Abstract
Background
Patients with burns present with different clinical features depending on the types of burn injury and burn patients with lower levels of vitamin D have worse prognoses and more complications. The study aims to investigate the association between vitamin D levels and burn factors according to each burn type in relation to early intensive rehabilitation therapy initiated for inpatients with burns.
Methods
In this retrospective study, we enrolled 757 of 1716 inpatients who underwent rehabilitative therapy between May 2013 and April 2017. Burn types were divided into flame burn, electrical burn and other burns, including scalding, contact and chemical burns. Age, burned body surface area (BSA), wound healing time (WHT), length of hospital stay (LOS) and body mass index were analysed between vitamin D deficient and non-deficient patient groups using Student’s t-tests, or Mann-Whitney U test and among three burn types using one-way analysis of variance (ANOVA) or Kruskal-Wallis one-way ANOVA. The relationship between vitamin D levels and burn factors was evaluated using Pearson's or Spearman's correlation coefficient tests, and multiple linear regression analysis in different burn groups.
Results
In total, 88.9% patients were vitamin D deficient, and these patients had a larger burned BSA (p = 0.015) and longer WHT and LOS (all p < 0.001) than non-deficient patients. Burned BSA, WHT and vitamin D levels showed significant differences in their mean values according to three burn types (all p < 0.001). WHT was a communal factor significantly associated with vitamin D levels in all three burn types (p < 0.05). The WHT cut-off points to predict vitamin D deficiency were 55 days for flame burn (p < 0.001) and 62.5 days for electrical burn (p = 0.001).
Conclusions
WHT across all three burn types was a common factor associated with vitamin D levels for inpatients with burns who had undergone rehabilitative therapy. Electrical burn patients with vitamin D deficiency, even those with a low burned BSA percentage, showed prolonged wound healing over a two-month post-burn period. Independent of burned BSA, nutritional intervention concerning vitamin D in relation to burn wound healing should be considered to guide early initiation of intensive rehabilitation therapy.
Keywords: Burn factors, Vitamin D deficiency, Burn type, Wound healing time, Rehabilitation
Highlights.
The highlight of the current study:
This study is the first to examine an association between vitamin D levels and burn factors according to burn injury types.
This study is the first to show that would healing time across all burn types was a common factor associated with vitamin D level for inpatients with burns who had undergone rehabilitative therapy.
Background
The loss of healthy skin following a burn injury can decrease epidermal vitamin D production. The conversion of 7-dehydrocholesterol to previtamin D in patients with burn injuries is attenuated in both hypertrophic scars and healthy skin adjacent to scars [1]. Additionally, low vitamin D levels have been reported to have continued for 7 years post-burn in pediatric outpatients [2]. It has been reported that 1,25 hydroxyvitamin D3 converted from 25(OH) vitamin D is associated with regulation of the permeability barrier of the epidermis [3] and stimulates proliferation and differentiation of epidermal keratinocytes in the skin [4]. Vitamin D stimulates wound healing [5] and increases immunity through vitamin D receptors detected in B and T lymphocytes, monocytes and macrophages [6]. Moreover, vitamin D levels in inpatients with burn injuries undergoing rehabilitative therapy have been found to be related to the biomechanical properties of hypertrophic scars [7,8]. Therefore, vitamin D is a critical nutrient in burn care [9,10].
Patients with burn injuries have different clinical features depending on burn types, which can be divided into flame burns, electrical burns and scalding, chemical or contact burns, according to the mechanism of burn injury. In particular, electrical burn have been found to cause considerable damage to nerves, blood vessels, muscles and organs due to their high water content, even when involving only 1% of the burned body surface area (BSA) [11]. Electrical burn are known to result in further complications such as vital organ damage and dysfunction of the sympathetic nervous system [11,12], when compared to other burn types with a similar burned BSA. Flame burn is the most common type of burn injury and frequently have a larger percentage of burned BSA [13,14].
In some burn injury studies, a relationship between vitamin D deficiency and burned BSA, the healing time for burn wounds and various complications following a burn injury have been reported [1,2,10,15]. Vitamin D levels have been reported to decrease proportionally depending on the percentage of burned BSA [1] and the hypermetabolic and catabolic response, which leads to a reduction in the body mass index (BMI), and these levels are also proportionate to the depth and extent of thermal injury [16,17]. In one study, patients with burn injuries with low vitamin D levels on admission were found to have prolonged wound healing and long-term institutionalization [18]. However, these studies did not consider whether vitamin D levels could have been affected due to the types of burn injury sustained in pediatric or adult patients with burn injuries. Moreover, there are a few studies that have demonstrated an association between vitamin D deficiency and burn factors, such as burned BSA, wound healing time (WHT), length of hospital stay (LOS) and BMI, according to each burn type. Therefore, we hypothesized that different mechanisms of burn injuries could affect the relationship between vitamin D levels and burn factors during the period of burn care. To help intensive rehabilitation therapy initiated earlier, inpatients with burn injuries who had undergone rehabilitative therapy were divided according to the type of burn injury, and we investigated the association between vitamin D levels and burn factors separately according to each burn type.
Methods
Patients
We undertook a retrospective study of inpatients with burn injuries who had been admitted to the rehabilitation unit of our burn center between May 2013 and April 2017 for rehabilitative therapy as soon as complete wound healing had occurred. Wound healing was defined as the complete covering of a denuded epithelial surface, namely re-epithelialization [19]. The confirmation of wound healing for WHT was determined according to the agreement of one surgeon and one rehabilitation specialist following examination of a burn wound. Due to the close association between the departments of surgery and rehabilitation in our hospital, burn patients were transferred directly to the rehabilitation unit within a period of approximately 5 days from confirmation of wound healing without returning home. Information about each patient concerning types of burn injury, burned BSA, WHT, LOS, BMI, 25(OH) vitamin D level and history of smoking, depression, pain and itching was obtained by a nurse as part of a routine admission evaluation. We analysed the clinical data of the patients using medical records held in a clinical database. Initially, 1716 patients (1407 men, 309 women) were enrolled, and 584 patients aged <19 or >50 years were excluded to eliminate the possible influence of age on vitamin D levels. Furthermore, 375 patients who met any one of the following criteria were excluded: (1) patients with heart, kidney, lung or parathyroid disease (n = 79); (2) patients taking vitamin D supplements (n = 170); (3) patients taking medication that affected bone metabolism (n = 7); and (4) patients with data missing from their clinical records (n = 119). Finally, data from 757 patients (635 men, 122 women) were obtained for the analysis. The study protocol was approved by the institutional review board and informed consent for participants was waived (IRB No. 2017–110).
Burn types and burn factors related to burn injury
Burn injury types were divided primarily into flame burn, electrical burn and other burns, including scalding, contact and chemical burns. To the best of our knowledge, no previous study has divided burn injury types into flame burn, electrical burn and other types to demonstrate an association between vitamin D levels and burn injury. This study is the first to examine an association between vitamin D levels and burn factors according to burn injury types. Although the overall study sample size was sufficient, some burn types were not sufficiently numerous to be analysed statistically. Therefore, flame burn, electrical burn and other burns were used as three representative types of burn injuries for statistical analysis considering clinical characteristics and the sample size of each burn type.
The capacity to synthesize vitamin D is known to decrease proportionally with age [20] and as the percentage of burned BSA increases [1]. A low vitamin D level on admission has been associated with prolonged wound healing and hospital stay [18]. Hypermetabolic and hypercatabolic responses following thermal injury have been reported to decrease the BMI through the loss of adipose tissue and muscle [21]. Moreover, a reduced BMI may affect vitamin D levels because adipocytes have been shown to store and release vitamin D [20]. Therefore, age, burned BSA, WHT, LOS and BMI were variables for burn factors used in the statistical analysis. Observational epidemiological studies have reported that low vitamin D levels are associated with a history of smoking, depression, pain, atopic dermatitis and elevated immunoglobulin E levels [22–26]. However, some recent studies have found no evidence to suggest that a reduction in 25(OH) vitamin D level confers an increase in atopic dermatitis or elevated immunoglobulin E levels [27,28]. Therefore, we evaluated the association between vitamin D deficiency and these factors.
Measurements of 25(OH) vitamin D levels
When patients were admitted to the department of rehabilitation following complete re-epithelialization, blood sampling for vitamin D plasma levels was obtained after fasting for over 8 hours as routine admission chemistry. Vitamin D levels were confirmed through measuring the plasma concentration of 25(OH) vitamin D using a radioimmunoassay analyser (ADVIA Centaur XPT, Siemens, Germany) because 25(OH) vitamin D has been shown to be a detectable circulating form [30]. This method was designed to detect serum 25-OH-D values from 5 to 100 ng/ml, which was beyond the normal range of values expected to be observed for human serum 25-OH-D concentration. Any values less than the lowest standard, 5 ng/ml, were reported as “<4.2” and the lowest value was determined as 4.2 ng/ml. Vitamin D deficiency was defined as a 25(OH) vitamin D level of <20 ng/ml, and non-deficiency was defined as a 25(OH) vitamin D level of ≥20 ng/ml, in accordance with a review published by Holick [20].
Statistical analysis
Data are expressed as mean ± standard deviation or median (range) for continuous variables where appropriate and number (%) for categorical variables. The Kolmogorov–Smirnov test was performed to test the normality assumption. Data concerning age, burned BSA, WHT, LOS and BMI were analysed using Student’s t-tests between vitamin D deficient and non-deficient patient groups for the total number of patients, and using one-way analysis of variance with the Tukey post hoc test among the three burn types. A Fisher’s exact test was used to analyse history of smoking, depression, pain and itching between the vitamin D deficient and non-deficient patient groups. The relationship between vitamin D levels and burn factors in flame burn, electrical burn and other burns was evaluated separately using Pearson’s and Spearman’s correlation coefficient tests and multiple linear regression analysis. Because the correlation coefficient between WHT and LOS was approximately 1.0, only WHT could be used for multiple linear regression analysis. Burn factors are quietly correlated one another, not independent. Therefore, in order to evaluate whether our regression results were acceptable, the variance inflation factor, which detects multicollinearity in regression analysis, was checked. Multicollinearity is when there is correlation between predictors in a model; its presence can adversely affect the regression results. If a variance inflation factor is >10, this indicates high multicollinearity. To determine the cut-off values of burn factors for predicting vitamin D deficiency according to the three burn types, a receiver operating characteristic (ROC) curve was constructed. Statistical analyses were conducted using SPSS 21.0 software (IBM Corp., Armonk, NY, USA). A p value of <0.05 was considered statistically significant.
Results
Patient demographics
In total, 635 (83.9%) men and 122 (16.1%) women were included in this study, of whom those with flame burn, electrical burn and other burns comprised 375 (49.5%), 193 (25.5%) and 189 (25%) patients, respectively. Other burns included 96 patients with scald burns, 63 patients with contact burns and 30 patients with chemical burns. The mean values of burned BSA, WHT and LOS were 20.5 ± 16.1%, 81.2 ± 53.7 days and 86.3 ± 53.7 days, respectively. The mean 25(OH) vitamin D level was 14.2 ± 4.9 ng/ml. The baseline characteristics concerning all 757 study patients are presented in Table 1.
Table 1.
Baseline demographics of 757 patients
| Variable | |
|---|---|
| Sex (male : female), n(%) | 635 (83.9) : 122 (16.1) |
| Age (years) | 39.5 ± 8.2 |
| Height (cm) | 171.4 ± 7.3 |
| Weight (kg) | 68.0 ± 11.0 |
| FB : EB : Other burns, n(%) | 375 (49.5) : 193 (25.5) : 189 (25) |
| Burned BSA (%) | 20.5 ± 16.1 |
| WHT (days) | 81.2 ± 53.7 |
| LOS (days) | 86.3 ± 53.7 |
| BMI (kg/m2) | 23.5 ± 3.1 |
| 25(OH) vitamin D level (ng/mL) | 14.2 ± 4.9 |
| Smoking, n(%) | 118 (15.6) |
| Depression, n(%) | 10 (0.01) |
| Pain, n(%) | 114 (15.1) |
| Itching, n(%) | 24 (3.2) |
Data are expressed as mean ± standard deviation for continuous variables and number (%) for categorical variables. Other burns include scalding, contact, and chemical burns
Scar pain or itching of ≥5 points on a numerical rating scale
FB flame burn, EB electrical burn, BSA body surface area, WHT wound healing time, LOS length of hospital stay, BMI body mass index
Burn factors associated with vitamin D deficiency
In total, 88.9% of patients were found to have a vitamin D deficiency and 11.1% of patients had a 25(OH) vitamin D level of >20 ng/mL at admission to the department of rehabilitation. The mean 25(OH) vitamin D level was 13.1 ± 3.5 ng/ml in the vitamin D deficient patient group and 23.6 ± 4.5 ng/ml in the non-deficient patient group. The vitamin D deficient patient group had significantly larger burned BSA (p = 0.015) and longer WHT (p < 0.001) than the non-deficient patient group. No significant association with age (p = 0.887), BMI (p = 0.775), history of smoking (p = 0.282), depression (p = 0.795), pain (p = 0.744) and itching (p = 0.993) was observed in the vitamin D deficient patient group. Demographic characteristics of the two vitamin D patient groups are shown in Table 2.
Table 2.
Demographic characteristics concerning the vitamin D deficient and non-deficient groups
| 25(OH) vitamin D levels | ||
|---|---|---|
| Deficiency | Non-deficiency | |
| Variable | n = 673 (88.9%) | n = 84 (11.1%) |
| Age (years) | 39.5 ± 8.3 | 39.4 ± 8.2 |
| Burned BSA (%) | 21.0 ± 16.2* | 16.5 ± 15.2 |
| WHT (days) | 84.2 ± 54.3* | 57.4 ± 41.3 |
| LOS (days) | 89.4 ± 54.2* | 62.3 ± 41.4 |
| BMI (kg/m2) | 23.4 ± 3.0 | 23.5 ± 3.4 |
| History of smoking, n (%) | 37(21.5) | 5(6.0) |
| Depression, n (%) | 16(2.4) | 1 (1.2) |
| Pain, n (%) | 190 (28.2) | 13(15.5) |
| Itching, n (%) | 41(6.1) | 2 (2.4) |
Data are expressed as mean ± standard deviation for continuous variables and number (%) for categorical variables
Age, burned BSA, WHT, LOS, and BMI were analyzed using student’s t-test or Mann-Whitney U test, and history of smoking, depression, pain, and itching were analyzed using Fisher’s exact test
BSA body surface area, WHT wound healing time, LOS length of hospital stay, BMI body mass index
* p < 0.05
Burned BSA, 25(OH) vitamin D level, WHT, LOS, and BMI according to burn type Flame burn (22 (10~36)%) had a larger burned BSA than electrical burn (10 (6~24)%) and other burns (12 (5~20)%) (p < 0.001) (Figure 1). 25(OH) vitamin D levels were significantly lower in electrical burn group (12.1 ± 6.0 mg/mL) than in flame burn and other burns groups (14.7 ± 6.0 mg/mL, 13.8 ± 6.3 mg/mL) (p < 0.001) and were significantly different among three burn types in post-hoc test (all p < 0.05). Electrical burn had longer WHT (71.2 ± 33.3 days) and LOS (76.3 ± 33.2 days) than flame burn (56.1 ± 30.7 days, 61.1 ± 30.8 days) and other burns (47.1 ± 20.3 days, 52.2 ± 20.3 days) (p < 0.001 and p < 0.001). The mean values of BMI were also significantly different among the all burn types in post-hoc test (all p < 0.05) (Figure 2).
Figure 1.
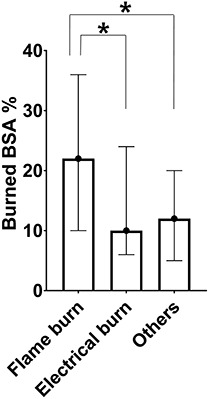
Burned body surface area of different burn groups. The bar of each group represents median values and interquartile range for the percentage of burned BSA. The burned BSA of flame burn group was larger than that of electrical burn group and other burns group. BSA body surface area, Others other burns *p < 0.05
Figure 2.

Vit D, WHT, LOS, and BMI in different burn groups. The bar of each group represents mean and standard deviation for 25(OH) vitamin (a) Vit D, (b) WHT, (c) LOS and (d) BMI. One-way ANOVA or Kruskal-Wallis one-way ANOVA with the post-hoc test was used for statistical analysis of data. Vit D 25(OH) vitamin D, WHT wound healing time, LOS length of hospital stay, BMI body mass index. *p < 0.05
The relationship between vitamin D levels and burn factors among the three burn types
Table 3 shows the correlations between vitamin D levels and age, burned BSA, WHT and BMI among the three burn type groups. In the flame burn and other burns groups, vitamin D levels had weakly negative correlations with burned BSA (p < 0.001, p = 0.002), WHT (p < 0.001, p < 0.001) and LOS (p < 0.001, p < 0.001). In the electrical burn patient group, vitamin D levels had a significant negative correlation with WHT and LOS (all p < 0.001) but there was no correlation between burned BSA and vitamin D levels (p = 0.054). Moreover, the association between vitamin D levels and burn factors according to the types of burn injury are shown in Table 4. The variance inflation factor values to detect multicollinearity ranged from 1.0 to 1.3 (Table S1). As our variance inflation factor is not >10, our regression results were acceptable despite the correlation between each factor. Vitamin D levels were negatively associated with burned BSA (p = 0.009) and WHT (p = 0.007) for flame burn, with WHT (p < 0.001) for electrical burn and with burned BSA (p = 0.023) and WHT (p < 0.001) for other burns. These results showed that WHT was a common factor significantly associated with vitamin D levels across all three burn types.
Table 3.
Correlations between vitamin D levels and burn factors in three burn groups
| Correlation coefficient, r | ||||||
|---|---|---|---|---|---|---|
| Flame burn | Electrical burn | Other burns | ||||
| r | P value | r | P value | r | P value | |
| Age (years) | −0.015 | 0.381 | 0.049 | 0.251 | −0.113 | 0.063 |
| Burned BSA (%) | −0.189 | <0.001 | −0.116 | 0.054 | −0.203 | 0.002 |
| WHT (days) | −0.192 | <0.001 | −0.483 | <0.001 | −0.321 | <0.001 |
| LOS (days) | −0.192 | <0.001 | −0.483 | <0.001 | −0.321 | <0.001 |
| BMI (kg/m2) | 0.142 | 0.058 | 0.023 | 0.374 | −0.086 | 0.120 |
Other burns include scalding, contact, and chemical burns
Pearson’s correlation coefficients were used for age, LOS, and BMI in flame burn group; age, WHT, LOS, and BMI in electrical burn group; and WHT, LOS, and BMI in other burns
Spearman’s correlation coefficients were used for burned BSA and WHT in flame burn group; burned BSA in electrical burn groud; and age and burned BSA in other burns
BSA body surface area, WHT wound healing time, LOS length of hospital stay, BMI body mass index
Table 4.
Relationship between vitamin D levels and burn factors in three burn groups
| 25(OH) Vitamin D | ||||||
|---|---|---|---|---|---|---|
| Flame burn | Electrical burn | Other burns | ||||
| β | P value | β | P value | β | P value | |
| Age (years) | 0.015 | 0.606 | 0.056 | 0.193 | −0.049 | 0.286 |
| Burned BSA (%) | −0.041 | 0.009 | −0.036 | 0.261 | −0.058 | 0.023 |
| WHT (days) | −0.012 | 0.007 | −0.043 | <0.001 | −0.045 | <0.001 |
| BMI (kg/m2) | 0.123 | 0.129 | −0.065 | 0.571 | −0.157 | 0.098 |
| Adjusted R | 0.257 | 0.249 | 0.264 | |||
Other burns include scalding, contact, and chemical burns
Multiple linear regression analyses were used to demonstrate the effect of burn factors on vitamin D levels in flame burn, electrical burn, and other burns groups
BSA body surface area, WHT wound healing time, BMI body mass index
Cut-off points of burned BSA and WHT for predicting vitamin D deficiency among the three burn types
The cut-off values of burned BSA and WHT for predicting significant vitamin D deficiency according to burn types, and their sensitivity and specificity, are shown in Table 5. Using ROC analysis, the cut-off points for burned BSA was 21% for flame burn, 7% for electrical burn, and 9% for other burns. The cut-off points for WHT to predict vitamin D deficiency were 55 days for lame burn, 63 days for electrical burn, and 60 days for other burns.
Table 5.
Cut-off points for burned BSA and WHT in predicting vitamin D deficiency
| Flame burn |
Electrical burn | Other burns | |
|---|---|---|---|
| Burned BSA (%) | |||
| Cut-off point (%) | 21 | 7 | 9 |
| AUC | 0.598 | 0.565 | 0.649 |
| Sensitivity | 0.618 | 0.531 | 0.657 |
| Specificity | 0.600 | 0.606 | 0.727 |
| 95% CI | 0.505–0.690 | 0.444–0.685 | 0.464–0.834 |
| WHT (days) | |||
| Cut-off point (days) | 55 | 63 | 60 |
| AUC | 0.738 | 0.685 | 0.556 |
| Sensitivity | 0.728 | 0.625 | 0.562 |
| Specificity | 0.675 | 0.606 | 0.545 |
| 95% CI | 0.659–0.817 | 0.570–0.800 | 0.372–0.740 |
Other burns include scalding, contact, and chemical burns
The cut-off points for burned BSA and WHT were calculated to predict 25(OH) vitamin D deficiency using receiver operating characteristic curve (ROC) analysis
BSA body surface area, WHT wound healing time, AUC area under the curve, CI confidence interval
Discussion
Our findings showed that 88.9% of the post-burn inpatients in our study were vitamin D deficient and that patients without vitamin D deficiency had a significantly lower burned BSA. A lower burned BSA could be a factor for lower incidence of vitamin D deficiency and shorter duration of healing, as indicated in previous studies [4,15,19,20,30]. The prevalence of vitamin D deficiency in the Korean general population has been reported to be 47.3% in men and 64.5% in women [31]. The high prevalence of vitamin D deficiency among the inpatients in this study might have been due to reduced sun exposure to protect against hyperpigmentation, as well as longer immobilization because of burn wound dressings, long-term hospitalization for wound healing, decreased cutaneous conversion of vitamin D at and around burn scars and decreased level of vitamin D binding protein [20]. Recently, vitamin D receptors that are present in most cells and tissues have been the focus of medical studies with regard to their role in the regulation of vitamin D in the endocrine system [20]. Vitamin D deficiency may affect wound healing, thereby leading to prolonged hospital stay. No significant association was found with a history of smoking, depression, pain and itching in the vitamin D deficient patient group. However, these findings do not correspond with results of previous epidemiological studies that showed low vitamin D levels were related to a history of smoking, depression, pain, itching in atopic dermatitis and elevated immunoglobulin levels [20,22–26]. In the present study, these variables could not be related to vitamin D deficiency separately because the various mechanisms of burn injuries and burn factors could more strongly influence vitamin D levels. Recently, some studies have demonstrated no evidence suggesting that a reduction in 25(OH) vitamin D levels confers an increase in atopic dermatitis or elevated immunoglobulin E levels [27,28].
Although there were some similarities with previous studies that reported patients with higher levels of vitamin D had a better prognosis and improved wound healing, this study is the first to show that WHT across all three burn types was a common factor associated with vitamin D levels for inpatients with burns who had undergone rehabilitative therapy. Moreover, this study focused on burn types and showed that vitamin D levels were inversely associated with WHT but not with burned BSA for electrical burn; whereas for flame burn and other burns, burned BSA, as well as WHT, had a negative relationship with vitamin D levels. These findings are explicable because it has been reported that electrical burn impair vitamin D biosynthesis and transformation in various internal tissues and organs, as well as in the skin, despite a small burned BSA [11]. Additionally, a local electrical field has been reported to damage cell membranes and cell lysis through non-thermal mechanisms [32], and dysfunction of the autonomic nervous system might result from nerve injury accompanied with an electrical burn [12]. Therefore, electrical burn with vitamin D deficiency could have a significantly longer WHT as compared to the other burn types with the same percentage of burned BSA.
Furthermore, in the present study, we found that vitamin D deficiency was associated with unhealed wounds of approximately 2 months in inpatients with burns undergoing rehabilitative therapy, even when electrical burn patients had a small percentage of burned BSA. The cut-off points of WHT associated with vitamin D deficiency were 55 days for flame burn and 62.5 days for electrical burn. Vitamin D stimulates wound healing mediated by dermal fibroblasts in vitro through interaction with low levels of transforming growth factor beta 1 [5]. It has been reported that 1,25 hydroxyvitamin D3 induces Toll-like receptor function in the skin post-injury, which in turn increases the expression of the antimicrobial peptide cathelicidin [33]. Additionally, antimicrobial peptides have been reported to play a role in the stimulation of fibroblast and keratinocyte proliferation, neovascularization and collagen production [34]. As a result, vitamin D has been shown to increase immunity and promote wound healing via Toll-like receptor 2 function and vitamin D receptor function [20,33]. Vitamin D supplementation in patients with diabetes mellitus and foot ulcers has been reported to decrease the length, depth, width and erythema rate of wounds indirectly through the improvement of glycemic homeostasis [35]. Early initiation of vitamin D therapy may decrease WHT in patients with flame burn and electrical burn with unhealed wounds over a post-burn duration of 2 months. Further study for nutritional intervention is warranted to demonstrate this hypothesis.
This study has some limitations. First, the time taken prior to blood sampling following burn injury differed because the WHT for each patient varied. Second, this study could not determine the causality between vitamin D levels and WHT because there was no data on the vitamin D levels of burn patients at the time of admission to the burn surgery unit. However, it is noteworthy that the relationship between vitamin D levels and burn factors according to burn types was investigated and, for electrical burn, low vitamin D levels measured at the initiation time of intensive rehabilitation therapy following re-epithelialization were related more to WHT than the percentage of burned BSA. A prospective cohort study focusing on dynamic changes in vitamin D levels between the time of admission to the burn surgery unit and wound healing should be conducted. Third, the exposure of patients to the sun was not controlled because not all activities could be restricted during hospital admission. However, our hospital has a close relationship between the departments of surgery and rehabilitation such that the burn patients were transferred directly from the department of surgery to the department of rehabilitation as soon as the burn wounds had completely healed without returning home. This process minimized the exposure of patients to outdoor sun prior to blood sampling and evaluation. Fourth, decreased vitamin D binding protein following burn injury might influence total levels of 25(OH) vitamin D. Finally, other burns were not analysed separately according to each burn type because the sample sizes concerning scalding, chemical or contact burns were too small. Therefore, further studies are warranted to elucidate the relationship and causality between vitamin D levels and burn factors, and to determine how much vitamin D should be supplemented according to each type of burn injury.
Conclusions
In conclusion, WHT across all three burn types was a common factor associated with vitamin D levels for inpatients with burns who had undergone rehabilitative therapy. Particularly, electrical burn with vitamin D deficiency showed prolonged wound healing over a post-burn period of 2 months despite having a small percentage of burned BSA. Independent of burned BSA, nutritional intervention concerning vitamin D in relation to burn wound healing should be considered to guide early initiation of intensive rehabilitation therapy. In future, prospective cohort multicenter studies are warranted to elucidate the association and causality between vitamin D levels and burn factors according to each burn type.
Abbreviations
BSA: body surface area; BMI: body mass index; WHT: wound healing time; LOS: length of hospital time; ROC: receiver operating characteristic; AUC: area under the curve
Conflicts of interest
None declared.
Funding
This study was not supported by any funding or grant.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Authors’ contributions
YS Cho and SH Ohn contributed to the study concept and design. YS Cho, CH Seo and SY Joo collected and assembled the data. YS Cho drafted the manuscript. CH Seo, SY Joo and SH Ohn critically revised the manuscript for important intellectual content. YS Cho performed the statistical analysis. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study protocol was approved and the requirement for informed consent from the participants was waived by the Institutional Review Board of Hangang Sacred Heart Hospital (IRB No. 2017–110).
Consent for publication
Not applicable.
Supplementary Material
References
- 1. Klein GL, Chen TC, Holick MF, Langman CB, Price H, Celis MM, et al. Synthesis of vitamin D in skin after burns. Lancet. 2004;363:291–2. [DOI] [PubMed] [Google Scholar]
- 2. Klein GL, Langman CB, Herndon DN. Vitamin D depletion following burn injury in children: a possible factor in post-burn osteopenia. J Trauma. 2002;52:346–50. [DOI] [PubMed] [Google Scholar]
- 3. Bikle DD, Chang S, Crumrine D, Elalieh H, Man MQ, Choi EH, et al. 25 Hydroxyvitamin D 1 alpha-hydroxylase is required for optimal epidermal differentiation and permeability barrier homeostasis. J Invest Dermatol. 2004;122:984–92. [DOI] [PubMed] [Google Scholar]
- 4. Piotrowska A, Wierzbicka J, Żmijewski M. Vitamin D in the skin physiology and pathology. Acta Biochim Pol. 2016;63:17–29. [DOI] [PubMed] [Google Scholar]
- 5. Ding J, Kwan P, Ma Z, Iwashina T, Wang J, Shankowsky HA, et al. Synergistic effect of vitamin D and low concentration of transforming growth factor beta 1, a potential role in dermal wound healing. Burns. 2016;42:1277–86. [DOI] [PubMed] [Google Scholar]
- 6. Baeke F, Takiishi T, Korf H, Gysemans C, Mathieu C. Vitamin D: modulator of the immune system. Curr Opin Pharmacol. 2010;10:482–96. [DOI] [PubMed] [Google Scholar]
- 7. Cho YS, Seo CH, Joo SY, Song J, Cha E, Ohn SH. The association between postburn vitamin D deficiency and the biomechanical properties of hypertrophic scars. J Burn Care Res. 2019;40:274–80. [DOI] [PubMed] [Google Scholar]
- 8. Cho YS, Joo SY, Seo CH. Crosstalk among adipose tissue, vitamin D level, and biomechanical properties of hypertrophic burn scars. Burns. 2019;45:1430–7. [DOI] [PubMed] [Google Scholar]
- 9. Rech MA, Hidalgo DC, Larson J, Zavala S, Mosier M. Vitamin D in burn-injured patients. Burns. 2019;45:32–41. [DOI] [PubMed] [Google Scholar]
- 10. Al-Tarrah K, Hewison M, Moiemen N, Lord JM. Vitamin D status and its influence on outcomes following major burn injury and critical illness. Burns Trauma. 2018; 6:11. https://doi.org/ 10.1186/s41038-018-0113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Koumbourlis AC. Electrical injuries. Crit Care Med. 2002;30:424–30. [DOI] [PubMed] [Google Scholar]
- 12. Roshanzamir S, Dabbaghmanesh MH, Daggaghmanesh A, Nejati S. Autonomic dysfunction and osteoporosis after electrical burn. Burns. 2016;42:583–8. [DOI] [PubMed] [Google Scholar]
- 13. Byrom RR, Word EL, Tewksbury CG, Edlich RF. Epidemiology of flame burn injuries. Burns. 1984;11:1–10. [DOI] [PubMed] [Google Scholar]
- 14. Bang RL, Sharma PN, Sanyal SC, Al NI. Septicemia after burn injury: a comparative study. Burns. 2002;28:746–51. [DOI] [PubMed] [Google Scholar]
- 15. Gottschlich MM, Mayes T, Khoury J, Warden GD. Hypovitaminosis D in acutely injured pediatric burn patients. J Am Diet Assoc. 2004;104:931–41. [DOI] [PubMed] [Google Scholar]
- 16. Rousseau AF, Losser MR, Ichai C, Berger MM. ESPEN endorsed recommendations: nutritional therapy in major burns. Clin Nutr. 2013;32:497–502. [DOI] [PubMed] [Google Scholar]
- 17. Clark AT, Christie A, Wolf SE. Weight changes and patterns of weight measurements in hospitalized burn patients: a contemporary analysis. Burns Trauma. 2018;6:30. https://doi.org/ 10.1186/s41038-018-0131-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Blay B, Thomas S, Coffey R, Jones L, Murphy CV. Low vitamin D level on admission for burn injury is associated with increased length of stay. J Burn Care Res. 2017;38:e8–13. [DOI] [PubMed] [Google Scholar]
- 19. Pastar I, Stojadinovic O, Yin NC, Ramirez H, Nusbaum AG, Sawaya A, et al. Epithelialization in wound healing: a comprehensive review. Adv Wound Care. 2014;3:445–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. [DOI] [PubMed] [Google Scholar]
- 21. Hart DW, Wolf SE, Chinkes DL, Gore DC, Mlcak RP, Beauford RB, et al. Determinants of skeletal muscle catabolism after severe burn. Ann Surg. 2000;232:455–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kassi EN, Stavropoulos S, Kokkoris P, Galanos A, Moutsatsou P, Dimas C, et al. Smoking is a significant determinant of low serum vitamin D in young and middle-aged healthy males. Hormones (Athens). 2015;14:245–50. [DOI] [PubMed] [Google Scholar]
- 23. Kerr DC, Zava DT, Piper WT, Saturn SR, Frei B, Gombart AF. Associations between vitamin D levels and depressive symptoms in healthy young adult women. Psychiat Res. 2015;227:46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gloth FM, III, Lindsay JM, Zelesnick LB, Greenough WB .. Can vitamin D deficiency produce an unusual pain syndrome? Arch Intern Med. 1991;151:1662–4. [PubMed] [Google Scholar]
- 25. Wang SS, Hon KL, Kong APS, Pong HNH, Wong GWK, Leung TF. Vitamin D deficiency is associated with diagnosis and severity of childhood atopic dermatitis. Pediatr Allergy Immunol. 2014;25:30–5. [DOI] [PubMed] [Google Scholar]
- 26. Hypponen E, Berry DJ, Wjst M, Power C. Serum 25-hydroxyvitamin D and IgE—a significant but nonlinear relationship. Allergy. 2009;64:613–20. [DOI] [PubMed] [Google Scholar]
- 27. Manousaki D, Paternoster L, Standl M, Moffatt MF, Farrall M, Bouzigon E, et al. Vitamin D levels and susceptibility to asthma, elevated immunoglobulin E levels, and atopic dermatitis: a Mendelian randomization study. PLoS Medicine. 2017;14:e1002294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Thuesen BH, Heede NG, Tang L, Skaaby T, Thyssen JP, Friedrich N. No association between vitamin D and atopy, asthma, lung function or atopic dermatitis: a prospective study in adults. Allergy. 2015;70:1501–4. [DOI] [PubMed] [Google Scholar]
- 29. Tsuprykov O, Chen X, Hocher CF, Skoblo R. Lianghong Yin, Hocher B. why should we measure free 25(OH) vitamin D? J Steroid Biochem Mol Biol. 2018;180:87–104. [DOI] [PubMed] [Google Scholar]
- 30. Lee P, Eisman A, Center JR. Vitamin D deficiency in critically ill patients. N Engl J Med. 2009;360:1912–4. [DOI] [PubMed] [Google Scholar]
- 31. Choi HS, Oh HJ, Choi H, Choi WH, Kim JG, Kim KM, et al. Vitamin D insufficiency in Korea—a greater threat to younger generation: the Korea National Health and nutrition examination survey (KNHANES) 2008. J Clin Endocrinol Metab. 2011;96:643–51. [DOI] [PubMed] [Google Scholar]
- 32. Lee RC, Kolodney MS. Electrical injury mechanisms: electrical breakdown of cell membranes. Plast Reconstr Surg. 1987;80:672–9. [DOI] [PubMed] [Google Scholar]
- 33. Schauber J, Dorschner RA, Coda AB, Büchau AS, Liu PT, Kiken D, et al. Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D-dependent mechanism. J Clin Invest. 2007;117:803–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mangoni ML, McDermott AM, Zasloff M. Antimicrobial peptides and wound healing: biological and therapeutic considerations. Exp Dermatol. 2016;25:167–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Razzaghi R, Pourbagheri H, Momen-Heravi M, Bahmani F, Shadi J, Soleimani Z, et al. The effects of vitamin D supplementation on wound healing and metabolic status in patients with diabetic foot ulcer: a randomized, double-blind, placebo-controlled trial. J Diabetes Complicat. 2017;31:766–72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.


