predict need for bowel resection in patients with IGH. This study identifies risk factors of bowel resection based on multivariable logistic regression analysis results for 323 patients with IGH.
This study revealed that NLR and bowel obstruction were independent risk factors for bowel resection in patients with IGH. First, we found a relationship between an increased NLR and high risk of bowel resection. Moreover, our study revealed that if the NLR >6.5, the probability of bowel necrosis was higher, suggesting that an NLR cutoff value of 6.5 could indicate that emergency surgery should be done immediately. Second, we identified bowel obstruction as an independent risk factor for bowel resection. Similar results were reported by several authors.[6,7] Bowel ischemia caused by bowel obstruction may be responsible for this finding.
Available studies have provided strong evidences that when inflammation induces neutrophils rise and lymphocytes fall, leading to an elevated NLR.[18–21] Previous researches have reported that NLR can be a good and valuable diagnostic indicator of the severity of inflammation. Ishizuka et al[10] found that NLR (≤8/>8) appeal to be more sensitive and valuable than white blood cell count, neutrophil count, and C-reactive protein (CRP). Kahramanca et al[22] reported that an NLR of 4.68 can aid to diagnose acute appendicitis and an NLR of 5.74 can differentiate between simple and complicated appendicitis. Shimizu et al[12] discovered that NLR (<5/>5) may be more accurate and useful than the white blood cell count or CRP for diagnosis of severe appendicitis. In addition, Lee et al[23] reported that preoperative NLR ≥3.0 was significantly associated with severe cholecystitis in patients undergoing cholecystectomy. In our study, we found that NLR (≤6.5/>6.5) was the independent risk factor for bowel resection in patients with IGH. When incarcerated groin hernia is associated with bowel necrosis, which can lead to severe inflammatory response and an elevated NLR.
Currently, there are few reports on the relationship between NLR and bowel resection. Zhou et al[24] found that NLR (≤6.5/>6.5) was more closely associated with SGH than white blood cell count and neutrophils with higher sensitivity and specificity (0.75 and 0.689, respectively). Xie et al[25] reported that NLR (<11.5/≥11.5) could be used as the best predictor for diagnosing SGH. Koksal et al[26] found that the risk of bowel necrosis was significantly higher when the NLR increased to 7.72 ± 5.69 (P = .019) and failed to give a cutoff value of NLR. However, the sample size included in these 3 studies was relatively small, whereas the total number of samples included in our study was 323 and our results might be more reliable. We discovered that NLR (≤6.5/>6.5) can be used as a good predictor for diagnosing bowel resection in patients with IGH. Still, a prospective cohort study is needed to evaluate NLR value in the diagnosis of bowel resection in patients with IGH.
Previous research has found statistically significant higher rates of bowel ischemia for females with IGH.[6,8,27–30] However, other studies have argued that sex is not a risk factor for bowel resection in patients with IGH.[4,7,9,25,31] Our results revealed that bowel resection occurred more often in female IGH patients than that in male patients with IGH; however, female sex was not an independent risk factor of bowel resection among patients with IGH according to our multivariable logistic regression analysis.
Although via multivariable regression analysis, our study found that age is not an independent risk factor for bowel resection, there has been significant debate on this issue. Previous research studies have found that advanced age was a predictor of bowel resection in patients with IGH,[4,9,25,30] with Kurt et al's finding that the rate of bowel resection in IGH patients older than 65 years was significantly higher than that in patients aged ≤65 years.[8] Possible explanations include atypical signs and symptoms for elderly presentations of IGH, leading to delays in treatment and operation. Other studies have shown that advanced age was not a risk factor for bowel resection in patients with IGH.[6–8,27,28,31]
There is also significant debate regarding duration of incarceration as a risk factor for bowel ischemia and subsequent bowel resection for patients with IGH.[4,6,7,25,27,31] Our findings found late hospitalization to be a risk factor for bowel resection in patients with IGH in the analysis of continuous variables, but analysis of categorical variables revealed that duration of incarceration was not a risk factor for bowel resection in patients with IGH. This variable may be difficult to analyze, as patients may not remember the exact hour of onset for incarceration.
In addition, many studies have shown that femoral hernia is a risk factor for bowel resection in patients with IGH.[4,6,8,9,27,28,30] Femoral hernias account for only 2.3% of elective hernias,[32] but are usually present as incarcerated or strangulated with evidence of bowel necrosis, leading to severe morbidity and mortality.[12,29,33,34] Anatomic reasons for the high incidences of incarceration may include the hard lacunar ligament, long and narrow femoral canal, and the structure of the femoral canal with small upper and large lower orifices. However, our study showed that although incarcerated femoral hernia patients were more susceptible to bowel resection, femoral hernia was not an independent risk factor for bowel resection. Additionally, the differences in the admission season, presence of previous abdominal surgery, presence of recurrent hernia, and presence of concomitant disease were not statistically significant between the 2 groups in our study.
Lastly, there are inconsistent results regarding the predictive effects of white blood cell count and neutrophilic leukocyte count for bowel necrosis in patients with IGH. The results of Koizumi et al[7] show that there was no statistically significant difference in white blood cell count and neutrophilic leukocyte count between the bowel resection group and no bowel resection group. However, Ge et al[6] found that high levels of hematological markers such as white blood cell count and neutrophilic leukocyte count were associated with an increased risk of bowel resection. In our study, univariate analysis suggested that increased preoperative white blood cell count and preoperative neutrophilic leukocyte count were risk factors associated with bowel necrosis in IGH patients. Although multivariate analysis suggested that the above 2 factors were not independent predictors of bowel resection, the 2 indicators may help clinicians diagnose SGH.
The principal limitation of our study is that this was not a large-scale, multicenter prospective study. Due to the retrospective nature of our study, patient selection bias is inevitable, and future studies with large population samples and prospective study design are necessary for a better assessment of the risk factors discussed above.
5. Conclusions
An elevated NLR >6.5 and bowel obstruction are independent risk factors for bowel resection among patients with IGH. Surgeons should pay more attention on these patients and perform surgery promptly.
Acknowledgments
The authors thank Wordvice Company and Bonnie O. Wong (School of Medicine, Stanford University, 291 Campus Drive, Stanford, California, 94305, USA) for their assistance of language editing.
Author contributions
Conceptualization: Lie Yang, Yong Wang.
Data curation: Peng Chen, Wenming Yang, Jianhao Zhang.
Formal analysis: Lie Yang, Yong Wang.
Funding acquisition: Lie Yang.
Methodology: Peng Chen, Wenming Yang, Lie Yang, Yong Wang.
Project administration: Lie Yang.
Software: Peng Chen, Wenming Yang, Jianhao Zhang.
Supervision: Lie Yang, Yong Wang.
Writing – original draft: Peng Chen.
Writing – review & editing: Lie Yang, Yong Wang, Cun Wang, Yongyang Yu, Zongguang Zhou.
Footnotes
Abbreviations: BPH = benign prostatic hyperplasia, CI = confidence interval, COPD = chronic obstructive pulmonary disease, CRP = C-reactive protein, CT = computed tomography, IGH = incarcerated groin hernia, NLR = neutrophil-to-lymphocyte ratio, OR = odds ratio, ROC = receiver-operating characteristic, SD = standard deviation, SGH = strangulated groin hernia.
How to cite this article: Chen P, Yang W, Zhang J, Wang C, Yu Y, Wang Y, Yang L, Zhou Z. Analysis of risk factors associated bowel resection in patients with incarcerated groin hernia. Medicine. 2020;99:23(e20629).
PC, WY, and JZ contributed equally to this work, and above 3 authors should be considered first author.
This study was supported by National Natural Science Foundation of China (grant numbers 81472304); National Key Research and Development Program of China (grant numbers 2016YFC0906000 [2016YFC0906003]); Sichuan Science and Technology Program (grant numbers 2017JY0020); and 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University.
The authors report no conflicts of interest.
The datasets generated during and/or analyzed during the current study are publicly available.
References
- [1].Bay-Nielsen M, Kehlet H, Strand L, et al. Quality assessment of 26 304 herniorrhaphies in Denmark: a prospective nationwide study. Lancet 2001;358:0–1128. [DOI] [PubMed] [Google Scholar]
- [2].Dai W, Chen Z, Zuo J, et al. Risk factors of postoperative complications after emergency repair of incarcerated groin hernia for adult patients: a retrospective cohort study. Hernia 2019;23:267–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kulah B, Duzgun AP, Moran M, et al. Emergency hernia repairs in elderly patients. Am J Surg 2001;182:455–9. [DOI] [PubMed] [Google Scholar]
- [4].Abd Ellatif ME, Negm A, Elmorsy G, et al. Feasibility of mesh repair for strangulated abdominal wall hernias. Int J Surg 2012;10:153–6. [DOI] [PubMed] [Google Scholar]
- [5].Derici H, Unalp HR, Bozdag AD, et al. Factors affecting morbidity and mortality in incarcerated abdominal wall hernias. Hernia 2007;11:341–6. [DOI] [PubMed] [Google Scholar]
- [6].Ge BJ, Huang Q, Liu LM, et al. Risk factors for bowel resection and outcome in patients with incarcerated groin hernias. Hernia 2010;14:259–64. [DOI] [PubMed] [Google Scholar]
- [7].Koizumi M, Sata N, Kaneda Y, et al. Optimal timeline for emergency surgery in patients with strangulated groin hernias. Hernia 2014;18:845–8. [DOI] [PubMed] [Google Scholar]
- [8].Kurt N, Oncel M, Ozkan Z, et al. Risk and outcome of bowel resection in patients with incarcerated groin hernias: retrospective study. World J Surg 2003;27:741–3. [DOI] [PubMed] [Google Scholar]
- [9].Sawayama H, Kanemitsu K, Okuma T, et al. Safety of polypropylene mesh for incarcerated groin and obturator hernias: a retrospective study of 110 patients. Hernia 2014;18:399–406. [DOI] [PubMed] [Google Scholar]
- [10].Bessa SS, Abdel-fattah MR, Al-Sayes IA, et al. Results of prosthetic mesh repair in the emergency management of the acutely incarcerated and/or strangulated groin hernias: a 10-year study. Hernia 2015;19:909–14. [DOI] [PubMed] [Google Scholar]
- [11].Venara A, Hubner M, Le Naoures P, et al. Surgery for incarcerated hernia: short-term outcome with or without mesh. Langenbecks Arch Surg 2014;399:571–7. [DOI] [PubMed] [Google Scholar]
- [12].Alimoglu O, Kaya B, Okan I, et al. Femoral hernia: a review of 83 cases. Hernia 2006;10:70–3. [DOI] [PubMed] [Google Scholar]
- [13].Chen P, Huang L, Yang W, et al. Risk factors for bowel resection among patients with incarcerated groin hernias: A meta-analysis. Am J Emerg Med 2020;38:376–83. [DOI] [PubMed] [Google Scholar]
- [14].Youden WJ. Index for rating diagnostic tests. Cancer 1950;3:32–5. [DOI] [PubMed] [Google Scholar]
- [15].Compagna R, Rossi R, Fappiano F, et al. Emergency groin hernia repair: implications in elderly. BMC Surg 2013;13: suppl 2: S29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Elsebae M. Tension-free repair versus Bassini technique for strangulated inguinal hernia: A controlled randomized Study. Int J Surg 2008;6:302–5. [DOI] [PubMed] [Google Scholar]
- [17].Kulah B, Polat Duzgun A, Moran M, et al. Emergency hernia repairs in elderly patients. Am J Surg 2001;182:455–9. [DOI] [PubMed] [Google Scholar]
- [18].Kay HD, Smith DL. Regulation of human lymphocyte-mediated natural killer (NK) cell activity. I. Inhibition in vitro by peripheral blood granulocytes. J Immunol 1983;130:475–83. [PubMed] [Google Scholar]
- [19].Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol 2006;6:173–82. [DOI] [PubMed] [Google Scholar]
- [20].Patek PQ, Collins JL, Cohn M. Evidence that cytotoxic T cells and natural cytotoxic cells use different lytic mechanisms to lyse the same targets. Eur J Immunol 1983;13:433–6. [DOI] [PubMed] [Google Scholar]
- [21].Petrie HT, Klassen LW, Kay HD. Inhibition of human cytotoxic T lymphocyte activity in vitro by autologous peripheral blood granulocytes. J Immunol 1985;134:230–4. [PubMed] [Google Scholar]
- [22].Kahramanca S, Ozgehan G, Seker D, et al. Neutrophil-to-lymphocyte ratio as a predictor of acute appendicitis. Ulus Travma Acil Cerrahi Derg 2014;20:19–22. [DOI] [PubMed] [Google Scholar]
- [23].Lee SK, Lee SC, Park JW, et al. The utility of the preoperative neutrophil-to-lymphocyte ratio in predicting severe cholecystitis: a retrospective cohort study. BMC Surg 2014;14:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zhou H, Ruan X, Shao X, et al. Clinical value of the neutrophil/lymphocyte ratio in diagnosing adult strangulated inguinal hernia. Int J Surg 2016;36:76–80. [DOI] [PubMed] [Google Scholar]
- [25].Xie X, Feng S, Tang Z, et al. Neutrophil-to-lymphocyte ratio predicts the severity of incarcerated groin hernia. Med Sci Monit 2017;23:5558–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Koksal H, Ates D, Nazik EE, et al. Predictive value of preoperative neutrophil-to-lymphocyte ratio while detecting bowel resection in hernia with intestinal incarceration. Ulus Travma Acil Cerrahi Derg 2018;24:207–10. [DOI] [PubMed] [Google Scholar]
- [27].Tanaka N, Uchida N, Ogihara H, et al. Clinical study of inguinal and femoral incarcerated hernias. Surg Today 2010;40:1144–7. [DOI] [PubMed] [Google Scholar]
- [28].Alvarez JA, Baldonedo RF, Bear IG, et al. Incarcerated groin hernias in adults: presentation and outcome. Hernia 2004;8:121–6. [DOI] [PubMed] [Google Scholar]
- [29].Kulah B, Kulacoglu IH, Oruc MT, et al. Presentation and outcome of incarcerated external hernias in adults. Am J Surg 2001;181:101–4. [DOI] [PubMed] [Google Scholar]
- [30].Tatar C, Tüzün İS, Karşidağ T, et al. Prosthetic Mesh Repair for Incarcerated Inguinal Hernia. Balkan Med J 2016;33:434–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Atila K, Guler S, Inal A, et al. Prosthetic repair of acutely incarcerated groin hernias: a prospective clinical observational cohort study. Langenbecks Arch Surg 2010;395:563–8. [DOI] [PubMed] [Google Scholar]
- [32].Glassow F. Femoral hernia. Review of 2,105 repairs in a 17 year period. Am J Surg 1985;150:353–6. [DOI] [PubMed] [Google Scholar]
- [33].Alhambra-Rodriguez de Guzmán C, Picazo-Yeste J, Tenías-Burillo JM, et al. Improved outcomes of incarcerated femoral hernia: a multivariate analysis of predictive factors of bowel ischemia and potential impact on postoperative complications. Am J Surg 2013;205:188–93. [DOI] [PubMed] [Google Scholar]
- [34].Oishi SN, Page CP, Schwesinger WH. Complicated presentations of groin hernias. Am J Surg 1991;162:568–71. [DOI] [PubMed] [Google Scholar]


