Supplemental Digital Content is available in the text
Keywords: case-control study, gastric carcinoma (GC), genetic variations, relative telomere length (RTL), single nucleotide polymorphism
Abstract
This study aimed to further understand the role of relative telomere length (RTL) in susceptibility to gastric carcinoma (GC) and investigate the association between genetic polymorphisms in the telomere length related genes and GC risk.
RTL was measured using the real-time quantitative polymerase chain reaction from 1000 patients and 1100 healthy controls. Genotyping was performed using the Agena MassARRAY platform. The statistical analysis was performed using the chi-square/ Welch T tests, Mann–Whitney U test, and logistic regression analysis.
The association analysis of telomere length and GC showed that the RTL in the case group was shorter than in the controls, and the shorter RTL was associated with an increased risk of GC. The association analysis between telomere length related genes polymorphisms and genetic susceptibility to GC indicated that: In the allele models and genetic models, TERT (rs10069690, rs2242652 and rs2853676) and TN1F1 (rs7708392 and rs10036748) were significantly associated with an increased risk of GC. In addition, the haplotype "Grs10069690Crs2242652” of TERT and the haplotype "Grs7708392Trs10036748” of TNIP1 were associated with an increased risk of GC
Our results suggested that shorter RTL was associated with an increased risk of GC; The association analysis have identified that the TERT (rs10069690, rs2242652 and rs2853676) and TN1P1 (rs7708392 and rs10036748) were associated with GC risk.
1. Introduction
Gastric carcinoma (GC), one of the most common human cancers, is a heterogeneous disease with high morbidity and mortality. Although the incidence has been declining in most parts of the world in the last decades, stomach carcinoma remains a prominent cancer worldwide and is responsible for over 1,000,000 new cases in 2018 and an estimated 783,000 deaths, making it the fifth most frequently diagnosed cancer and the third leading cause of cancer death.[1] Smoking, high salt intake and a familial genetic component are also recognized as predisposing factors.[2] Meanwhile, in recent years, many studies have shown that telomere length variation is strongly implicated in the process of carcinogenesis, although the current findings are still in debate.[3,4] Additionally, various genetic and epigenetic alterations are associated with GC.[5,6] Previously, genome-wide association analysis studies have identified many genes involved in gastric carcinogenesis and prognosis.[7]
Telomeres are specific structures located at the ends of eukaryotic chromosomes and are crucial in maintaining chromosome integrity and genomic stability.[8] Telomere length progressively shortens during somatic-cell replication, because of the inability of DNA polymerase to fully replicate the 3′ end of the DNA.[8] Telomere length is determined by the balance of processes that shorten and lengthen the telomere, thus leading to telomere variation in individuals at the same age.[9] The maintenance of telomere length relies on the activity of telomerase, a reverse transcriptase complex that adds DNA sequence repeats (‘TTAGGG’ in all vertebrates) to the 3′ end of DNA strands in the telomere regions.[10] The available evidence suggests that distinct cancer phenotypes are associated with both short and long telomere extremes. Telomeres also shorten in humans with age, and in the past decade, it has become clear that abnormally short telomeres can cause several age-related disease phenotypes.[11] When telomeres become critically short, they activate a dnadeoxyribonucleic acid (DNA) damage response, which provokes cellular senescence or apoptosis.[12] In the past 2 years, mutations that appear to lengthen telomeres have been linked to an increased risk of cancer. Unrestricted proliferation when telomeres are long would increase the likelihood of sustaining driver mutations that eventually promote a cancer clonal advantage and metastasis.[13] Nowadays, little research has been done on telomere length and GC, the association between leukocyte telomere length and GC risk has not yet been assessed. Whether the incidence of GC is related to longer telomeres or shorter telomeres is worthy of systematic exploration.
The activity of telomerase can affect the telomere length, which in turn can affect the incidence of cancer or other diseases.[14] However, telomerase activity and relative telomere length (RTL) can be directly or indirectly affected by many telomere related genes.[15] Genetic association studies have indicated that polymorphisms in the telomerase reverse transcriptase-encoding gene TERT and related genes such as TERC, MYNN, NAF1, TNIP1, STN1, ZNF208, and RTEL1 are associated with the variation of telomere length.[16] However, there are few studies on telomere related genes and genetic susceptibility of GC. Therefore, it is necessary to explore the telomere related genes and the susceptibility of GC.
To identify the associations between telomere length and telomere related genes (TERT, TERC, MYNN, NAF1, TNIP1, STN1, ZNF208, and RTEL1) and GC risk in previous studies, we conduct a case-control study including 1000 cases and 1100 controls to further clarify their potential roles in GC risk in the Chinese population. To the best of our knowledge, this is the first epidemiological study to investigate the role of telomere length and telomere related genes in GC etiology.
2. Materials and methods
2.1. Participants and ethics statement
This case-control study involved 1000 GC patients and 1100 control subjects. All participants were conducted at the People's Hospital of Xinjiang Uygur Autonomous Region, and the healthy controls were the same race as the GC patients. Patients diagnosed with other types of cancer or underwent radiotherapy or chemotherapy were excluded. Healthy control subjects were recruited from the physical examination center at the same hospital. All control patients had no history of cancer. Additionally, healthy subjects were the same race as the GC patients and were age- and sex-matched with GC patients.
All participants were informed, both in writing and verbally, of the procedures and purpose of the study, and each participant signed informed consent document. The protocols for this study were approved by the Ethical Committee of the People's Hospital of Xinjiang Uygur Autonomous Region. All subsequent research analyses were carried out in accordance with the approved guidelines and regulations.
2.2. Dnadeoxyribonucleic acid extraction and relative telomere length measurement
Genomic DNA was isolated from whole-blood samples using the GoldMag-Mini Purification Kit (Gold-Mag Co. Ltd., Xi’an, People's Republic of China), and DNA concentrations were measured using the NanoDrop 2000 (Thermo Scientific, Waltham, MA). RTL was measured using the real-time quantitative polymerase chain reaction (PCR) method as described by Cawthon.[17] Gene-specific amplification was performed in a ViiATM7 Dx Real-Time PCR Instrument (AB). The intra-assay or inter-assay differences were controlled by assaying each sample in 2 to 3 replicates or a calibrator DNA sample in different plates and the acceptable coefficient of variation (CV) was lower than 5% for cycle threshold values. 36B4 on chromosome 12, encoding acidic ribosomal phosphoprotein P0, was used as the single copy gene. All samples for both the telomere and 36B4 gene amplifications were always done in duplicate in separate 96- well plates. The cycle threshold is the number of cycles required for the fluorescent signal to cross the threshold. Ct values generated were used to calculate the telomere (T) repeat copy number to a single gene (S) copy number (T/S ratio) for each sample using the equation: T/S = 2−(ΔCt), (ΔCt = Cttelomere − Ct36B4). The relative ratio of T/S was defined as the ratio of each sample 2−ΔCt to a calibrator DNA 2−ΔCt, 2−(ΔΔCt). The primer sequences are shown in Supplementary Table S1.
2.3. Single-nucleotide polymorphism (SNP) selection and genotyping
In this study, 15 SNPs in TERC, MYNN, NAF1, TNIP1, RTEL1, ZNF208 were selected from the 1000 Genomes Project (http://www.1000genomes.org/) for analysis and each had minor allele frequency >5% in Chinese Han population. The primers were designed online (https://agenacx.com/online-tools/). The PCR primers for each SNP are shown in Supplementary Table S2. Agena MassARRAY Assay Design 3.0 software was used to design a multiplexed SNP Mass EXTENDED assay. Genotyping was performed on an Agena MassARRAY RS1000 platform using the manufacturer's protocol. Data management and analysis were performed using the Agena Typer 4.0 Software.
2.4. Statistical analyses
Pearson test was used to examine differences of categorical variables between different groups. The chi-square test and the Welch T test was used to examine differences of categorical variables and continuous variables between cases and controls, respectively. Mann–Whitney U test was used for RTL comparison between different groups. To evaluate the association between RTL and GC risk, unconditional logistic regression was used to determine odds ratios (ORs) and 95% confidence intervals (CIs). The variable of age and gender were adjusted in multivariate unconditional logistic regression analysis in order to eliminate these residual confounding effects. Statistical analyses were performed using the Microsoft Excel (Microsoft Corp., Redmond, WA) and Statistical Package for the Social Sciences (SPSS) statistics 19.0 version software (SPSS Inc, Chicago, IL). P values <.05 were considered statistically significant.
Allele and genotype frequencies were determined using direct counts. SNP allele frequencies in the controls were tested for departure from Hardy–Weinberg Equilibrium (HWE) before analysis. Allele and genotype frequencies in GC patients and controls were calculated using chi-squared and Fisher exact tests. Associations between SNPs and the risk of GC were tested in genetic models using PLINK software (Version 1.07). Unconditional logistic regression analysis was used to examine the ORs and 95% CIs in order to assess the association between SNPs and GC risk. Four models (co-dominant, dominant, recessive, and log-additive) were used to test the association between SNPs and GC risk. Finally, the Haploview software package (version 4.2) and SHEsis software platform (http://shesisplus.bio-x.cn/SHEsis.html) were used to estimate pairwise linkage disequilibrium (LD), haplotype construction, and genetic association at polymorphism loci. All P values were 2-sided, and P < .05 indicates statistical significance.
3. Results
3.1. Association analysis of telomere length and risk of gastric carcinoma
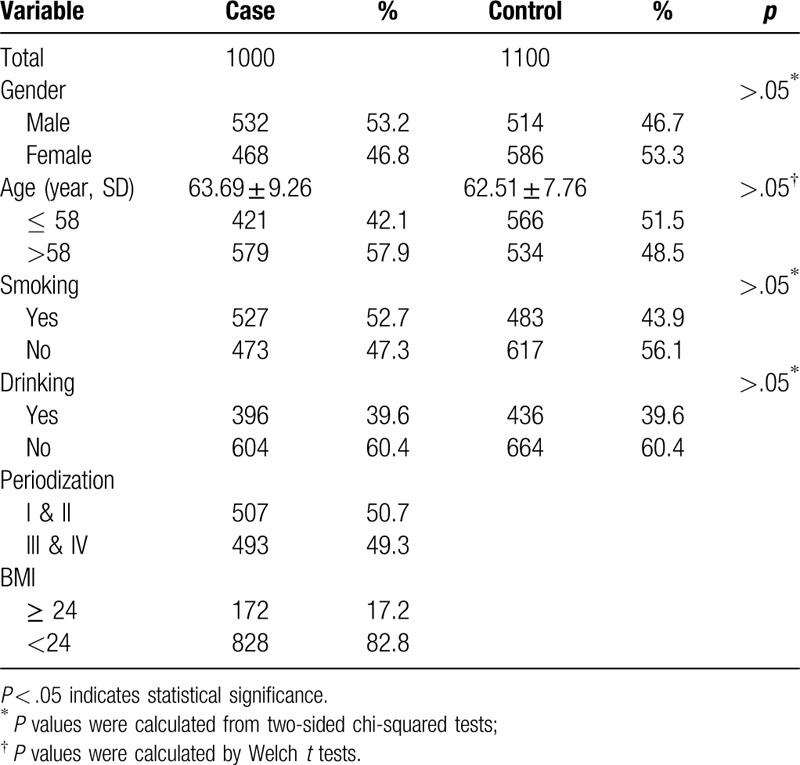
A total of 1000 GC cases (532 males and 468 females) and 1100 healthy controls (514 males and 586 females) were included in this study. The epidemiological and clinical characteristics of the participants were summarized in Table 1. The ages of controls and cases were 63.69 ± 9.26 years and 62.51 ± 7.76 years (P > .05), respectively. There was no significant difference in either smoking status or drinking status between cases and controls (P > .05).
Table 1.
Basic characteristic of cases and controls.
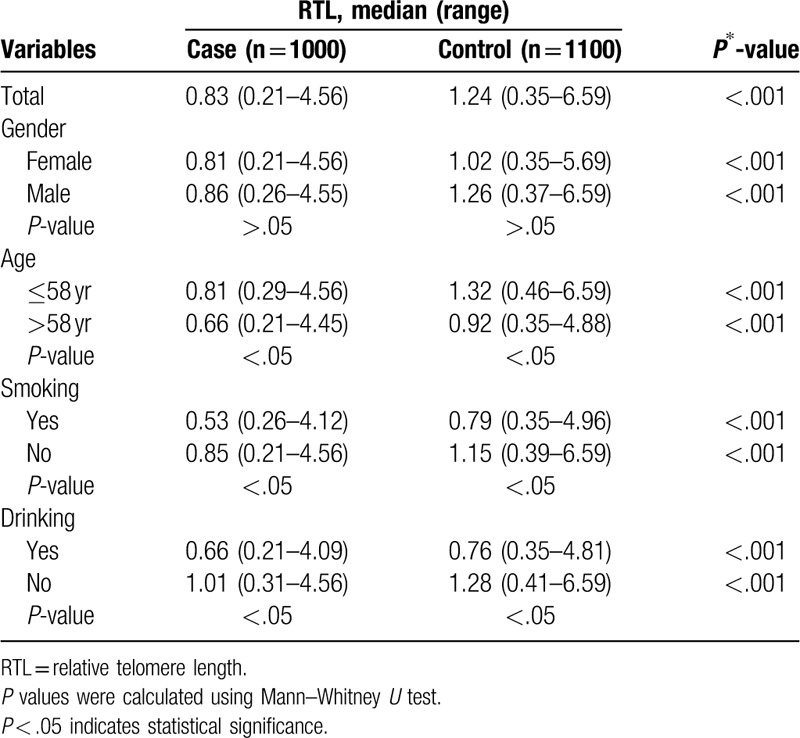
We performed real-time quantitative PCR to measure the RTL of peripheral blood leukocytes (PBLs) from cases and controls. The mean inter-assay CV of real-time PCR reaction was 6.2% (range, 3.6%–9.5%), whereas intra-assay CV was 5.3% (range, 2.8%–7.1%). The results indicated that GC patients had notably shorter median RTL than healthy controls (0.83 vs 1.24; P < .001) (Table 2). When comparing RTL according to gender stratification, age of 58 years, smoking status and drinking status, Mann–Whitney U test showed that both groups of GC patients in male and female had statistically shorter median RTL than relevant healthy controls (0.81 vs 1.02, P < .001; 0.86 vs 1.26, P < .001). The analysis results indicate that both groups of GC patients in age ≤58 years and age >58 had statistically shorter median RTL than relevant healthy controls (0.81 vs 1.32, P < .001; 0.66 vs. 0.92, P < .001). We also found that the groups of GC patients in smoking and no-smoking or drinking and no-drinking had statistically shorter median RTL than relevant healthy controls.
Table 2.
Distributions of RTL by host characteristic in all participants.
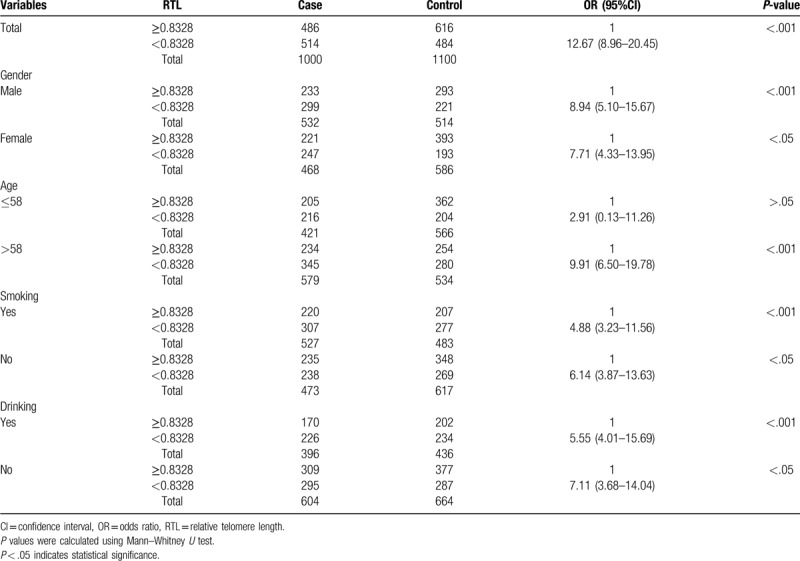
We performed an unconditional multivariate regression analysis to investigate the association between the RTL and GC risk. The participants were divided into 2 groups based on the median RTL, we observed that the shorter RTL (<0.8328) significantly increased risk of GC as compared with the longer RTL (≥0.8328) when adjusted by age, gender, smoking and drinking (OR = 12.67, 95% CI 8.96–20.45, P < .001) (Table 3). To explore whether age, sex, smoking, and drinking influenced the observed associations, we conducted stratified analyses by sex (male and female), age (≤58 years and >58 years), smoking status and drinking status for case-control samples as shown in Table 3. As compared with the longer RTL (≥0.8326), we observed that the shorter RTL (<0.8326) significantly increased risk of GC in male (OR = 8.94, 95% CI: 5.10–15.67, P < .001), female (OR = 7.71, 95% CI: 4.33–13.95, P < .05), age >58 years (OR = 9.91, 95% CI: 6.50–19.78, P < .001), smoking (OR = 4.88, 95% CI: 3.23–11.56, P < .001), no-smoking (OR = 6.14, 95% CI: 3.87–13.63, P < .05), drinking (OR = 5.55, 95% CI: 4.01–15.69, P < .001), and no-drinking (OR = 7.11, 95% CI: 3.68–14.04, P < .05), except for the age ≤58 years subjects (P > .05).
Table 3.
Stratified analysis of the association between the RTL and the risk of GC.
3.2. The association between telomere length-related genes polymorphisms and gastric carcinoma risk
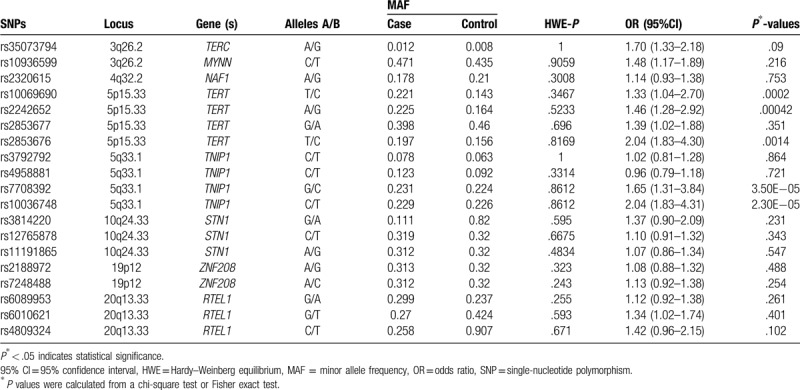
Table 4 summarized the basic information of candidate SNPs in our study, such as chromosomal position, gene, allele, HWE test results, and minor allele frequency, 95% CI, and the P values. In control groups, all SNPs were in line with HWE (P > .05). Pearson chi-squared test was used to assess the associations between SNPs variants and the risk of GC in the allele models. We found that the SNPs rs10069690, rs2242652, and rs2853676 in the TERT were significantly associated with increased GC risk (rs10069690: OR = 1.33, 95% CI: 1.04–2.70, P = .0002; rs2242652: OR = 1.46, 95% CI: 1.28–2.92, P = .00042; rs2853676: OR = 2.04, 95% CI: 1.83–4.30, P = .0014). The other 2 SNPs rs7708392 and rs10036748 (in the TNIP1) were also associated with increased GC risk (rs7708392: OR = 1.65, 95% CI: 1.31–3.84, P = 3.5e–5; rs10036748: OR = 2.04, 95% CI: 1.83–4.31, P = 2.3e–5).
Table 4.
Allele frequencies in cases and controls and OR estimates for GC risk.
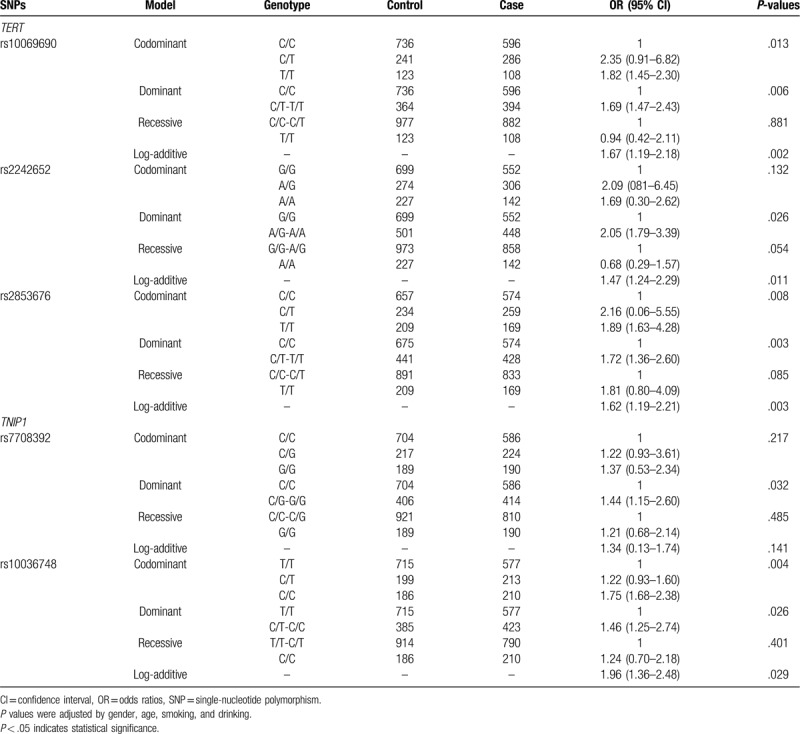
As is shown in Table 5, logistic regression analyses revealed that the rs6010620 (TERT) polymorphism conferred an increased risk of GC in the codominant model (OR = 1.82, 95% CI: 1.45–2.30, P = .013 for the "T/T” genotype), the dominant model (OR = 1.69, 95% CI: 1.47–2.43, P = .006 for the "C/T-T/T” genotype) and log-additive model (OR = 1.67, 95% CI: 1.19–2.18, P = .002), respectively. The rs2242652 (TERT) polymorphism was associated with increased risk of GC in the dominant model (OR = 2.05, 95% CI: 1.79–3.39, P = .026 for the "A/G-A/A” genotype) and log-additive model (adjusted: OR = 1.47, 95% CI: 1.24–2.29, P = .011), respectively. The rs2853676 (TERT) polymorphism was associated with increased risk of GC in the codominant model (OR = 1.89, 95% CI: 1.63–4.28, P = .008 for the “T/T” genotype), the dominant model (OR = 1.72, 95% CI: 1.36–2.60, P = .003 for the "C/T-T/T” genotype) and log-additive model (OR = 1.62, 95% CI: 1.19–2.21, P = .003), respectively. The rs7708392 (TNIP1) polymorphism was associated with increased risk of GC in the dominant model (OR = 1.44, 95% CI: 1.15–2.60, P = .032 for the "C/G-C/C” genotype). The rs10036748 (TNIP1) polymorphism was associated with increased risk of GC in the codominant model (OR = 1.75, 95% CI: 1.68–2.38, P = .004 for the "C/C” genotype), the dominant model (OR = 1.46, 95% CI: 1.25–2.74, P = .026 for the "C/T-C/C” genotype) and log-additive model (adjusted: OR = 1.96, 95% CI: 1.36–2.48, P = .029), respectively.
Table 5.
Association between candidate SNPs and the risk of GC under genotype models.
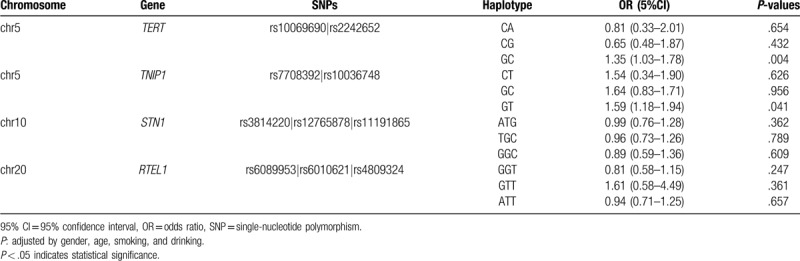
Haploid blocks were obtained by Haploview4.2 software for haploid analysis of candidate SNP sites in control population. We observed that the SNPs rs10069690 and rs2242652 in the TERT had very strong linkage disequilibria, it forms 1 LD block. One block was detected in studied TNIP1 SNPs (rs7708392 and rs10036748) by haplotype analyses. The SNPs (rs3814220, rs12765878, and rs11191865) on the STN1 gene and the SNPs (rs6089953, rs6010621 and rs4809324) on the RTEL1 gene formed 1 LD block, respectively (Fig. 1). Finally, the haplotypes with frequencies of more than 0.05 were selected for further research (Table 6). Haplotype analysis revealed the block in the TERT gene, the "GC” haplotype was associated with increased risk of GC (OR = 1.35, 95% CI: 1.03–1.78, P = .004) (Table 6). The association between the TNIP1 haplotype and the risk of GC was shown in the Table 6. The result showed that the "Grs7708392Trs10036748” haplotype was associated with increased the risk of GC (OR = 1.59, 95% CI: 1.18–0.94, P = .041).
Figure 1.
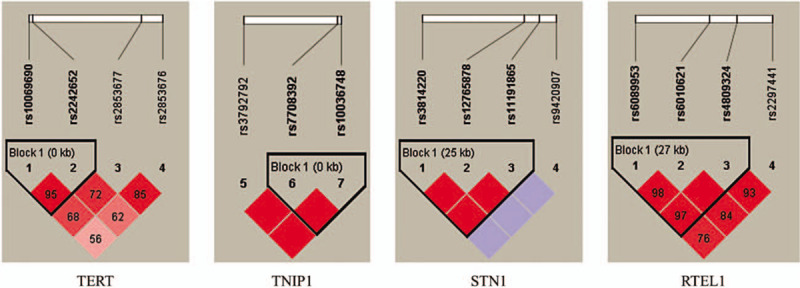
Haplotype block map for single-nucleotide polymorphisms in the TERT, TNIP1, STN1, and RTEL1 genes.
Table 6.
Haplotype analysis results of this study.
4. Discussion
Several studies showed that the etiology and pathogenesis of GC were likely to comprise a multifactorial disorder resulting from environmental and genetic factors and their interaction. In the present case–control study, we studied the role of RTL in susceptibility to GC and investigate the association between genetic polymorphisms in the telomere length related genes and GC risk. The results showed that the RTL in the case group was shorter than in the controls, and the shorter RTL was associated with increasing the risk of GC. In addition, smoking, drinking and different age range may also affect the telomere length. Association analysis between telomere length related genes polymorphisms and GC indicated that TERT (rs10069690, rs2242652, and rs2853676) and TN1F1 (rs7708392 and rs10036748) were significantly increasing the risk of GC. The results indicated that the telomere length and the TERT and TNIP1 genes may play important roles in GC risk in the Chinese population.
To date, many studies have examined telomere length in PBLs and its association with cancer risks.[18–20] However, the results remain inconsistent with positive, negative, or null associations between telomere length and cancer risks. The majority studies have shown that short telomere length is significantly associated with increased risks of cancers such as breast cancer,[21] papillary thyroid carcinoma,[22] lymphoblastic leukemia,[23] glioma,[24] etc. On the contrary, longer telomere has also been found to be associated with increased risks of colorectal adenoma,[25] prostate cancer,[26] esophageal cancer,[27] and renal cell carcinoma,[28] etc. Interestingly, our findings indicate that the shorter RTL are associated with higher risk of GC, suggesting a significant association between RTL in PBLs and GC risk consistent with the report of Liu et al, who conducted a case-control study consisting of 524 gastric cardia adenocarcinoma (GCA) cases and 510 controls samples in Chinese Han population, the result indicated that short RTL was associated with increasing the susceptibility of GCA.[29] In the meanwhile, another research reported that short leukocyte RTL significantly associated with poor prognosis of GC patients.[30] In addition, the present study found that smoking, drinking and different age range may also be risk factors affecting the telomere length. These findings indicated that RTL might be a promising marker to identify high-risk individuals. Certainly, differences in study design, specific cancer site, limited statistical power, variability in confounding factors, and laboratory measurement of telomere length maybe contributing factors to these discrepancies.
In addition to the TERT, the TERC gene plays an important role in encoding the telomere RNA.[31] The STN1 gene is specifically involved in telomere replication and end sealing.[32] The NAF1 gene can change telomere length by affecting the level of telomerase RNA transcription.[33] The RTEL1 gene also plays an important role in the stability, protection, and elongation of telomeres.[33] The TNIP1 and ZNF208 were identified by genome-wide association studies (GWAS) with affecting mean telomere length and their association diseases. Until now, many researches have reported that polymorphisms in these genes may affect the predisposition to telomere dysfunction-related malignancies, including GC.[34–36] Zhang et al, found that TERT (rs10069690 and rs2853676) was significantly associated with increasing the GCA development.[37] Zhang et al, found that the rs2736100 and rs2853669 in TERT gene were associated with increased GC risk.[38] In the present study, we identified that the TERT (rs10069690, rs2242652 and rs2853676) was associated with increased risk of GC, which was consist with the report of Zhang et al. The current findings also suggested that the TNIP1 (rs7708392 and rs10036748) can be considered as a risk factor for GC. However, we have not found the biological relevance between the polymorphisms of other telomere length related genes (TERC, MYNN, NAF1, STN1, ZNF208, and RTEL1) and GC risk. Until now, little research has been done on the correlation between TERC, MYNN, NAF1, TNIPI, STN1, ZNF208, and RTEL1 gene polymorphism and GC risk.
To sum up, we provide new evidence for the association between RTL and RTL-related genes variants and GC risk in Chinese population for the first time, which may provide new data to facilitate earlier diagnosis and promote early prevention, and shed light on the new candidate genes and new ideas for the study. Nevertheless, there are limitations that need to be noticed. Our current research is fundamental, further studies in larger samples and biological functional assays are warranted to validate our findings.
5. Conclusion
The results indicated that the RTL in the case group was shorter than in the controls, and the shorter RTL was associated with increased risk of GC. The polymorphisms of TERT (rs10069690, rs2242652, and rs2853676) and TNIP1(rs7708392 and rs10036748) were significantly associated with increased GC risk.
Acknowledgment
We thank all the patients and individuals for their participation. We thank the physicians and nurses of the People's Hospital of Xinjiang Uygur Autonomous Region for their offers of gastric carcinoma blood samples.
Author contributions
Data curation: Fan Yuxiang.
Formal analysis: Xu Rong.
Investigation: Su Ying.
Methodology: Xu Rong.
Project administration: Chen Ru.
Resources: Han Zhongcheng, Chen Ru.
Software: Han Zhongcheng.
Supervision: Su Ying.
Visualization: Su Ying.
Validation: Jiang Liu.
Writing – original draft: Ma Lili.
Writing – review & editing: Fan Yuxiang, Jiang Liu.
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: CV = coefficient of variation, HWE = Hardy–Weinberg equilibrium, GC = gastric carcinoma, GCA = gastric cardia adenocarcinoma, LD = linkage disequilibrium, OR = odds ratio, PBLs = peripheral blood leukocytes, PCR = polymerase chain reaction, RTL = relative telomere length.
How to cite this article: Lili M, Yuxiang F, Zhongcheng H, Ying S, Ru C, Rong X, Jiang L. Genetic variations associated with telomere length affect the risk of gastric carcinoma. Medicine. 2020;99:23(e20551).
ML and FY contributed equally to this work.
This work was supported by The Natural Science Foundation of Xinjiang Uygur Autonomous Region (2019D01C162).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
- [1].Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- [2].Kelley JR, Duggan JM. Gastric cancer epidemiology and risk factors. J Clin Epidemiol 2003;56:1–9. [DOI] [PubMed] [Google Scholar]
- [3].Pan W, Du J, Shi M, et al. Short leukocyte telomere length, alone and in combination with smoking, contributes to increased risk of gastric cancer or esophageal squamous cell carcinoma. Carcinogenesis 2016;38:12. [DOI] [PubMed] [Google Scholar]
- [4].Bakács T, Mehrishi JN. Breast and other cancer dormancy as a therapeutic endpoint: speculative recombinant T cell receptor ligand (RTL) adjuvant therapy worth considering? BMC Cancer 2010;10:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].González CA, Sala N, Capellá G. Genetic susceptibility and gastric cancer risk. Int J Cancer 2010;100:249–60. [DOI] [PubMed] [Google Scholar]
- [6].Hamajima N, Naito M, Kondo T, et al. Genetic factors involved in the development of Helicobacter pylori-related gastric cancer. Cancer Sci 2010;97:1129–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Yu F, Tian T, Deng B, et al. Multi-marker analysis of genomic annotation on gastric cancer GWAS data from Chinese populations. Gastric Cancer 2018;22:1–9. [DOI] [PubMed] [Google Scholar]
- [8].Baur JA, Zou Y, Shay JW, et al. Telomere position effect in human cells. Science 2001;292:2075. [DOI] [PubMed] [Google Scholar]
- [9].Xu L, Li S, Stohr BA. The role of telomere biology in cancer. Ann Rev Pathol Mech Dis 2013;8:49–78. [DOI] [PubMed] [Google Scholar]
- [10].Ornish D, Lin J, Daubenmier J, et al. Increased telomerase activity and comprehensive lifestyle changes: a pilot study. Lancet Oncol 2008;9:1023–4. [DOI] [PubMed] [Google Scholar]
- [11].Bär C, Blasco MA. Telomeres and telomerase as therapeutic targets to prevent and treat age-related diseases. F1000research 2016;5:F1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Fabrizio DADF, Reaper PM, Lorena CF, et al. A DNA damage checkpoint response in telomere-initiated senescence. Nature 2003;426:194–8. [DOI] [PubMed] [Google Scholar]
- [13].Stanley SE, Armanios M. The short and long telomere syndromes: paired paradigms for molecular medicine. Curr Opin Genet Dev 2015;33:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Rubio MA, Davalos AR, Campisi J. Telomere length mediates the effects of telomerase on the cellular response to genotoxic stress. Exp Cell Res 2004;298:17–27. [DOI] [PubMed] [Google Scholar]
- [15].Atzmon G, Cho M, Cawthon RM, et al. Colloquium paper: genetic variation in human telomerase is associated with telomere length in Ashkenazi centenarians. Proc Natl Acad Sci U S A 2010;107: Suppl 1: 1710–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Veryan C, Nelson CP, Eva A, et al. Identification of seven loci affecting mean telomere length and their association with disease. Nat Genet 2013;45:422–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Cawthon RM. Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic Acids Res 2009;37:e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Skinner HG, Gangnon RE, Litzelman K, et al. Telomere length and pancreatic cancer: a case-control study. Cancer Epidemiol Biomarkers Prev 2012;21:2095–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Qin Q, Sun J, Yin J, et al. Telomere length in peripheral blood leukocytes is associated with risk of colorectal cancer in Chinese population. PloS One 2014;9:e88135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Terry KL, Tworoger SS, Vitonis AF, et al. Telomere length and genetic variation in telomere maintenance genes in relation to ovarian cancer risk. Cancer Epidemiol Biomarkers Prev 2012;21:504–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wang Z, Zhang Z, Guo Y, et al. Shorter telomere length is associated with increased breast cancer risk in a Chinese Han population: a case-control analysis. J Breast Cancer 2018;21:391–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Li J, An C, Zheng H, et al. Leukocyte telomere length and risk of papillary thyroid carcinoma. J Clin Endocrinol Metab 2019;104:2712–8. [DOI] [PubMed] [Google Scholar]
- [23].Eskandari E, Hashemi M, Naderi M, et al. Leukocyte telomere length shortening, hTERT genetic polymorphisms and risk of childhood acute lymphoblastic leukemia. Asian Pac J Cancer Prev 2018;19:1515–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wang S, Chen Y, Qu F, et al. Association between leukocyte telomere length and glioma risk: a case-control study. Neuro-oncology 2014;16:505–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Peacock SD, Massey TE, Vanner SJ, et al. Telomere length in the colon is related to colorectal adenoma prevalence. PloS One 2018;13:e0205697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Renner W, Krenn-Pilko S, Gruber HJ, et al. Relative telomere length and prostate cancer mortality. Prostate Cancer Prostatic Dis 2018;21:579–83. [DOI] [PubMed] [Google Scholar]
- [27].Lv Y, Zhang Y, Li X, et al. Long telomere length predicts poor clinical outcome in esophageal cancer patients. Pathol Res Pract 2017;213:113–8. [DOI] [PubMed] [Google Scholar]
- [28].Machiela MJ, Hofmann JN, Carreras-Torres R, et al. Corrigendum re “genetic variants related to longer telomere length are associated with increased risk of renal cell carcinoma” [Eur Urol 2017;72:747-54]. Eur Urol 2018;74:e85–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Liu Y, Lei T, Zhang N, et al. Leukocyte telomere length and risk of gastric cardia adenocarcinoma. Sci Rep 2018;8:14584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Qu F, Li R, He X, et al. Short telomere length in peripheral blood leukocyte predicts poor prognosis and indicates an immunosuppressive phenotype in gastric cancer patients. Molecular Oncol 2015;9:727–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Du H, Pumbo E, An JP, et al. TERC and TERT gene mutations in patients with bone marrow failure and the significance of telomere length measurements. Blood 2009;113:309–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Levy D, Neuhausen SL, Hunt SC, et al. Genome-wide association identifies OBFC1 as a locus involved in human leukocyte telomere biology. Proc Natl Acad Sci U S A 2010;107:9293–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Stanley SE, Gable DL, Wagner CL, et al. Loss-of-function mutations in the RNA biogenesis factor NAF1 predispose to pulmonary fibrosis–emphysema. Sci Trans Med 2016;8:351ra107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Pellatt AJ, Wolff RK, Torres-Mejia G, et al. Telomere length, telomere-related genes, and breast cancer risk: the breast cancer health disparities study. Genes Chromosomes Cancer 2013;52:595–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ning X, Yang S, Wang R, et al. POT1 deficiency alters telomere length and telomere-associated gene expression in human gastric cancer cells. Eur J Cancer Prev 2010;19:345. [DOI] [PubMed] [Google Scholar]
- [36].Du J, Zhu X, Xie C, et al. Telomere length, genetic variants and gastric cancer risk in a Chinese population. Carcinogenesis 2015;36:963–70. [DOI] [PubMed] [Google Scholar]
- [37].Zhang N, Zheng Y, Liu J, et al. Genetic variations associated with telomere length confer risk of gastric cardia adenocarcinoma. Gastric Cancer 2019;22:1089–99. [DOI] [PubMed] [Google Scholar]
- [38].Zhang J, Ju H, Gao JR, et al. Polymorphisms in human telomerase reverse transcriptase (hTERT) gene, gene- gene and gene-smoking interaction with susceptibility to gastric cancer in Chinese Han population. Oncotarget 2017;8:20235–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


