Abstract
Etiology of complex diseases, such as breast cancer, involves multiple genetic, behavioral and environmental factors. Gene sequencing enabled detection of genetic risks with relatively small effect size, and high-resolution metabolomics (HRM) to provide omics level data for exposures is poised to do the same for environmental epidemiology. Coupling HRM to the Child Health and Development Studies (CHDS) cohort combines two unique resources to create a prototype for exposome epidemiology, in which omics scale measures of exposure are used for study of distribution and determinants of health and disease. Using this approach, exposures and biologic responses during pregnancy have been linked to breast cancer in the CHDS. With improved chemical coverage and extension to larger populations and other disease processes, development of exposome epidemiology portends discovery of new disease-associated environment factors with small effect size as well as new capabilities to disentangle these from behavioral and other risk factors.
1. Introduction
The cumulative measure of environmental influences and associated biological responses throughout the lifespan, i.e., the human exposome [1, 2], is becoming tangible as analytic methods become available to provide omics scale measures of exposures and biologic responses [3, 4]. These developments have profound implications for applications in epidemiology of environmental exposures, human development and long-term health outcomes. In this brief commentary, we summarize convergence of redox theory of development [5], detailed analysis of environmental and metabolic space termed “the million metabolome [6]”, and application of advanced mass spectrometry methods with emerging integrative bioinformatics tools to Child Health Development Study (CHDS) serum samples with associated health outcomes, to provide a vision for exposome epidemiology. Exposome epidemiology is herein defined as “Use of omics scale measures of exposure and biologic response for study of distribution and determinants of health and disease”.
The need for new approaches is evident from the historical identification of environmental risk factors for breast cancer, where knowledge of environmental risks was recognized from small populations with high exposures. The availability of unique birth cohorts such as the Child Health and Development Studies (CHDS), with breast cancer outcome data for children and existing maternal samples from pregnancy, provides a window into the past to test for potential environmental exposures during pregnancy which are associated with disease outcome. The value of this longstanding multigenerational health cohort to environmental health is amplified by application of new analytical methods which enable untargeted measurement of tens of thousands of chemical signals in those biologic samples.
From knowledge built in this special issue, we identify potential for this combination of unique resources to promote rigorous development and widespread applications for exposome epidemiology. At the same time, we recognize challenges and critical barriers to implementation and elaboration to fulfill this potential. Most importantly, as outlined below, we can build upon emerging knowledge and big data approaches to achieve important goals in understanding environmental contributions to breast cancer risk, and progress toward these goals will have broader applications to other important health conditions. The approaches are based upon knowledge that in evolution of complex organisms, developmental programs evolved mechanisms to respond to the external redox environment. Evidence that redox-sensitive signaling mechanisms function throughout embryogenesis and fetal development, and that a broad spectrum of environmental agents impacts the redox sensing and signaling mechanisms, lead to the conclusion that systematic and comprehensive methods are needed to study the exposome in pregnancy, development and subsequent disease risk. This provides justification, and indeed, necessity, to extend environmental epidemiology tools to more powerful exposome epidemiology capabilities.
Exposome Biology
About 700 million years ago, a dramatic rise in O2 in the Earth’s atmosphere created a substantial redox gradient between living organisms and their environment [7, 8]. This redox gradient provided a driving force for evolution of the animal kingdom and persists today as a lifelong interface between us and our environment (Fig. 1A). Importantly, this provides founding principles for exposome biology because the evolution of multicellular organisms was driven by risks and benefits of life within a changing redox environment. Complex multicellular animals evolved genetically controlled developmental programs in response to the redox environment [5]. These systems supported differential expression of a common genome to create multiple cell types and functions in response to external signals. These dynamic systems, which include both the epigenetic code [9] and the redox code [7], define the operations required to maintain dynamic organizational structure, proliferation, differentiation, and adaptation to environment [5]. This is manifest in a broad array of redox-sensitive transcriptional and epigenetic controls for gene expression that operate throughout embryonic and fetal development [5] The acquired ability to sense fluctuations of the environment and successfully adapt to these conditions resulted in signaling systems that are universally responsive to environmental exposures. As a consequence, we continuously respond to our environment, with both beneficial as well as harmful consequences. In this context, adverse biologic consequences as well established for endocrine disrupting environmental chemicals [10, 11], can be considered exemplar for all environmental influences on form and function in complex organisms. We are continuously interacting with our environment; our scientific challenge is to systematically and comprehensively separate those factors which cause adverse outcomes to support improved prospects for health.
Figure 1. Environmental driving force for evolution of complex multicellular systems provides foundation for systematic and comprehensive approaches for exposome epidemiology.
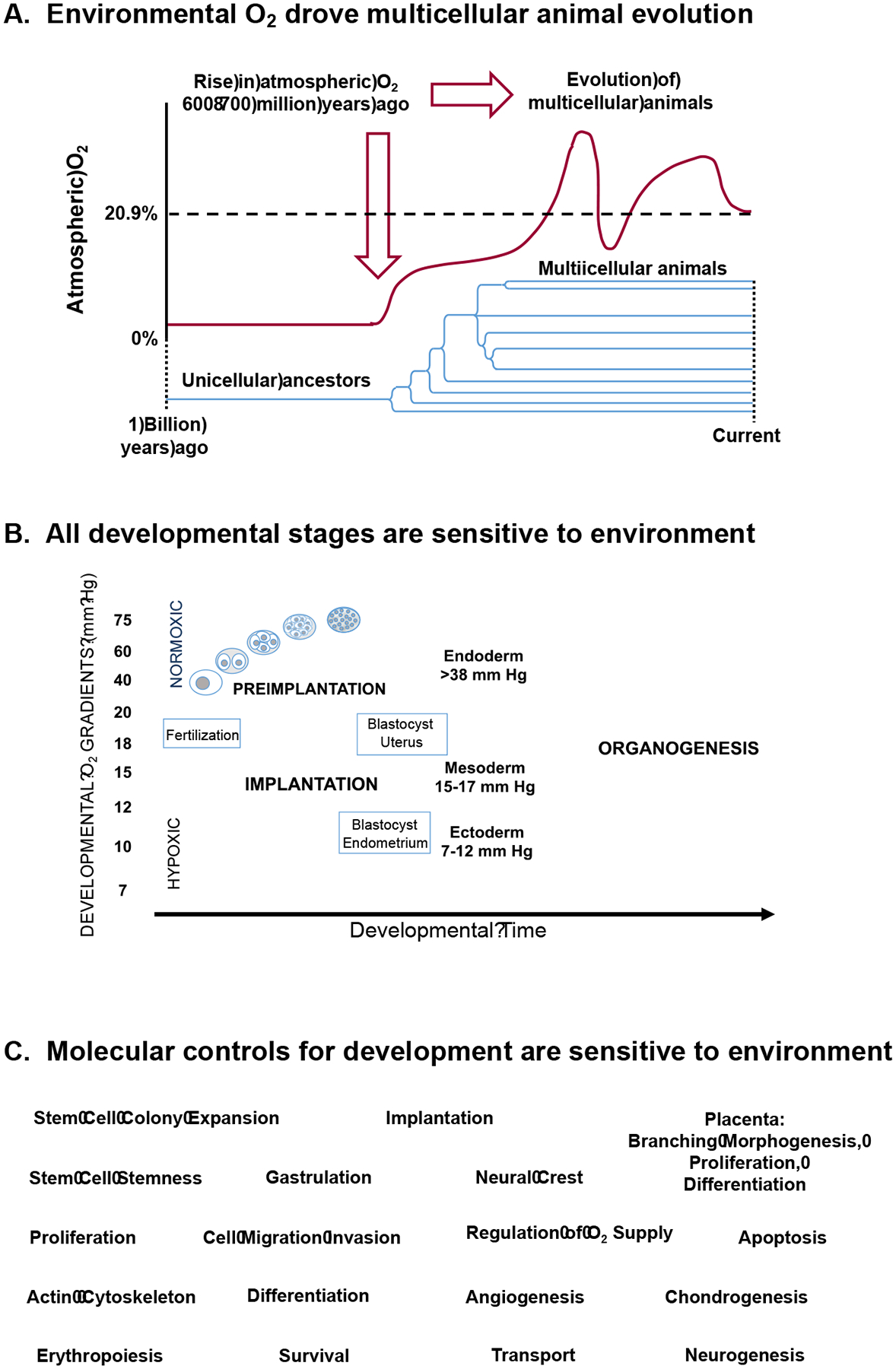
A. The rise in atmospheric O2 about 600 to 700 million years ago provided a key environmental driving force for evolution of animal life. B. All stages of embryonic and fetal development are sensitive to the environment, indicating that research on environmental causes of disease must be systematic and cover the entire life span. C. Molecular signaling and regulatory systems are required for every aspect of development, and these are responsive to environmental agents. Comprehensive approaches are needed to identify adverse environmental exposures impacting these systems.
The developing embryo and fetus depend upon progressive changes in the redox environment which occur through control of O2 delivery and associated maintenance of energetics and redox signaling systems (Fig 1B). The redox signaling systems require O2 to generate reactive oxygen species (ROS), most importantly H2O2, which activate and deactivate sulfur switches in the cysteine residues of proteins [7]. Successive activation/deactivation of these switches thus provides a generalized series of operators to control the complex spatial and time-dependent sequences of events required for multicellular differentiation and development. The redox signaling mechanisms are highly integrated with other reversible switching mechanisms, including phosphorylation, acetylation, methylation, and others. This provides an extensive array of epi-proteome controls linked to central bioenergetic systems which depend upon high-energy systems, ATP, acetyl-CoA, S-adenosylmethionine, etc [12]. The availability of these precursors relies upon the partitioning of activities of the mitochondrial and glycolytic bioenergetic systems, and this partitioning changes during pregnancy, during development, and throughout life, with different demands in different organ systems.
Pleiotropy of ROS signaling has recently been reviewed [7], as have the key systems of the epi-metabolome which control these critical switching mechanisms [12]. Importantly, the exposome, defined broadly in terms of the environmental stressors impacting a biologic system, includes an extensive array of agents causing oxidative stress: air pollution, trace metals, herbicides, fungicides, commercial products, ionizing and non-ionizing radiation, heat stress, psychosocial stress [13]. Major mechanisms for elimination of environmental chemicals are also oxidative in nature, resulting in generation of reactive intermediates which can disrupt developmental programs. Thus, the developmental systems for all of animal life are intimately tied to ongoing interaction with environment. Indeed, an elaboration of these same systems operate throughout lifespan to support adaptation to an uncertain and changing environment.
Child Health and Development Studies
Longitudinal cohort studies enable development of systematic studies of the exposome in breast cancer risk. The fact that endocrine-disrupting chemicals have long-term health outcomes [11, 14] means that any new knowledge obtained concerning pregnancy and breast cancer risk provides foundation for more systematic, in-depth study of other early life exposures and adult-onset disease. Thus, research on breast cancer in the Child Health and Development Studies (CHDS) [15–32] provides a prototype for a generalized approach to systematically address environmental health challenges.
As indicated above, the rationale for application of new untargeted ultra-high resolution mass spectrometry, termed high-resolution metabolomics (HRM), to CHDS, specifically addressing links of exposures in pregnancy to subsequent development of breast cancer, was based upon the innovative paring of the unique resource available in CHDS with the unique resource available from HRM. As a project of the independent, non-profit organization, Public Health Institute, the CHDS is committed to investigating how health and disease are passed on between generations--not just by genes, but also through social, personal, and environmental surroundings. Of critical value, this is a three generational cohort which is now poised to be a four generational cohort, enabling true multigenerational studies in humans.
This is possible because nearly 60 years ago, over 15,000 families in the Kaiser Foundation Health Plan in Oakland, California, voluntarily enrolled when a woman first thought that she was pregnant. Comprehensive interviews were obtained about health, lifestyle, and experiences, and biologic samples were collected, which are useful today to enable scientists to investigate the relationships between biologic, behavioral, genetic, and environmental factors in early family life to health outcomes in adults. The biologic samples collected over a 60-y period from more than 50,000 individuals with associated health outcome data provides a unique resource to understand the pregnancy exposome and its relationship to long-term health outcomes.
Omics level measures of exposures and biologic responses
During the past decade considerable advances were made in use of mass spectrometry-based methods for biomonitoring low abundance environmental chemicals in biologic samples and linking those to biologic responses in metabolism [6, 33]. A critical change, which is currently ongoing, is a transition from assays measuring only a small number of chemicals to methods providing information on hundreds to thousands of chemicals in a single analysis. Development of new data extraction algorithms with multiple extractions using different parameter settings, followed by data merger with statistical filtering, and averaging of multiple technical replicates, improves depth of coverage and quantitative reliability [34]. Use of automated, computerized data extraction algorithms substantially decreases variation due to subjectivity of humans in signal identification and quantification.
Presently, there are no adopted standards for omics scale analyses of environmental chemicals and metabolism. Analytical issues have been summarized [6], and progress in applications has been reviewed [3, 35, 36]. Briefly, in alignment with principles for omics analytics, assays should be simple, automated and high throughput, reproducible, quantitative, suitable for entry into data libraries and affordable (Fig 2). To address these issues, we adopted methods using 1) a single step sample extraction with inclusion of internal standards, 2) an automated, continuous analysis of samples along with pooled reference materials in a 24 h/day, 7 day/week workflow with automated data extraction, 3) quality control procedures to assure reproducibility of technical replicates and signals of internal standards, 4) quantifiability relative to concurrently analyzed pooled reference materials, and 5) assembly into a standardized format with accurate mass, retention time and ion intensity [37]. Currently this has a cost of about $125 per sample including three technical replicates analyzed on each of two complementary platforms [HILIC with positive electrospray ionization (ESI); C18 with negative ESI]. Inclusion of a comparable gas chromatography analysis increases this to about $250 per sample. In principle, this combination of methods will provide coverage of up to a million metabolic and chemical signals in relatively small biologic samples, e.g., <0.5 ml plasma. To keep this in perspective, the least expensive clinical test is probably glucose, which costs about $1/sample; targeted environmental chemical analysis can cost more than hundred dollars per assay. To measure 1000 chemicals at $1/assay would cost at least ten times that for HRM, which currently measures >1000 metabolites with confirmed identification, including >170 confirmed environmental chemicals. Inclusion of gas chromatography more than doubles the number of total confirmed chemicals to over 2000, including over 1200 environmental chemicals with confirmed identity. Thus, from a cost standpoint, high-throughput HRM methods can deliver much greater environmental coverage for a lower cost than provided by targeted methods.
Figure 2. Omics Scale Data for Exposome Epidemiology.
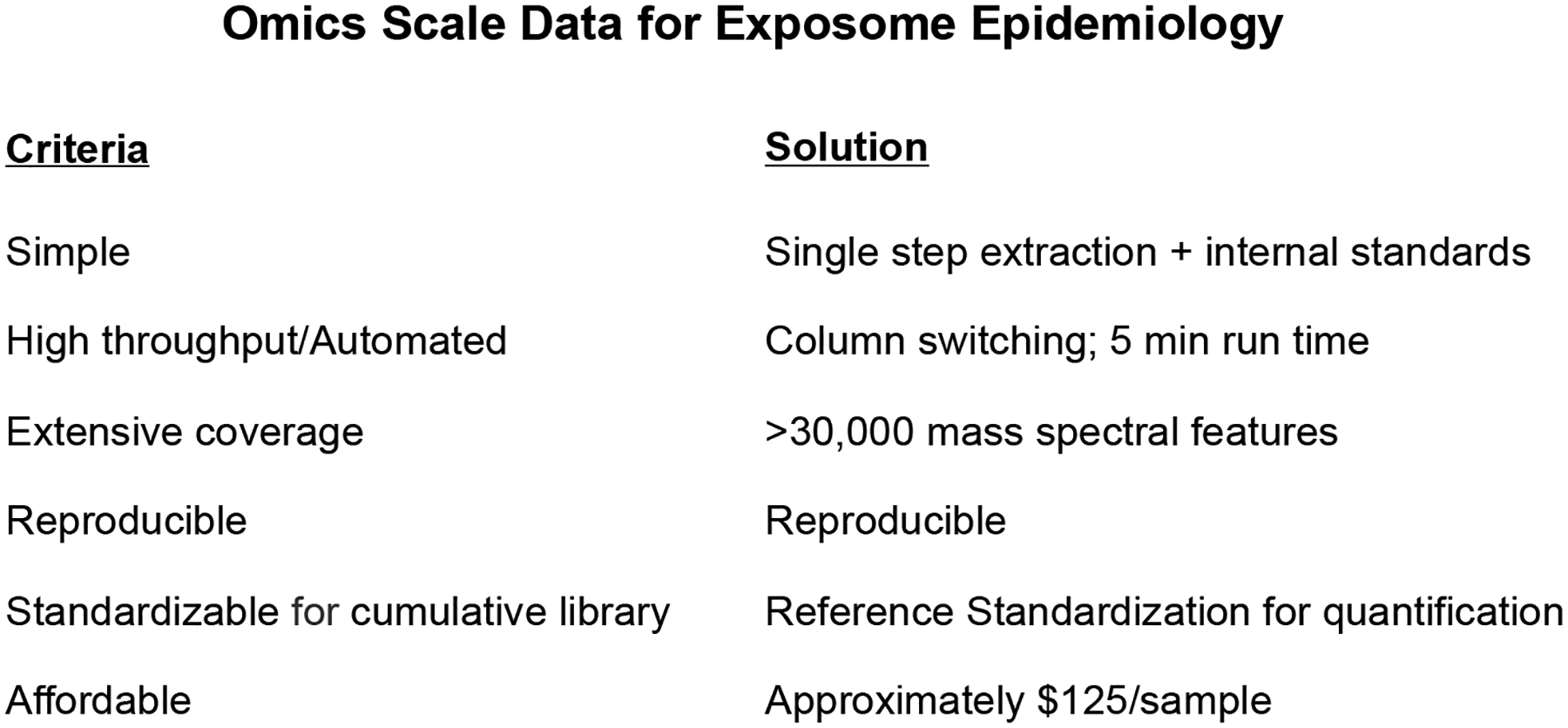
Criteria for omics scale data are provided on the left based upon success of the Human Genome Project in providing genomics data meeting these criteria. For high-resolution metabolomics (HRM), a single step sample treatment was found to effectively remove protein and prepare sample for analysis by liquid chromatography with ultra-high-resolution mass spectrometry detection. By use of an autosampler, a column switching valve and a short run time, a relatively high throughput analytic capability was obtained. The assay gives extensive coverage, with routine detection of >30,000 mass spectral features. Analyses with three technical replicates enables reproducible data detection for each feature. With concurrent analysis of a pooled reference standard, a single point calibration method, reference standardization, provides quantitative analysis which are suitable for inclusion into cumulative data libraries. The current cost is approximately $125/sample.
Demonstration that these methods provide measures of metabolism as well as diverse elements of the exposome (foods, microbiome, drugs and supplements, commercial products, environmental chemicals) was available a decade ago [37]. A relatively early comparison of 7 mammalian species showed that the analyses included information on xenobiotics as well as associated biologic responses [38]. A complete example of an exposome research analysis from external exposure to internal dose to metabolic response to biomarker of health outcome (Fig. 3) was provided in 2016 [39]. This showed that exposure to trichloroethylene (TCE) was associated with metabolites of TCE in the plasma; these TCE metabolites were associated with biologic effects, as measured by metabolic changes; and these metabolic changes were associated with biomarkers of renal injury and immune dysfunction [39]. Current capabilities combine ultra-high resolution mass spectrometry with different separation and ionization methods [liquid chromatography with positive or negative electrospray ionization (ESI); and gas chromatography with electron ionization] to optimize coverage of chemical space and provide capabilities to obtain approximately a million chemical signals. Data using these methods have supported dozens of environmental epidemiology studies, yet full incorporation to support exposome epidemiology has remaining hurdles.
Figure 3. Omics Scale Measurement of Environmental Chemicals and Metabolic Responses Provides a Foundation for Exposome Epidemiology.
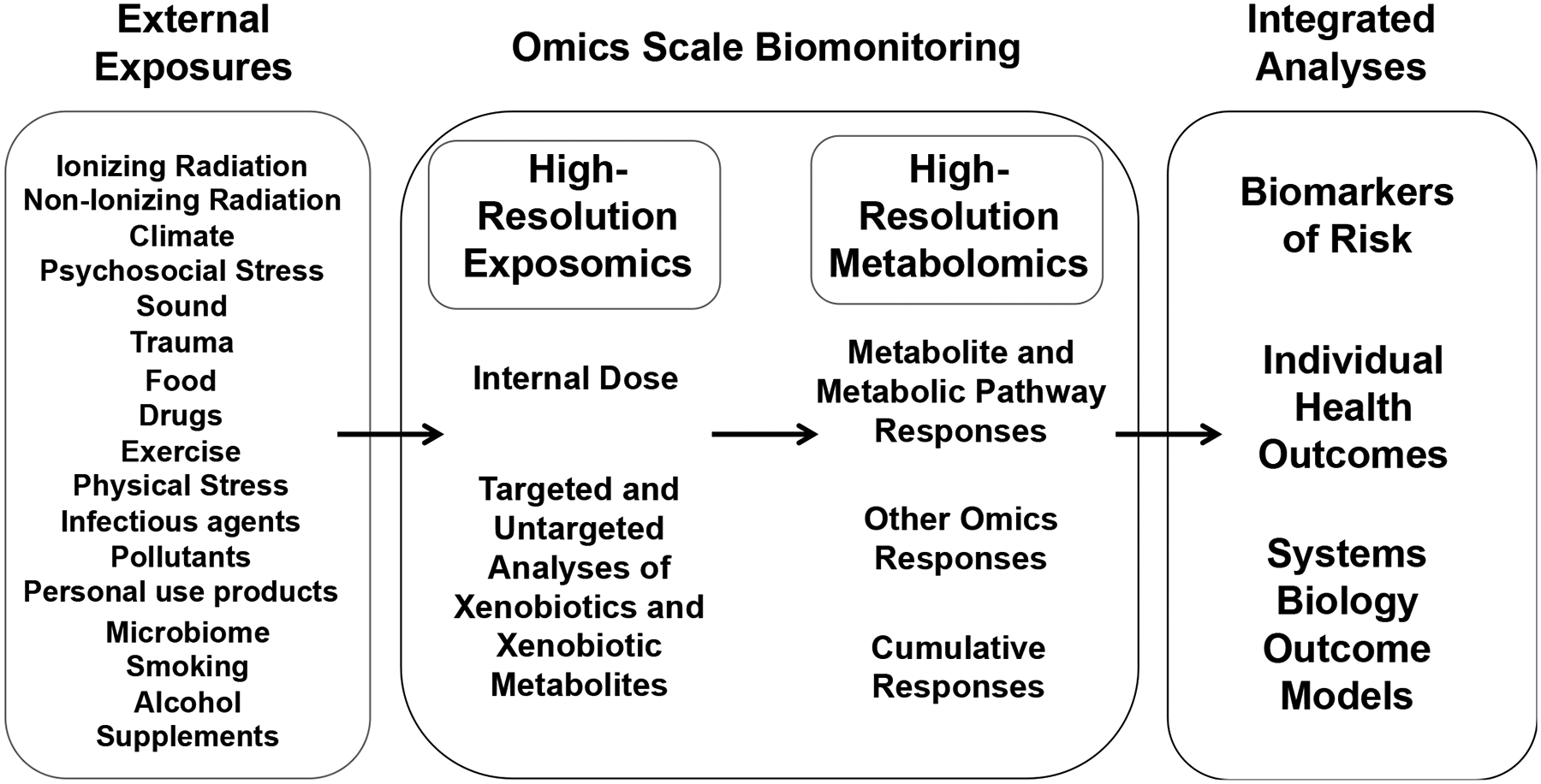
Untargeted ultra-high resolution mass spectrometry methods are greatly expanding the capabilities to obtain omics scale measures of environmental chemical exposure and associated biologic responses in metabolites and metabolic pathways (Center). Coupled to an expanding range of methods for personal exposure monitoring, including improved geospatial monitoring of air pollution and improved availability of sensors and cell phone apps, these methods are providing epidemiology research with expanded capabilities to test a wide range of exposures and separate contributions of different stressors. Integration of these data with other omics data provide new capabilities to identify associations linked to health and develop predictive models of disease outcome.
Transitioning to Exposome Epidemiology
Although environmental or gene x environment factors have been suggested to account for most human disease risk [40, 41], to date, most environmental disease risk factors are recognized because of relatively large effects in individuals having high exposures. Detection of risks from long term exposure to low-dose or exposures with small effect size is more difficult because of needs for sensitive detection of lower exposures and avoidance of mis-interpretation due to other confounding factors. Detection of environmental factors with small effect size also require larger population sizes, and intermittent or transient exposures require that monitoring is frequent enough to allow connection of exposure to outcome. These latter needs place demands for exposome epidemiology which are not fully met by current exposure surveillance methods, i.e., omics scale exposure and metabolic response data are not currently being collected with sufficient frequency or in large enough populations to support the potential of exposome epidemiology.
As indicated above, HRM methods have been used in epidemiology research to link external exposures to internal dose, external dose to metabolic pathway responses, internal dose to metabolic pathway responses, and metabolic pathway responses to health and disease phenotype measures (Fig 3). In the present special issue, this approach complements the other extensive phenotypic and outcome measures used to understand environmental effects on breast cancer. As an example, the study by Hu et al [42] shows the importance of the methods to link different PFAS, in the same chemical class and having similar chemical structures, to different metabolic pathway effects. This finding has been confirmed by analyses of additional PFAS in an independent study [43], suggesting that interactive effects of multiple exposures can be detected and potentially deconvoluted for mechanistic interpretation. Similar findings have been recently reported for air pollution [44] and polycyclic aromatic hydrocarbon exposures [45], with the latter confirmed by HRM analysis using - cell culture system [46]. The conclusion from these studies is that environmental epidemiology can be extended to studies involving simultaneous exposures to larger and more variable environmental mixtures.
Importantly, the newer HRM methods have potential to support exposome epidemiology through rapid expansion of the list of identified environmental chemicals associated with health outcomes. In the short term, cross comparison of untargeted analyses with targeted analyses provides a secure path forward [47]. In the analytic framework to address needs for omics scale analysis of exposure and metabolism (Fig 2), triplicate analyses of individual samples directly support evaluation of reproducibility of chemical detection even for environmental chemicals only detected within a small fraction of a study population. Additionally, the use of triplicates supports use of average or median intensity for replicate assays, improving quantification of low abundance environmental chemicals. By inclusion of concurrent analysis of pooled reference samples, a reference is available for quantification of signals also found within this reference. By having this reference calibrated against a widely available pooled reference, such as National Institute of Standards and Technology Standard Reference Material 1950 (SRM-1950), an analytical structure is made available for deposition into cumulative reference databases. For substances where the pooled reference does not contain the same m/z feature of interest, the intensity can be referenced to intensities for stable isotopic internal standards to facilitate future identification and quantification.
The sensitivity of methods supports analysis of exposures over nearly eight orders of magnitude [6, 47]. This supports detection of large numbers of environmental chemicals in a moderately high-throughput assay at sub-nanomolar concentrations and means that expanded use for exposome epidemiology is possible to test for disease associations. Additionally, reliable detection of unidentified mass spectral features, defined by accurate mass m/z, retention time and intensity, means that disease associations can be detected whether or not the identity of a chemical is known. Such associations provide rationale for studies to identify relevant chemicals and source.
By using a standardized method with rigorous standard operating procedures, collection of data for large populations is possible to support detection of disease associations even for chemicals with small effect size. Because many signals with small effect size are simultaneously detected, biostatistical and bioinformatic approaches can be used to detect interacting or confounding effects by other chemicals. This will demand improved computational and biostatistical methods, especially when attempting to use such information-rich data for large populations. In this regard, it should be noted that HRM databases containing >20,000 m/z features in 10,000 or more biologic samples are not yet available. Thus, maturation of exposome epidemiology will require improved methods for deconvolution of low intensity signals and associations with small effect size in health outcomes.
Next Generation: Genome-Exposome Epidemiology
Gene-environment (G × E) interactions are key drivers of human health and disease. The genetically encoded systems that evolved to allow individuals to adapt to environment are supported by memory systems to improve fitness for survival and reproduction during lifespan [7, 48]. Ultimately, genetic epidemiology and exposome epidemiology must fully embrace this interaction, not at the level of individual genes and individual exposures, but rather at the level that defines precision health and precision medicine, i.e., at the level of the complex cumulative gene-environment interactions that occur in individuals over a lifespan. From the definition of the exposome as the cumulative measure of environmental influences and associated biological responses throughout the lifespan [2], one can recognize that predictive modeling of health and disease will require detailed understanding of the memory systems which allow an individual genome to benefit from its history of exposures by having improved responses to subsequent exposures.
All homeostatic mechanisms, which function to drive an individual to maintain daily health, can be considered as evolved exposure memory systems because these exist within the organism to facilitate survival with prevailing resources (See Fig. 1). These exposure memory systems also include longer-term mechanisms, however, such as cell selection in the immune system, differential gene expression by epigenetic mechanisms, reprogramming of mitochondrial, fatty acid and glycolytic energy metabolisms, and others [7]. These also include structural changes, such as resulting from wounds, physical activity and dietary imbalances, which are often not fully integrated within metabolic and functional models. Omics tools are now widely available to help examine the cumulative lifelong changes in the associated network structure and function that occur as a consequence of these G × E interactions. Methods are not yet in place, however, to routinely capture information on cell populations or long-term structural changes within an individual. The study on placental morphometry and breast density in daughters in this special issue [49] provides clear evidence for the importance of capturing information on physical structures as we move forward with maturation of exposome epidemiology.
Exposome epidemiology is necessary to address the integral of G × E (∫G×E), which changes continuously as a function of E within a lifespan. Systematic investigation of all hypotheses is impractical when the number of interacting elements in a mammalian system is more than 1018 [20,000 genes, 20,000 transcripts with splice variants, 100,000 or more proteins with 20 or more posttranslational modifications, and 10,000 or more metabolites [50]; 2019)]. An exposome epidemiology solution will be to obtain information-rich analyses longitudinally within individuals for whom genetic information is available. While initially limited in power, this will provide a platform to achieve the long-term goal to elucidate exposures impacting outcome at a population level [51].
In the near term, existing cohorts for which multi-omics can be applied to individuals may offer the best opportunity to rapidly advance understanding of the connections between exposures and disease [52]. Differential network analysis tools for multi-omics probe the interplay between molecules rather than changes in single molecules [53], and these approaches enable systematic multiple hypothesis testing to identify central hubs in the organizational structure [50, 54] which provide stability [55]. Omics technologies and bioinformatics tools for integrative omics include both data-driven and knowledge-driven analytic approaches, both of which are represented within papers in this special issue. xMWAS is a data-driven integrative network analysis tool designed for integration of multiple layers of omics data with other data types [54], while MMRN is a multiscale, multifactorial response network method to discover associations between transcriptomics, metabolomics, cytokine, and vaccine response data [56]. Such integrative methods allow deep phenotyping of individuals; when applied across multiple time-points, resolution is obtained to discover and understand multi-level dynamic changes related to health and disease [57]. While there are many gaps in the overall framework for development of exposome epidemiology, this special issue illustrates the state of the art. Li et al [58] bring together data for 39 environmental exposures visualized in networks associated with breast cancer outcome. This was obtained with a very small number of individuals and has not yet benefited from the newer untargeted environmental chemical analytical tools which are becoming available. The models have not yet incorporated the full spectrum of big data tools and databases which are rapidly becoming more accessible and interoperable.
Prospects and Challenges
Among the key innovations provided by the CHDS is the opportunity to address unanswered questions about multigenerational consequences of environmental exposures. Application of the new exposome and metabolome analytic capabilities to this cohort in a systematic way could enable epidemiologic studies to test for association of environmental or metabolic signals with any health outcome in the cohort. The size of the cohort is too small to allow validation of multigenerational effects, but small enough to do studies in an unbiased, systematic manner. With such an approach, the studies would provide critical first steps in discovery of multigenerational environmental effects which can subsequently targeted for validation in larger cohorts.
Exposome epidemiology cannot arrive overnight but will require continued theoretical and practical development. Some of the most critical limitations are presented in Table 1. Among these, current approaches are using omics scale metabolomics data but have not yet achieved omics scale environmental measurements. In fairness, the untargeted analyses include hundreds of known environmental chemicals and could also include thousands of unidentified environmental chemicals and related xenobiotic metabolites, but identification of low intensity mass spectral signals is a major bottleneck. To develop the epidemiologic approaches and fund the sample analyses, it will be necessary to provide compelling arguments to skeptical reviewers and funding agencies of the need to support analyses of unidentified chemical signals as well as development of epidemiologic methods to study them for possible associations with health outcomes. The present collection of articles provide strong argument that this should be high priority.
Table 1.
Needs for Exposome Epidemiology
| >Extension of environmental epidemiology to analysis of omics scale exposure data |
| >Improved environmental chemical and xenobiotic metabolite identification |
| >Improved quantification procedures for low abundance environmental chemicals |
| >Assembly of omics scale xenobiotics and metabolites data for pregnancy exposome of more than 10,000 individuals across multiple generations |
| >Coupling of exposome data to epigenetic data to develop systematic knowledge of the best characterized mechanism for exposure memory |
| >Maintenance of cohorts with coverage of critical developmental exposure windows and consequences for multiple subsequent generations to link ancestral developmental exposures to current health problems; Record exposures in real time, or link to resources to recorded exposures (e.g. newborn blood spots, stored prenatal sera) for multiple generations to comprehend E × E × G impacts on current populations |
| Expansion of high-resolution exposomics and metabolomics analyses |
| Initiation of pilot G × E and predictive modeling studies that result from exposomics studies |
| Integration of ‘omics within informative populations—tracing G × E revealed by the metabolome to track the epigenome, genome, proteome, transcriptome effects—use for pilot testing of approaches for mitigation |
The needs for improved methods for chemical identification are apparent, even though journal reviews frequently discourage or prevent discussion of unidentified signals. This challenge is being addressed by the US National Institutes of Health Metabolomics Common Fund through Chemical Identification Development Cores. This effort may be insufficient to address the large number of unidentified signals, and similar efforts will be needed to address the challenges to obtain reliable quantification of low abundance environmental chemicals at an acceptable cost. Both identification and quantification will allow assembly of data for large populations, and databases for large populations will be needed to allow detection of environmental contributions with small effect sizes. To truly facilitate progress, these resources will need to be publicly available, and specific efforts will be needed to remove barriers and disincentives to collaboration, especially between the public and private sectors.
Resources will be needed to maintain important assets such as CHDS. Cohorts that have been followed for 60 years cannot be replaced for another 60 years. Hence, among the top priorities for exposome epidemiology is the need to assure continued data collection on the relevant cohorts. At the same time, expansion of exposomics and metabolomics analyses and development of resources for investigators to use the resulting omics scale data can function catalytically in research to understand environmental exposures in pregnancy and contributions to breast cancer and other top priority disease risks. As precision medicine further develops, integration of exposome data with genetic data will naturally follow and provide, for the first time, a glimpse into individual exposome health risks.
With this, research into disease etiology for precision medicine will become possible. Challenging questions concerning windows of susceptibly (pregnancy early life), contribution of ancestral exposures, heterogeneity by group (e.g., race and biologic sex at birth, social class, familial risk) and discrimination between behavioral health and environmental health (e.g. metabolic disruption/obesogens + calories) can be addressed. Sufficient interest is present in both the public and private sectors, but higher society level planning will be needed to assure cooperation and efficiency in attainment of the desired new capabilities.
Summary and Conclusions
Research on the pregnancy exposome in relation to breast cancer in the CHDS cohort provides a prototype for a generalized approach to address the key challenge to identify environmental contributions to disease. Application of high-resolution metabolomics to CHDS brings together two unique resources to link exposures in pregnancy to subsequent development of breast cancer. Ultrahigh-resolution mass spectrometry methods for untargeted metabolomics and environmental chemical surveillance enables data-driven approaches to associate unknown, as well as known, chemicals with health outcome. At the same time, analysis of internal metabolic correlations provides leads to potential disease mechanisms. This is critical because there are large numbers of environmental chemicals, and exposures occur with variable intensity and duration and in combination with other health exposures. Biologic effects may be transient or persistent, and health outcomes may become apparent only decades after exposure. By applying these methods to the CHDS to understand environmental factors in pregnancy contributing to subsequent breast cancer, the studies not only provide critical new understanding of environmental contributors to breast cancer but also provide a foundation to extend understanding of prenatal exposures and breast cancer by clarifying interactions with other exposures, health behaviors and health outcomes. Future studies can build upon these successes through application to understand other complex disease etiologies. Incorporation of new computational methods within a more conventional epidemiologic framework demands new considerations to ensure statistical rigor while, at the same time, extending capabilities to discover/infer variable interactions, especially those which occur in subpopulations.
In conclusion, development of exposome epidemiology will require investment. The results demonstrated in this special issue demonstrate that the returns will be substantial and completely worthy of this investment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Referencces
- [1].Wild CP, The exposome: from concept to utility, Int. J. Epidemiol 41(1) (2012) 24–32. [DOI] [PubMed] [Google Scholar]
- [2].Miller GW, Jones DP, The nature of nurture: refining the definition of the exposome, Toxicological Sciences 137(1) (2013) 1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Niedzwiecki MM, Walker DI, Vermeulen R, Chadeau-Hyam M, Jones DP, Miller GW, The Exposome: Molecules to Populations, Annual Review of Pharmacology and Toxicology 59(1) (2019) 107–127. [DOI] [PubMed] [Google Scholar]
- [4].Vermeulen R, Schymanski EL, Barabási A-L, Miller GW, The exposome and health: Where chemistry meets biology, Science 367(6476) (2020) 392–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hansen JM, Jones DP, Harris C, The Redox Theory of Development, Antioxid Redox Signal 32(10) (2020) 715–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Uppal K, Walker DI, Liu K, Li S, Go Y-M, Jones DP, Computational Metabolomics: A Framework for the Million Metabolome, Chemical research in toxicology 29(12) (2016) 1956–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Jones DP, Sies H, The redox code, Antioxidants & redox signaling 23(9) (2015) 734–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Santolini J, Wootton SA, Jackson AA, Feelisch M, The Redox architecture of physiological function, Current opinion in physiology (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Turner BM, Defining an epigenetic code, Nature cell biology 9(1) (2007) 2–6. [DOI] [PubMed] [Google Scholar]
- [10].Schug TT, Johnson AF, Birnbaum LS, Colborn T, Guillette LJ Jr, Crews DP, Collins T, Soto AM, Vom Saal FS, McLachlan JA, Minireview: endocrine disruptors: past lessons and future directions, Molecular Endocrinology 30(8) (2016) 833–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gore AC, Chappell V, Fenton S, Flaws JA, Nadal A, Prins G, Toppari J, Zoeller R, EDC-2: the Endocrine Society’s second scientific statement on endocrine-disrupting chemicals, Endocr Rev 36(6) (2015) E1–E150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hu X, Go YM, Jones DP, Omics Integration for Mitochondria Systems Biology, Antioxid Redox Signal (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sies H, Berndt C, Jones DP, Oxidative stress, Annual review of biochemistry 86 (2017) 715–748. [DOI] [PubMed] [Google Scholar]
- [14].Diamanti-Kandarakis E, Bourguignon J-P, Giudice LC, Hauser R, Prins GS, Soto AM, Zoeller RT, Gore AC, Endocrine-Disrupting Chemicals: An Endocrine Society Scientific Statement, Endocr Rev 30(4) (2009) 293–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Cohn BA, Cirillo PM, Christianson RE, van den Berg BJ, Siiteri PK, Placental characteristics and reduced risk of maternal breast cancer, J Natl Cancer Inst 93(15) (2001) 1133–40. [DOI] [PubMed] [Google Scholar]
- [16].Cohn BA, Wolff MS, Cirillo PM, Sholtz RI, DDT and breast cancer in young women: new data on the significance of age at exposure, Environ Health Perspect 115(10) (2007) 1406–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Cohn BA, Developmental and environmental origins of breast cancer: DDT as a case study, Reproductive Toxicology 31(3) (2011) 302–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Cohn BA, Terry MB, Plumb M, Cirillo PM, Exposure to polychlorinated biphenyl (PCB) congeners measured shortly after giving birth and subsequent risk of maternal breast cancer before age 50, Breast Cancer Research and Treatment 136(1) (2012) 267–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Cohn BA, La Merrill M, Krigbaum NY, Yeh G, Park JS, Zimmermann L, Cirillo PM, DDT Exposure in Utero and Breast Cancer, Journal of Clinical Endocrinology and Metabolism 100(8) (2015) 2865–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Cirillo PM, Benz CC, Cohn BA, Comment on: ‘Hypertensive diseases in pregnancy and breast cancer risk’, British Journal of Cancer 114(11) (2016) e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cohn BA, Cirillo PM, Hopper BR, Siiteri PK, Third Trimester Estrogens and Maternal Breast Cancer: Prospective Evidence, Journal of Clinical Endocrinology 102(10) (2017) 37393748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Krigbaum NY, Rubin RA, Cirillo PM, Terry MB, Habel LA, Morris C, Cohn BA, Feasibility of collecting tumor samples of breast cancer patients diagnosed up to 50 years ago in the Child Health and Development Studies, J Dev Orig Health Dis (2017) 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Tehranifar P, Cohn BA, Flom JD, Protacio A, Cirillo P, Lumey LH, Michels KB, Terry MB, Early life socioeconomic environment and mammographic breast density, BMC Cancer 17(1) (2017) 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Cohn B, Cirillo P, La Merrill M, Correlation of body mass index with serum DDTs predicts lower risk of breast cancer before the age of 50: prospective evidence in the Child Health and Development Studies, J Expo Sci Environ Epidemiol 17 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Cirillo PM, Cohn BA, Gestational biomarkers of daughter’s breast cancer in the Child Health and Development Studies, Reprod. Toxicol (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Cohn BA, Cirillo PM, Terry MB, DDT and Breast Cancer: Prospective Study of Induction Time and Susceptibility Windows, J Natl Cancer Inst 111(8) (2019) 803–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Cohn BA, La Merrill MA, Krigbaum NY, Wang M, Park J-S, Petreas M, Yeh G, Hovey RC, Zimmermann L, Cirillo PM, In Utero Exposure to Poly and Perfluoroalkyl Substances (PFASs) and Subsequent Breast Cancer, Reproductive Toxicology (2019). [DOI] [PubMed] [Google Scholar]
- [28].Krigbaum NY, Cirillo PM, Flom JD, McDonald JA, Terry MB, Cohn BA, In utero DDT exposure and breast density before age 50, Reprod. Toxicol (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].McDonald JA, Cirillo PM, Tehranifar P, Krigbaum NY, Engman N, Cohn BA, Terry MB, In Utero DDT Exposure and Breast Density in Early Menopause by Maternal History of Breast Cancer, Reprod. Toxicol (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Terry MB, Schaefer CA, Flom JD, Wei Y, Tehranifar P, Liao Y, Buka S, Michels KB, Prenatal smoke exposure and mammographic density in mid-life, Journal of Developmental Origins of Health and Disease 2(Special issue 6) (2011) 340–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].McDonald JA, Michels KB, Cohn BA, Flom JD, Tehranifar P, Terry MB, Alcohol intake from early adulthood to midlife and mammographic density, Cancer Causes Control 27(4) (2016) 493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Michels KB, Cohn BA, Goldberg M, Flom JD, Dougan M, Terry MB, Maternal Anthropometry and Mammographic Density in Adult Daughters, Pediatrics 138(Suppl 1) (2016) S34–S41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Hebels DG, Georgiadis P, Keun HC, Athersuch TJ, Vineis P, Vermeulen R, Portengen L, Bergdahl IA, Hallmans G, Palli D, Performance in omics analyses of blood samples in long-term storage: opportunities for the exploitation of existing biobanks in environmental health research, Environmental health perspectives 121(4) (2013) 480–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Uppal K, Soltow QA, Strobel FH, Pittard WS, Gernert KM, Yu T, Jones DP, xMSanalyzer: automated pipeline for improved feature detection and downstream analysis of large-scale, non-targeted metabolomics data, BMC Bioinformatics 14(1) (2013) 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Walker DI, Go Y-M, Liu K, Pennell KD, Jones DP, Population screening for biological and environmental properties of the human metabolic phenotype: implications for personalized medicine, Metabolic Phenotyping in Personalized and Public Healthcare, Elsevier; 2016, pp. 167–211. [Google Scholar]
- [36].Johnson CH, Gonzalez FJ, Challenges and opportunities of metabolomics, Journal of cellular physiology 227(8) (2012) 2975–2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Johnson JM, Yu T, Strobel FH, Jones DP, A practical approach to detect unique metabolic patterns for personalized medicine, Analyst 135(11) (2010) 2864–2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Park YH, Lee K, Soltow QA, Strobel FH, Brigham KL, Parker RE, Wilson ME, Sutliff RL, Mansfield KG, Wachtman LM, High-performance metabolic profiling of plasma from seven mammalian species for simultaneous environmental chemical surveillance and bioeffect monitoring, Toxicology 295(1–3) (2012) 47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Walker DI, Uppal K, Zhang L, Vermeulen R, Smith M, Hu W, Purdue MP, Tang X, Reiss B, Kim S, Li L, Huang H, Pennell KD, Jones DP, Rothman N, Lan Q, High-resolution metabolomics of occupational exposure to trichloroethylene, Int J Epidemiol 45(5) (2016) 1517–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Hemminki K, Bermejo JL, Försti A, The balance between heritable and environmental aetiology of human disease, Nature Reviews Genetics 7(12) (2006) 958–965. [DOI] [PubMed] [Google Scholar]
- [41].Krewski D, Andersen M, Tyshenko M, Krishnan K, Hartung T, Boekelheide K, Wambaugh J, Jones D, Whelan M, Thomas R, Toxicity testing in the 21st century: progress in the past decade and future perspectives, Archives of Toxicology (2019) 1–58. [DOI] [PubMed] [Google Scholar]
- [42].Hu X, Li S, Cirillo PM, Krigbaum NY, Tran V, Jones DP, Cohn BA, Metabolome Wide Association Study of Serum Poly and Perfluoroalkyl Substances (PFASs) in Pregnancy and Early Postpartum, Reproductive Toxicology 87 (2019) 70–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kingsley SL, Eliot MN, Kelsey KT, Calafat AM, Ehrlich S, Lanphear BP, Chen A, Braun JM, Variability and predictors of serum perfluoroalkyl substance concentrations during pregnancy and early childhood, Environmental research 165 (2018) 247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Liang D, Moutinho JL, Golan R, Yu T, Ladva CN, Niedzwiecki M, Walker DI, Sarnat SE, Chang HH, Greenwald R, Use of high-resolution metabolomics for the identification of metabolic signals associated with traffic-related air pollution, Environment international 120 (2018) 145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Smith MR, Woeller CF, Uppal K, Thatcher TH, Walker DI, Hopke PK, Rohrbeck P, Mallon TM, Krahl PL, Utell MJ, Associations of benzo (ghi) perylene and heptachlorodibenzo-p-dioxin in serum of service personnel deployed to Balad, Iraq, and Bagram, Afghanistan correlates with perturbed Amino acid metabolism in human lung fibroblasts, Journal of occupational and environmental medicine 61 (2019) S35–S44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Smith MR, Walker DI, Uppal K, Utell MJ, Hopke PK, Mallon TM, Krahl PL, Rohrbeck P, Go Y-M, Jones DP, Benzo [a] pyrene perturbs mitochondrial and amino acid metabolism in lung epithelial cells and has similar correlations with metabolic changes in human serum, Journal of occupational and environmental medicine 61 (2019) S73–S81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Go YM, Walker DI, Liang Y, Uppal K, Soltow QA, Tran V, Strobel F, Quyyumi AA, Ziegler TR, Pennell KD, Miller GW, Jones DP, Reference Standardization for Mass Spectrometry and High-resolution Metabolomics Applications to Exposome Research, Toxicol Sci 148(2) (2015) 531–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Go Y-M, Jones DP, Exposure memory and lung regeneration, Annals of the American Thoracic Society 13(Supplement 5) (2016) S452–S461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Cohn BA, Cirillo PM, Krigbaum NY, Zimmermann LM, Flom JD, Terry MB, Placental morphometry in relation to daughters’ percent mammographic breast density at midlife, Reproductive Toxicology (2019). [DOI] [PubMed] [Google Scholar]
- [50].Go YM, Fernandes J, Hu X, Uppal K, Jones DP, Mitochondrial network responses in oxidative physiology and disease, Free Radic Biol Med 116 (2018) 31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Jones DP, Sequencing the exposome: a call to action, Toxicology reports 3 (2016) 29–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Hasin Y, Seldin M, Lusis A, Multi-omics approaches to disease, Genome Biol 18(1) (2017) 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Lichtblau Y, Zimmermann K, Haldemann B, Lenze D, Hummel M, Leser U, Comparative assessment of differential network analysis methods, Brief Bioinform 18(5) (2017) 837–850. [DOI] [PubMed] [Google Scholar]
- [54].Uppal K, Ma C, Go YM, Jones DP, Wren J, xMWAS: a data-driven integration and differential network analysis tool, Bioinformatics 34(4) (2018) 701–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Ravasz E, Somera AL, Mongru DA, Oltvai ZN, Barabasi AL, Hierarchical organization of modularity in metabolic networks, Science 297(5586) (2002) 1551–5. [DOI] [PubMed] [Google Scholar]
- [56].Li S, Sullivan NL, Rouphael N, Yu T, Banton S, Maddur MS, McCausland M, Chiu C, Canniff J, Dubey S, Liu K, Tran V, Hagan T, Duraisingham S, Wieland A, Mehta AK, Whitaker JA, Subramaniam S, Jones DP, Sette A, Vora K, Weinberg A, Mulligan MJ, Nakaya HI, Levin M, Ahmed R, Pulendran B, Metabolic Phenotypes of Response to Vaccination in Humans, Cell 169(5) (2017) 862–877 e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Chen R, Mias GI, Li-Pook-Than J, Jiang L, Lam HY, Miriami E, Karczewski KJ, Hariharan M, Dewey FE, Cheng Y, Clark MJ, Im H, Habegger L, Balasubramanian S, O’Huallachain M, Dudley JT, Hillenmeyer S, Haraksingh R, Sharon D, Euskirchen G, Lacroute P, Bettinger K, Boyle AP, Kasowski M, Grubert F, Seki S, Garcia M, Whirl-Carrillo M, Gallardo M, Blasco MA, Greenberg PL, Snyder P, Klein TE, Altman RB, Butte AJ, Ashley EA, Gerstein M, Nadeau KC, Tang H, Snyder M, Personal omics profiling reveals dynamic molecular and medical phenotypes, Cell 148(6) (2012) 1293–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Li S, Cirillo P, Hu X, Tran V, Krigbaum N, Yu S, Jones DP, Cohn B, Understanding mixed environmental exposures using metabolomics via a hierarchical community network model in a cohort of California women in 1960’s, Reproductive Toxicology (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]


