Abstract
Background:
Coronavirus disease 2019 (COVID-19) is a global pandemic caused by the Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2). Since the outbreak, the disease has caused more than 60,502 deaths worldwide. Lian-Hua Qing-Wen Granule (LHQWG) is widely used in treating COVID-19 in China. However, there is no evidence that LHQWG is effective for COVID-19.
Methods and analysis:
A comprehensive literature search will be conducted. Two methodological trained researchers will read the title, abstract and full texts and independently select the qualified literature according to inclusion and exclusion criteria. After assessment of the risk of bias and data extraction, we will conduct meta-analyses for outcomes related to COVID-19. The heterogeneity of data will be investigated by Cochrane X2 and I2 tests. Publication bias assessment will be conducted by funnel plot analysis and Egger test.
Results:
The results of our research will be published in a peer-reviewed journal.
Conclusion:
Our study aims to systematically present the clinical evidence of LHQWG in treating COVID-19, which will be of significant meaning for further research and clinical practice.
OSF registration number:
10.17605/OSF.IO/27SBU.
Keywords: COVID-19, Lian-Hua Qing-Wen granule, protocol, systematic review and meta-analysis
1. Introduction
Coronavirus disease 2019 (COVID-19) is a global pandemic caused by the Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2).[1,2] The patients of COVID-19 usually present with fever, cough, while about 23.7% of patients are accompanied by at least one coexisting disease.[3–7] The rapid rise in the number of patients has placed a heavy burden on the health care system.[8] Up to April 6, 2020, the disease has infected 836,769 people and caused 60,502 deaths all over the world.
At present, there is no effective treatment for this disease.[9] The clinical management of this disease mainly depends on supportive treatment. Studies found that some treatments may be effective, but the efficacy remains to be further evaluated.[10–12] Thus, effective treatment is still urgently needed.
In China, traditional Chinese medicine (TCM) is widely used in treating COVID-19.[13,14] It is reported that more than 85% of COVID-19 patients are treated with TCM.[15] Among the many commonly used TCMs, the most commonly used one is Lian-Hua Qing-Wen Granule (LHQWG). LHQWG was first used in the treatment of influenza, and studies have found that the drug is superior to oseltamivir in improving the symptoms of influenza A virus infection.[16] Since the outbreak of COVID-19, this medicine has been widely recommended for clinical treatment of patients with common symptoms. In the fifth edition of COVID-19's diagnosis and treatment guideline issued by the China Health Commission, LHQWG is listed as one of the recommended medicine candidates. Researchers carried out a series of studies on the efficacy of this medicine in the treatment of COVID-19. It was found that routine treatment combined with LHQWG could significantly improve the clinical symptoms including fever, fatigue, cough, sputum, shortness of breath, chest tightness, loss of appetite and so on; suggesting that LHQWG is an effective treatment for COVID-19 patients.[17–20] In addition, the experimental research on the mechanisms of LHQWG in the treatment of COVID-19 by means of cell experiment and network pharmacology is also being widely carried out.[21–24]
2. Methods and analysis
2.1. Study registration
This study has been registered in advance on the website of Open Science Framework (OSF, https://osf.io/) with a registration number of DOI: 10.17605/OSF.IO/27SBU. This systematic review protocol is reported in accordance with Cochrane reporting expectations, as recommended by the Cochrane handbook.[25]
2.2. Inclusion and exclusion criteria
2.2.1. Study design
In this study, both randomized studies and non-randomized studies will be included. Randomized studies can provide reliable clinical evidence, but it can be time-consuming and expensive. Non-randomize studies may lead to greater bias, but it is more convenient to obtain clinical data. Since COVID19 is an urgent public health event, it is difficult to carry out randomized studies, it is appropriate to include non-randomized studies in this systematic review and meta-analysis.
2.2.2. Participants
Participants with laboratory-confirmed COVID-19 will be included in this study. The assay was primarily RC-qPCR, and there were no restrictions on the age, sex or disease severity of the participants.
2.2.3. Intervention
Studies using LHQWG or Lian-Hua Qing-Wen Capsule will be included. There will be no restriction about the doses and methods of use of intervention. Also, there will be no limitation about control group.
2.2.4. Outcomes
Total clinical effective rate, effective rate of clinical symptoms, disappearance rate of clinical symptoms, treatment time, improvement rate of lung CT, adverse events.
2.3. Study search
Three English database including PubMed, EMBASE, Cochrane Library Central Register of Controlled Trials and four Chinese databases including China National Knowledge Infrastructure (CNKI) database, Wanfang Data Knowledge Service Platform, the VIP information resource integration service platform, China Biology Medicine Disc will be searched from its inception to April 6, 2020. There will be no language limitation. Preprinted website including arXiv (http://arxiv.org/), BioRxiv (https://www.biorxiv.org/), F1000 (https://f1000.com/) and PeerJ Preprints (https://peerj.com/preprints/) will also be searched to find out more unpublished manuscript. Chinese Clinical Trial Registry (ChiCTR) and ClinicalTrials.gov will also be searched to find out ongoing research. The references of included manuscript will be searched.
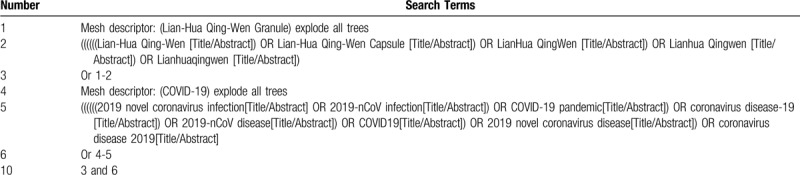
A search strategy of the combination of controlled vocabulary and text words will be adopted. Boolean operators will be used to concatenate search terms. This work will be conducted by 2 authors (Zhipeng Hu and Maoyi Yang) independently. The search strategy of PubMed is presented in Table 1.
Table 1.
Example of PubMed search strategy.
2.4. Study selection
EndNote X9 will be used to screen the citations independently according to inclusion and exclusion criteria by two reviewers (Zhipeng Hu and Maoyi Yang). Discrepancies between two authors will be solved by discussion with a third author (Chunguang Xie). A research flow chart will be drawn to show the whole process of research selection (Fig. 1).
Figure 1.
Flow chart of study selection.
2.5. Data extraction
Data extraction will be conducted by 2 independent authors (Maoyi Yang and Zhipeng Hu) according to a prespecified form and checked by a third author (Chunguang Xie). The following data will be extracted: the first author's name, publication time, country, article title, article type, interventions in experimental and control group, course of treatment, severity of disease, number of patients in each group, ages and sex of patients, outcomes and adverse effect. If the author does not report certain information in the article, we will then contact the authors by email for more detailed information. Once the extraction is complete, the 2 authors will check with each other to ensure the accuracy of the data.
2.6. Risk of bias assessment
Version 2 of the Cochrane risk-of-bias tool for randomized trials (RoB2) and the risk of bias in non-randomized studies of interventions (ROBINS-I) tool will be used to assess the risk of bias of randomized studies and non-randomized studies respectively, as recommended by the Cochrane handbook.[26,27]
2.7. Data analysis
Data analysis will be conducted using Stata 14.0 software. Risk ratio (RR) or odds ratio (OR) and 95% confidence interval (CI) will be used for dichotomous outcomes and 95% CI and mean difference (MD) or standardized mean difference (SMD) will be used for continuous outcomes. The number needed to treat will be calculated for the interpretation of results. Cochrane X2 and I2 tests will be conducted to assess the heterogeneity analysis between studies. A random effect model will be used if P < .05 and I2 > 50%. When P > .05 and I2 < 50%, then a fixed effect model will be used to. The results of randomized studies and non-randomized studies will be analyzed and presented independently. Subgroup analysis will be conducted to explore the subgroup effects and investigate the source of heterogeneity. If there is a substantial heterogeneity and quantitative synthesis is not appropriate, the results will be presented in the form of tables and figures.
Non-reporting bias will be evaluated by funnel plot and Egger test.[28] A P value less than .05 indicates the existence of publication bias.
3. Discussion
The aim of this study was to summarize the efficacy of LHQWG on COVID-19 to provide an accurate guide for further research and clinical application. This study has some highlights. First, in order to collect clinical evidence as comprehensively as possible, preprinted websites will be searched in addition to main databases for systematic review. In addition, given the speed at which the epidemic is developing and the difficulty of conducting clinical trials, we will include both randomized studies and non-randomized studies in the research. Non-randomized studies have more biases and confounding than randomized studies, so the results of the 2 types of studies will be presented separately. In conducting the risk of bias assessment, we will use the latest version of the tool recommended in the handbook, which will methodologically ensure the correctness of our study.
Author contributions
The protocol was designed by ZH and MY under the guidance of CX. All the authors participated in the study. The manuscript was drafted by ZH and revised by CX. All authors approved the final manuscript before submission. ZH and MY contributed equally to this work and should be regarded as co-first authors.
Conceptualization: Zhipeng Hu, Chunguang Xie.
Data curation: Zhipeng Hu, Maoyi Yang.
Formal analysis: Zhipeng Hu, Maoyi Yang.
Investigation: Zhipeng Hu, Maoyi Yang.
Methodology: Zhipeng Hu, Maoyi Yang.
Project administration: Chunguang Xie
Software: Zhipeng Hu, Maoyi Yang.
Visualization: Zhipeng Hu, Maoyi Yang.
Writing – original draft: Zhipeng Hu.
Writing – review and editing: Chunguang Xie.
Footnotes
Abbreviations: CI = confidence interval, CNKI = China National Knowledge Infrastructure, COVID-19 = Coronavirus disease 2019, LHQWG = Lian-Hua Qing-Wen Granule, MD = mean difference, OSF = open science framework, RoB2 = version 2 of the Cochrane risk-of-bias tool for randomized trials, ROBINS-I = the risk of bias in non-randomized studies of interventions tool, RR = risk ratio, SARS-CoV-2 = the severe acute respiratory syndrome Coronavirus-2, SD = Standard Deviation, TCM = traditional Chinese medicine.
How to cite this article: Hu Z, Yang M, Xie C. Efficacy and safety of Lian-Hua Qing-Wen granule for COVID-2019: A protocol for systematic review and meta-analysis. Medicine. 2020;99:23(e20203).
MY and ZH contributed equally to this work and are co-first authors.
Ethical approval is not needed for this study. The results of this study will be published in peer-reviewed journals.
We will update our protocol for any changes in the entire research process if needed.
This work will be supported by the first batch of science and technology emergency projects of Sichuan Provincial Science and technology department in 2020 (2020YFS0012, 2020YFS0013), special fund for 2020 financial science and technology projects of Chengdu Science and Technology Bureau (the first batch) (2020-YF05-00333-SN) and Chengdu University of traditional Chinese Medicine Foundation (XGZX2001, XGZX2002). The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Zhai P, Ding Y, Wu X, et al. The epidemiology, diagnosis and treatment of COVID-19. Int J Antimicrob Agents 2020;105955.Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Zhou M, Zhang X, Qu J. Coronavirus disease 2019 (COVID-19): a clinical update. Front Med 2020;Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England) 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Novel Coronavirus Pneumonia Emergency Response Epidemiology Team [The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China]. Zhonghua Liu Xing Bing Xue Za Zhi 2020;41:145–51.32064853 [Google Scholar]
- [6].Yu N, Li W, Kang Q, et al. Clinical features and obstetric and neonatal outcomes of pregnant patients with COVID-19 in Wuhan, China: a retrospective, single-centre, descriptive study. Lancet Infect Dis 2020;Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Zavascki AP, Falci DR. Clinical Characteristics of Covid-19 in China. N Engl J Med 2020;382.Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- [8].Verity R, Okell LC, Dorigatti I, et al. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect Dis 2020;Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Matthay MA, Aldrich JM, Gotts JE. Treatment for severe acute respiratory distress syndrome from COVID-19. Lancet Respir Med 2020;Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Cao B, Wang Y, Wen D, et al. A trial of Lopinavir-Ritonavir in adults hospitalized with severe covid-19. N Engl J Med 2020;Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gautret P, Lagier JC, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents 2020;105949.Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Holshue ML, DeBolt C, Lindquist S, et al. First Case of 2019 Novel Coronavirus in the United States. N Engl J Med 2020;382:929–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Luo H, Tang QL, Shang YX, et al. Can chinese medicine be used for prevention of corona virus disease 2019 (COVID-19)? A review of historical classics, research evidence and current prevention programs. Chin J Integr Med 2020;26:243–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ren JL, Zhang AH, Wang XJ. Traditional Chinese medicine for COVID-19 treatment. Pharmacol Res 2020;155:104743.Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Yang Y, Islam MS, Wang J, et al. Traditional Chinese Medicine in the treatment of patients infected with 2019-New Coronavirus (SARS-CoV-2): a review and perspective. Int J Biol Sci 2020;16:1708–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zhao P, Yang HZ, Lv HY, et al. Efficacy of Lianhuaqingwen capsule compared with oseltamivir for influenza A virus infection: a meta-analysis of randomized, controlled trials. Altern Ther Health Med 2014;20:25–30. [PubMed] [Google Scholar]
- [17].Cheng D, Wang W, Li Y, et al. Novel coronavirus pneumonitis patients treated with Chinese herbal medicine Lianhua Qingwen: a multicenter retrospective study of 51 cases. Tianjin Trad Chinese Med:1–6; Epub ahead of print. [Google Scholar]
- [18].Cheng Dezhong LY. Novel coronavirus pneumonia treated by Lianhua Qingwen Granule: a clinical analysis and a typical Novel coronavirus pneumonia treated by Lianhua Qingwen Granule: a clinical analysis and a typical case report of 54 cases. Tianjin Trad Chinese Med 2020;15:150–4. [Google Scholar]
- [19].Lv R, Wang W, Li X. Novel coronavirus pneumonia suspected cases treated with Lianhua Qingwen Decoction: a clinical observation of 63 cases. J Trad Chin Med:1–5; Epub ahead of print. [Google Scholar]
- [20].Yao K, Liu M, Li X, et al. Retrospective clinical analysis of novel coronavirus pneumonia treated with Lianhua Qingwen. Chin J Exp Prescr 2020;1–7. Epub ahead of print. [Google Scholar]
- [21].Ling X, Tao J, Sun X, et al. Study on the material basis and mechanism of Lianhua Qingwen formula against coronavirus based on network pharmacology. Chin Herbal Med:1–8. [Google Scholar]
- [22].Runfeng L, Yunlong H, Jicheng H, et al. Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2). Pharmacol Res 2020;104761.Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wang L, Yang Z, Zhang H, et al. Pharmacology study and novel coronavirus of Lianhua Qingwen for the treatment of new coronavirus (2019-nCoV) pneumonia. Chin Herbal Med 2020;772–8. Epub ahead of print. [Google Scholar]
- [24].Wang F, Shen B, He C, et al. Novel coronavirus pneumonia: clinical efficacy and mechanism of Lianhua Qingwen Granule: a network pharmacology study. Pharmacol Clinic Trad Chin Med:1–22. [Google Scholar]
- [25].Cochrane, Page MJ, Cumpston M, Chandler J. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, et al. Chapter III: Reporting the review. Cochrane Handbook for Systematic Reviews of Interventions version 6. 0 (updated August 2019) 2019. [Google Scholar]
- [26].Cochrane, Higgins JPT, Savović J, Page MJ. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, et al. Chapter 8: Assessing risk of bias in a randomized trial. Cochrane Handbook for Systematic Reviews of Interventions version 6. 0 (updated July 2019) 2019. [Google Scholar]
- [27].Cochrane, Sterne JAC, Hernán MA, McAleenan A. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, et al. Chapter 25: Assessing risk of bias in a non-randomized study. Cochrane Handbook for Systematic Reviews of Interventions version 6. 0 (updated July 2019) 2019. [Google Scholar]
- [28].Song F, Gilbody S. Bias in meta-analysis detected by a simple, graphical test. Increase in studies of publication bias coincided with increasing use of meta-analysis. BMJ (Clinical research ed) 1998;316:471. [PMC free article] [PubMed] [Google Scholar]


