Abstract
Background:
Continuation of dual antiplatelet therapy (DAPT) following coronary artery bypass grafting (CABG) after acute myocardial infarction is recommended by current guidelines. We sought to evaluate guideline adherence over time and factors associated with post-operative DAPT within a regional consortium.
Methods:
Isolated CABG patients from 2011–2017 that had a myocardial infarction within 21 days prior to surgery were included. Patients were stratified by DAPT prescription at discharge and by time period, early (2011–2014) vs. late (2015–2017). Hierarchical regressions were then performed to evaluate factors influencing DAPT use after CABG.
Results:
A total of 7,314 patients were included with an overall rate of DAPT utilization of 31.2% that increased from 29.6% in the early to 33.4% in the late era (p<0.01). There was significant variability in hospital rates of DAPT (Range: 9.5%−92.1%) and hospital level changes over time (26% increased, 11% decreased and 63% remained stable). After adjustment for clinical factors, era was not associated with DAPT use but treating hospital remained significantly associated with DAPT use. Other clinical factors associated with increased DAPT utilization included off-pump surgery (OR 4.48, p<0.01), and prior percutaneous coronary intervention (OR 2.02, p<0.01) while atrial fibrillation (OR 0.39, p<0.01) was associated with decreased utilization.
Conclusions:
Dual antiplatelet use has increased between 2011 and 2017, driven primarily by evolving patient demographics. Significant hospital level variability drives inconsistency in DAPT utilization. Efforts to promote DAPT use for patients treated with CABG after myocardial infarction in concordance with current guidelines should be targeted at the hospital level.
Keywords: CABG, Dual antiplatelet, myocardial infarction
Patients with acute myocardial infarction (AMI) are at significant risk for recurrent ischemic events and cardiovascular mortality.(1) Dual antiplatelet therapy (DAPT) has become a mainstay of antithrombotic therapy for secondary prevention following AMI and has been shown to reduce the risk of future cardiovascular events.(2,3) There is high utilization of DAPT in AMI patients treated with a noninvasive strategy and for those treated with percutaneous coronary intervention (PCI).(4,5) However, the rate of DAPT in patients who undergo surgical revascularization is lower.(6)
One of the factors contributing to this discrepancy is that the benefit of DAPT after coronary artery bypass grafting (CABG) is debated.(7) Recent multicenter, randomized evidence suggests that in elective CABG patients, DAPT improves vein graft patency.(8) In addition, retrospective studies and post-hoc analyses of AMI cohorts demonstrate a benefit of DAPT after CABG in this setting.(6,9,10) However, other investigations have found contradictory results and given the limited prospective, randomized data, debate on the appropriateness of DAPT after CABG remains.(11,12) Despite this being an area of active investigation, the 2016 American College of Cardiology (ACC)/ American Heart Association (AHA) Guidelines Focused Update on the Duration of Dual Antiplatelet Therapy in Patients with Coronary Artery Disease included a class I recommendation for completion of 1 year of DAPT after CABG in the setting of AMI.(13)
To evaluate factors associated with DAPT use after CABG in the setting of AMI, we analyzed rates of DAPT utilization and changes over time within a multicenter regional quality consortium. Specifically, we sought to determine the relative impact that clinical factors, individual surgeon, and hospital level practices have on guideline-directed antiplatelet agent use in the post-operative setting.
Patients and Methods
Patient Data
The Virginia Cardiac Services Quality Initiative (VCSQI) includes 19 hospitals and surgical groups in the region. Registry data includes 99% of all adult cardiac surgery in the region and methodologies for clinical data acquisition have been described previously.(14) Standard Society of Thoracic Surgeons (STS) definitions were used for all variables.(15) Institutional STS data is voluntarily submitted by each center and compiled in a central database to be used for quality improvement. Due to the de-identified nature of the quality database, this study was exempt from review by the University of Virginia Institutional Review Board.
All patients who underwent isolated CABG between July 1, 2011 and June 30, 2017 were extracted from the VCSQI database. Only patients with AMI within 21 days of surgery were included. Patients were excluded if data on discharge antiplatelet agents were missing. Patients were stratified by DAPT use or non-use and whether their operation occurred in the early (2011–2014) or late (2015–2017) era. These years were chosen to allow for similar size group comparisons between eras and specific sensitivity analyses were performed to evaluate the impact of the 2016 ACC/AHA guidelines. Dual antiplatelet therapy was defined as the use of aspirin and any adenosine diphosphate receptor inhibitor.
Statistical Analysis
Continuous variables with skewed distributions are presented as median [interquartile range (IQR)], normally distributed continuous variables as mean ± standard deviation, and categorical variables as count (percentage). Wilcoxon rank sum test was used for skewed continuous variables, independent t test for normally distributed continuous variables and the Chi-Square test was utilized for categorical variables. Hierarchical logistic regression with a generalized linear regression model was used to analyze the effect of center and surgeon level clustering on dual antiplatelet therapy use with both variables treated as random effects. Aggregate effect size for treating hospital and surgeon practice was calculated by summation of estimates and standard errors for each level. Operative year, as well as variables known to influence anti-thrombotic medication use from prior studies and other variables thought by the authors to be important for the decision making in prescribing DAPT were selected a priori and included in the models for risk-adjustment. These are listed in Table 2. Additional risk adjusted analyses were performed to evaluate the impact of the 2014 ACC/AHA Guideline for the Management of Non-ST Elevation Acute Coronary Syndrome and the 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy by evaluating adjusted DAPT use before and after these guideline releases.(13,16) SAS version 9.4 (SAS Institute, Cary, NC) statistical software was used for analysis with a statistical threshold 0.05 set for significance.
Table 2:
Results of hierarchical model predicting risk-adjusted dual antiplatelet use.
| OR | p-value | |
|---|---|---|
| Off-pump surgery | 4.48 (2.77–7.23) | <0.0001 |
| Prior percutaneous coronary intervention | 2.02 (1.63–2.49) | <0.0001 |
| Aggregate hospital effect | 1.93 (1.05–3.55) | 0.0026 |
| ADP Inhibitor with 5 days of Surgery | 1.81 (1.38–2.38) | <0.0001 |
| Peripheral arterial disease | 1.72 (1.31–2.27) | 0.0001 |
| Prior stroke | 1.52 (1.10–2.09) | 0.0110 |
| Aggregate surgeon effect | 1.24 (0.68–2.27) | 0.1784 |
| Major morbidity | 1.21 (0.84 −1.75) | 0.2981 |
| ST elevation myocardial infarction | 1.12 (0.85–1.48) | 0.4369 |
| Predicted risk of morbidity or mortality | 1.01 (1.00–1.02) | 0.0109 |
| Heart failure | 0.99 (0.77–1.28) | 0.9478 |
| Patient age | 0.98 (0.98–0.99) | 0.0024 |
| Post-operative length of stay | 0.96 (0.94–0.98) | 0.0003 |
| Year | 0.90 (0.81–1.00) | 0.0505 |
| Preoperative dialysis | 0.76 (0.44–1.32) | 0.3300 |
| Transfusion | 0.72 (0.56–0.93) | 0.0103 |
| Post-operative atrial fibrillation | 0.64 (0.50–0.81) | 0.0003 |
| History of atrial fibrillation/flutter | 0.39 (0.28–0.55) | <0.0001 |
(C-statistic 0.881) (ADP: adenosine diphosphate; CI: Confidence Interval; OR: Odds Ratio PCI: Percutaneous coronary intervention)
Results
A total of 7,314 patients who underwent isolated CABG in the setting of a recent AMI were included in the analysis after excluding 167 (2.2%) patients with missing discharge medication data (Figure 1). Of these, 4,164 (56.9%) underwent surgery in the early era and 3,149 (43.1%) in the late era (1). In the late era, patients were more likely to have diabetes (50.5% vs. 47.6%, p=0.014), hypertension (86.8% vs. 85.0, p=0.031) and heart failure (31.2% vs. 24.0%, p<0.001) and were more likely to have had a PCI (29.6% vs. 26.2%, p=0.001) in the past. Although rates of other guideline-directed medications including statin therapy and post-operative beta blockers were >95% throughout the study period, the overall rate of post-operative DAPT utilization was 31.2%. In 2011, DAPT use was 16.8% and increased over the subsequent 3 years. DAPT use peaked in 2014 at 35.4% and plateaued for the remainder of the study period (Figure 2).
Figure 1:
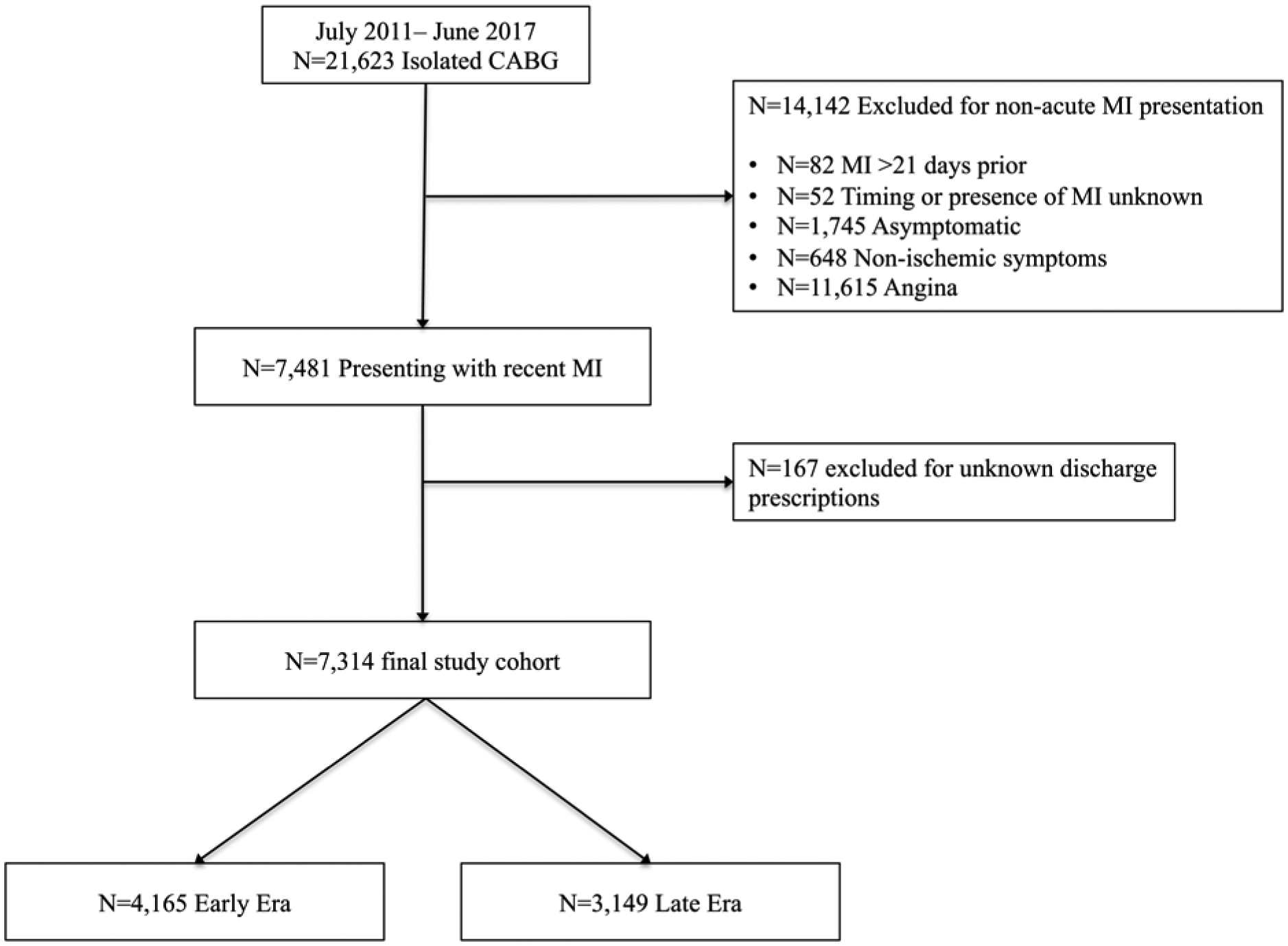
Consort diagram depicting derivation of study cohort and exclusion criteria. (CABG: Coronary Artery Bypass Grafting; MI: Myocardial Infarction)
Figure 2:
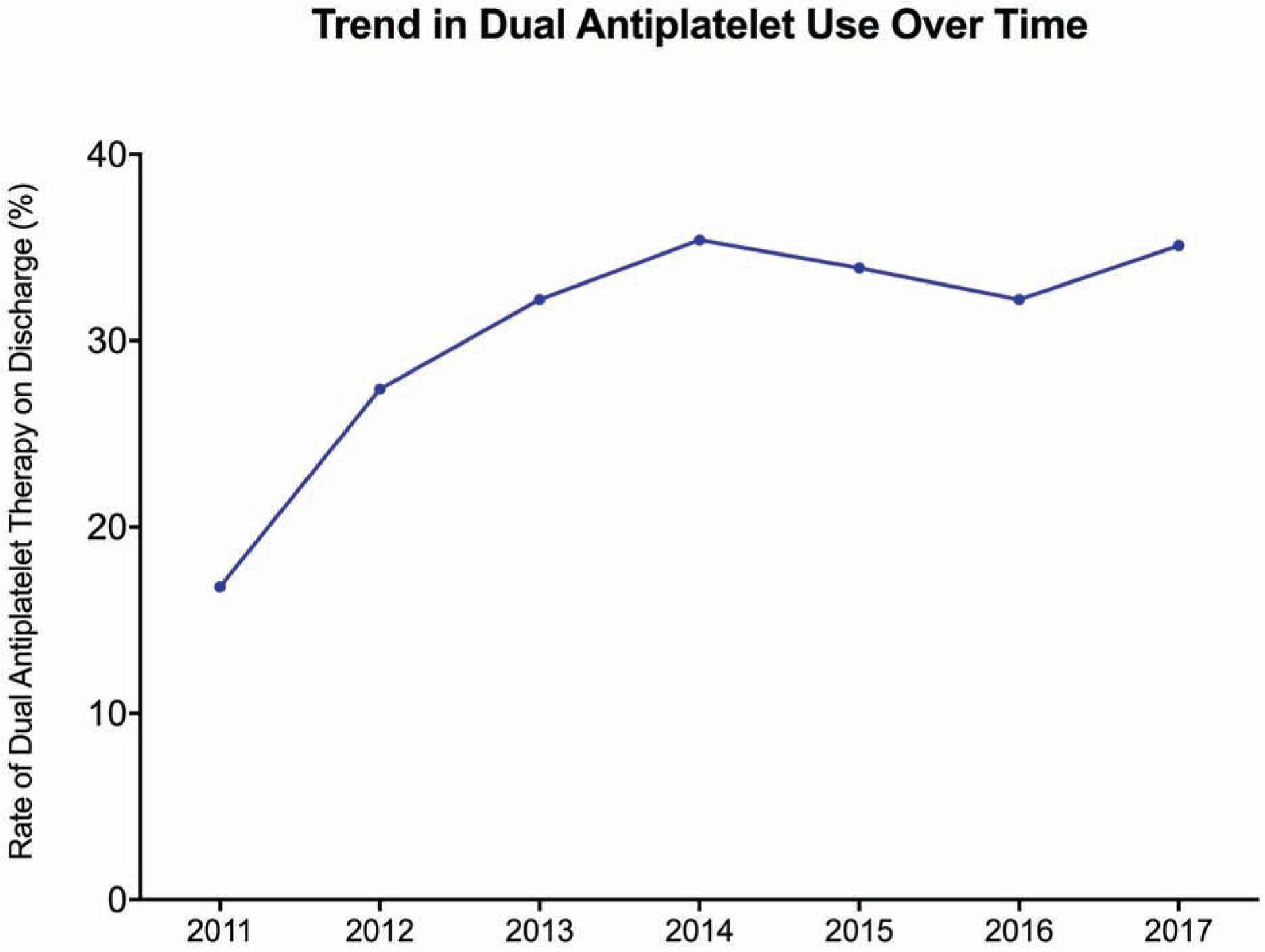
Trend in dual antiplatelet use over the study period.
Hospital level variability
There was substantial hospital level variability in the rates of DAPT utilization (Range: 9.5%−92.1%). Changes over time varied with 26% (5/19) of centers demonstrating a significant increase, 11% (2/19) demonstrating a significant decrease, and 63% (12/19) without significant change (Figure 3). The percent change in hospital-specific DAPT use varied from −44.6% to +217% over the time period of the study.
Figure 3:

Hospital level changes in dual antiplatelet therapy at discharge. A statistically significant increase (green) or decrease (red) in hospital level DAPT use is depicted by the colored lines. The relative hospital volume is signified by the size of the points. (CABG: Coronary Artery Bypass Grafting; DAPT: Dual antiplatelet use)
Comparison by DAPT status
Overall, 2285 (31.2%) of patients were discharged on DAPT. Patients receiving DAPT were younger (63.0±10.7 vs. 64.4±10.6, p<0.0001), had higher prevalence of peripheral arterial disease (17.5% vs. 12.8%, p<0.0001) and more likely to have undergone PCI in the past (37.1% vs. 23.4%, p<0.0001) (Table 1). In addition, those receiving DAPT were more likely to have had a ST-segment elevation MI (18.1% vs. 15.2%, p<0.0001), received ADP inhibitors preoperatively (22.4% vs. 16.5%, p<0.0001) and underwent off-pump surgery (12.7% vs. 3.7%, p<0.0001). Post-operative, in hospital outcomes by DAPT status are shown in Table 1.
Table 1:
Comparison of preoperative characteristics, operative variables and post-operative outcomes of patients stratified by dual antiplatelet utilization.
| No DAPT | DAPT | p-value | |
|---|---|---|---|
| n | 5029 (68.8%) | 2285 (31.2%) | |
| Preoperative Characteristics | |||
| Age | 64.4 ± 10.6 | 63 ± 10.7 | <0.0001 |
| Sex (Female) | 1335 (26.9%) | 669 (29.3%) | 0.0386 |
| Hypertension | 4258 (84.8%) | 2006 (87.9%) | 0.0005 |
| Diabetes Mellitus | 2428 (48.3%) | 1139 (49.9%) | 0.2184 |
| Peripheral Arterial Disease | 640 (12.8%) | 399 (17.5%) | <0.0001 |
| History of Atrial Fibrillation | 284 (12.4%) | 97 (8.2%) | 0.0002 |
| History of Heart Failure | 1394 (27.7%) | 586 (25.7%) | 0.0643 |
| History of Cerebrovascular Accident | 411 (8.2%) | 250 (11.0%) | 0.0001 |
| History of PCI | 1175 (23.4%) | 848 (37.1%) | <0.0001 |
| Preoperative Hemodialysis | 189 (3.8%) | 78 (3.4%) | 0.4664 |
| Predicted Risk of Mortality | 1.53% [0.79–3.35] | 1.51% [0.75–3.25] | 0.2863 |
| Presentation and Operative Factors | |||
| ST Segment Elevation Myocardial Infarction | 762 (15.2%) | 412 (18.1%) | 0.0016 |
| ADP inhibitor within 5 days | 829 (16.5%) | 511 (22.4%) | <0.0001 |
| Off-Pump Surgery | 188 (3.7%) | 289 (12.7%) | <0.0001 |
| Cross Clamp Time | 67 [52–84] | 74 [56–94] | <0.0001 |
| Cardiopulmonary Bypass Time | 93 [73–116] | 102 [79–126] | <0.0001 |
| Post-operative Medications | |||
| Aspirin at Discharge | 4878 (97%) | 2285 (100%) | <0.0001 |
| ADP Inhibitor at Discharge | 75 (1.5%) | 2285 (100%) | <0.0001 |
| Discharge Anticoagulant | 754 (15.0%) | 114 (5.0%) | <0.0001 |
| Post-operative Outcomes | |||
| Operative Mortality | 34 (0.7%) | 10 (0.4%) | 0.2216 |
| Major Morbidity | 660 (13.1%) | 218 (9.5%) | <0.0001 |
| Post-operative Atrial Fibrillation | 1192 (23.7%) | 426 (18.7%) | <0.0001 |
| Transfusion | 1552 (30.9%) | 603 (26.4%) | <0.0001 |
| Renal Failure | 120 (2.4%) | 27 (1.2%) | 0.0007 |
| Stroke | 69 (1.4%) | 34 (1.5%) | 0.695 |
| Cardiac Arrest | 68 (1.4%) | 21 (0.9%) | 0.1178 |
| DSWI | 15 (0.3%) | 8 (0.4%) | 0.7129 |
| Reoperation for Bleeding | 101 (2.0%) | 36 (1.6%) | 0.2057 |
| Prolonged Ventilation (>24 Hours) | 460 (9.2%) | 139 (6.1%) | <0.0001 |
| Post-operative Length of Stay | 6 [5–8] | 6 [4–8] | <0.0001 |
| Readmission | 535 (10.6%) | 242 (10.6%) | 0.9513 |
(ADP: Adenosine Diphosphate; DSWI: Deep Sternal Wound Infection; PCI: Percutaneous Coronary Intervention)
Risk-adjusted factors impacting DAPT use
The strongest clinical predictor of DAPT utilization by regression analysis was off-pump surgery (OR 4.48, p<0.0001). Other clinical factors strongly associated with increased DAPT use were prior PCI (OR 2.02, p<0.0001), and recent use of an ADP inhibitor (OR 1.81, p<0.0001, while a history of atrial fibrillation or flutter (OR 0.39, p<0.0001) was associated with decreased DAPT use (Table 2). The overall magnitude of the effect of the treating hospital was relatively important and statistically significant (OR 1.93, p=0.0026), whereas aggregate surgeon effect was not statistically significant (OR 1.24, p=0.1784). Due to the wide center level variability in DAPT use, the influence of each hospital’s practice ranged from odds ratios of 0.21 to 23.0 (Figure 4). In additional risk adjusted analyses, operation dates before or after the 2014 and 2016 guidelines did not significantly impact DAPT utilization (p=0.0634 and p=0.0507, respectively). Finally, due to the potential that discharge on anticoagulation or atrial fibrillation might impact these findings, a sensitivity analysis was performed excluding these populations. The model retains its excellent predictive ability with a C-statistic of 0.918 and while there are slight differences in the magnitude of the effect size, the variables that are significant on regression analysis are largely unchanged when these patients are excluded.
Figure 4:

Hospital specific adjusted odds ratios for dual antiplatelet use at discharge. (DAPT: Dual Antiplatelet Therapy).
Comment
In this analysis of a regional quality database containing over 7,000 patients who underwent CABG following AMI, we observed a relatively low rate of discharge on DAPT after CABG (31.2%). While there was a significant increase in DAPT over time, this plateaued in 2014. This change appears to be in part affected by evolving patient demographics, with a surgical population at higher overall ischemic risk and more patients presenting for CABG after previous PCI. Moreover, there was significant hospital level variability in DAPT use. Although anticipated clinical factors influenced medication choices, hospital culture or policy appears to have a dominant effect on patient treatment.
The primary finding of the study was that although there was an increase in the utilization of DAPT in post-AMI CABG patients, the overall penetration of this guideline-based therapy remains low. Further, neither the 2014 nor the 2016 ACC/AHA guidelines significantly influenced adjusted DAPT rates. These data suggest that there is a persistent gap between guideline-directed medical therapy and real world clinical practice. Although thought to be most important in patients treated with PCI, compliance with guideline-directed medical therapy significantly reduces cardiovascular events after CABG.(17,18) A review of guideline-directed medical adherence in 6 contemporary revascularization trials demonstrated poorer compliance rates in CABG patients compared to patients revascularized with PCI.(19) Given that these data are reported from well-executed clinical trials with significant oversight, one would expect an even greater deviation in real world practice. Therefore, more emphasis should be placed on ensuring that patients treated with CABG receive appropriate secondary prevention therapies to optimize long-term outcomes.(12) In order to promote guideline adherence, post-infarction care pathways need to be established when patients move from cardiology to surgical services in the setting of AMI. While there will certainly be a cohort of patients with clinical contraindications for DAPT and patients may have DAPT initiated at post-discharge follow up, approximately two thirds of the cohort was not receiving DAPT at discharge. Continuing DAPT for 1 year continues to be a class I recommendation, and the surgical community should take responsibility for ensuring that their patients receive optimal medical therapy at least at the time of discharge.(13)
The basis for the low rate of DAPT use is multifactorial, however, in this case, the lack of universal acceptance of DAPT following CABG is most likely the primary cause. Although the benefits of aspirin for secondary prevention are clear, surgeons are unsure whether there is a benefit to adding a second antiplatelet agent.(11,20) Recent data from Zhao et. al. provides some of the strongest evidence for the efficacy of DAPT to improve vein graft patency.(8) This study was done in an elective CABG population and used ticagrelor rather than the more commonly prescribed clopidogrel.(5) Ticagrelor is known to have a more rapid onset with a higher peak platelet inhibition and therefore may be more efficacious than clopidogrel.(21) In addition to the biochemical advantage of ticagrelor, its superior clinical potency has been shown in a subgroup analysis of patients treated with CABG in the PLATO trial. In this study, ticagrelor in combination with aspirin significantly reduced cardiovascular mortality at 1-year compared to clopidogrel with aspirin.(22) Prior single institution and multi-center randomized controlled trials with clopidogrel as the second antiplatelet agent have suggested the benefit of DAPT to both improve early graft patency and limit native coronary artery disease progression.(23–26) Multiple pooled analyses have also demonstrated superiority of DAPT to aspirin, with the most recent meta-analysis including over 11,000 patients and demonstrating the protective effect of DAPT with decreased graft occlusion (Relative Risk: 0.79) and reduction in all-cause mortality (Relative Risk: 0.67).(27–29)
Previous studies have demonstrated a wide variation of knowledge and attitudes surrounding dual antiplatelet use after CABG at the surgeon level.(30,31) However, in this study, we found that the treating hospital plays an unexpectedly large role in determining the antiplatelet regimen that patients receive. Most likely, this reflects the fact that surgeons at the same institution tend to follow similar protocols. Hospital level variation is not a new observation and has been demonstrated by other multicenter groups in several areas including pre-CABG antiplatelet discontinuation and transfusion rates after cardiovascular procedures.(32–34) However, in our current study, inter-hospital consistency in the utilization of other guideline-directed medications, such as statins and aspirin, suggest this is not indicative of a hospital quality gap, but rather a difference of perception as to the appropriateness of DAPT in post-CABG patients.
Beyond the treating hospital, there are a number of clinical factors that influence which patients receive post-operative DAPT. Patients that had received an ADP inhibitor prior to surgery and those who likely have a separate indication for treatment, such as those with peripheral arterial disease or prior PCI, were more likely to receive DAPT. In addition, those who underwent off-pump CABG were much more likely to receive DAPT. Patients that were at higher risk for bleeding events, such as older patients, those with atrial fibrillation and therefore, an indication for anticoagulation, or those requiring post-operative transfusions were less likely to receive DAPT. These findings are generally in concordance with a single center study that found age, preoperative ADP use, preoperative dialysis, off-pump surgery and anticoagulant use to be predictors of DAPT following CABG.(31) These findings suggest that clinicians currently weigh the relative likelihood of ischemic and bleeding events to determine who would most likely benefit from DAPT. Clinical risk scores similar to those that exist for the decision to extend DAPT beyond 1 year after PCI may be beneficial for this decision after CABG.(35)
This study is limited by its retrospective nature, which incorporates an element of selection bias. Regression modeling was used to control for these factors. In addition, the specific agents used are not identifiable, and there are known differences between medications within the same class. We only included a select subset of patients who underwent CABG, those with a recent AMI and therefore, are unable to draw conclusions about the larger population that requires CABG in other settings or with concomitant valve surgery. Finally, conclusions drawn from this regional consortium may not be generalizable to other regions.
In conclusion, although the rate of dual antiplatelet use for patients treated with CABG after AMI has increased in recent years, this has plateaued and much of the change can be attributed to evolving patient characteristics. There is substantial hospital-level prescribing variability that impacts the secondary prevention therapy that patients receive. Multidisciplinary efforts are needed to ensure patients receive optimal, guideline-directed secondary prevention following AMI and should be targeted at the hospital level.
Sources of Funding:
This work was supported by the National Heart, Lung, and Blood Institute (grant T32 HL007849).
Abbreviations:
- ACC
American College of Cardiology
- AHA
American Heart Association
- AMI
Acute Myocardial Infarction
- CABG
Coronary Artery Bypass Grafting
- DAPT
Dual Antiplatelet Therapy
- IQR
Interquartile Range
- PCI
Percutaneous Coronary Intervention
- STS
Society of Thoracic Surgeons
- VCSQI
Virginia Cardiac Services Quality Initiative
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Gorav Ailawadi discloses consulting for Abbott, Medtronic, Edwards Lifesciences, Admedus, and Cephea Valve Technologies. All other authors have nothing to disclose.
References
- 1.Jernberg T, Hasvold P, Henriksson M, Hjelm H, Thuresson M, Janzon M. Cardiovascular risk in post-myocardial infarction patients: nationwide real world data demonstrate the importance of a long-term perspective. European heart journal 2015;36:1163–70. [DOI] [PubMed] [Google Scholar]
- 2.Yusuf S, Zhao F, Mehta SR et al. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 2001;345:494–502. [DOI] [PubMed] [Google Scholar]
- 3.Wiviott SD, Antman EM, Gibson CM et al. Evaluation of prasugrel compared with clopidogrel in patients with acute coronary syndromes: design and rationale for the TRial to assess Improvement in Therapeutic Outcomes by optimizing platelet InhibitioN with prasugrel Thrombolysis In Myocardial Infarction 38 (TRITON-TIMI 38). Am Heart J 2006;152:627–35. [DOI] [PubMed] [Google Scholar]
- 4.Vermeer NS, Bajorek BV. Utilization of evidence-based therapy for the secondary prevention of acute coronary syndromes in Australian practice. J Clin Pharm Ther 2008;33:591–601. [DOI] [PubMed] [Google Scholar]
- 5.Dayoub EJ, Seigerman M, Tuteja S et al. Trends in Platelet Adenosine Diphosphate P2Y12 Receptor Inhibitor Use and Adherence Among Antiplatelet-Naive Patients After Percutaneous Coronary Intervention, 2008–2016. JAMA Intern Med 2018;178:943–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sorensen R, Abildstrom SZ, Hansen PR et al. Efficacy of post-operative clopidogrel treatment in patients revascularized with coronary artery bypass grafting after myocardial infarction. J Am Coll Cardiol 2011;57:1202–9. [DOI] [PubMed] [Google Scholar]
- 7.Levine GN, Bakaeen FG. Adding CABG to the Dual Antiplatelet Salad. J Am Coll Cardiol 2017;69:128–130. [DOI] [PubMed] [Google Scholar]
- 8.Zhao Q, Zhu Y, Xu Z et al. Effect of Ticagrelor Plus Aspirin, Ticagrelor Alone, or Aspirin Alone on Saphenous Vein Graft Patency 1 Year After Coronary Artery Bypass Grafting: A Randomized Clinical Trial. JAMA 2018;319:1677–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fox KA, Mehta SR, Peters R et al. Benefits and risks of the combination of clopidogrel and aspirin in patients undergoing surgical revascularization for non-ST-elevation acute coronary syndrome: the Clopidogrel in Unstable angina to prevent Recurrent ischemic Events (CURE) Trial. Circulation 2004;110:1202–8. [DOI] [PubMed] [Google Scholar]
- 10.Smith PK, Goodnough LT, Levy JH et al. Mortality benefit with prasugrel in the TRITON-TIMI 38 coronary artery bypass grafting cohort: risk-adjusted retrospective data analysis. J Am Coll Cardiol 2012;60:388–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kulik A Secondary prevention after coronary artery bypass graft surgery: a primer. Curr Opin Cardiol 2016;31:635–643. [DOI] [PubMed] [Google Scholar]
- 12.Kulik A, Ruel M, Jneid H et al. Secondary prevention after coronary artery bypass graft surgery: a scientific statement from the American Heart Association. Circulation 2015;131:927–64. [DOI] [PubMed] [Google Scholar]
- 13.Levine GN, Bates ER, Bittl JA et al. 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2016;68:1082–115. [DOI] [PubMed] [Google Scholar]
- 14.Ailawadi G, LaPar DJ, Speir AM et al. Contemporary Costs Associated With Transcatheter Aortic Valve Replacement: A Propensity-Matched Cost Analysis. The Annals of thoracic surgery 2016;101:154–60; discussion 160. [DOI] [PubMed] [Google Scholar]
- 15.Surgeons TSoT. Adult Cardiac Surgery Database Collection. 2018.
- 16.Amsterdam EA, Wenger NK, Brindis RG et al. 2014 AHA/ACC Guideline for the Management of Patients with Non-ST-Elevation Acute Coronary Syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;64:e139–e228. [DOI] [PubMed] [Google Scholar]
- 17.Rasmussen JN, Chong A, Alter DA. Relationship between adherence to evidence-based pharmacotherapy and long-term mortality after acute myocardial infarction. JAMA 2007;297:177–86. [DOI] [PubMed] [Google Scholar]
- 18.Kurlansky P, Herbert M, Prince S, Mack M. Coronary Artery Bypass Graft Versus Percutaneous Coronary Intervention: Meds Matter: Impact of Adherence to Medical Therapy on Comparative Outcomes. Circulation 2016;134:1238–1246. [DOI] [PubMed] [Google Scholar]
- 19.Pinho-Gomes AC, Azevedo L, Ahn JM et al. Compliance With Guideline-Directed Medical Therapy in Contemporary Coronary Revascularization Trials. J Am Coll Cardiol 2018;71:591–602. [DOI] [PubMed] [Google Scholar]
- 20.Mangano DT, Multicenter Study of Perioperative Ischemia Research G. Aspirin and mortality from coronary bypass surgery. N Engl J Med 2002;347:1309–17. [DOI] [PubMed] [Google Scholar]
- 21.Xu F, Feng W, Zhou Z et al. Antiplatelet effects of ticagrelor versus clopidogrel after coronary artery bypass graft surgery: A single-center randomized controlled trial. J Thorac Cardiovasc Surg 2018. [DOI] [PubMed] [Google Scholar]
- 22.Held C, Asenblad N, Bassand JP et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes undergoing coronary artery bypass surgery: results from the PLATO (Platelet Inhibition and Patient Outcomes) trial. J Am Coll Cardiol 2011;57:672–84. [DOI] [PubMed] [Google Scholar]
- 23.Sun JC, Teoh KH, Lamy A et al. Randomized trial of aspirin and clopidogrel versus aspirin alone for the prevention of coronary artery bypass graft occlusion: the Preoperative Aspirin and Postoperative Antiplatelets in Coronary Artery Bypass Grafting study. Am Heart J 2010;160:1178–84. [DOI] [PubMed] [Google Scholar]
- 24.Une D, Al-Atassi T, Kulik A, Voisine P, Le May M, Ruel M. Impact of clopidogrel plus aspirin versus aspirin alone on the progression of native coronary artery disease after bypass surgery: analysis from the Clopidogrel After Surgery for Coronary Artery DiseasE (CASCADE) randomized trial. Circulation 2014;130:S12–8. [DOI] [PubMed] [Google Scholar]
- 25.Gao G, Zheng Z, Pi Y, Lu B, Lu J, Hu S. Aspirin plus clopidogrel therapy increases early venous graft patency after coronary artery bypass surgery a single-center, randomized, controlled trial. J Am Coll Cardiol 2010;56:1639–43. [DOI] [PubMed] [Google Scholar]
- 26.Kulik A, Le May MR, Voisine P et al. Aspirin plus clopidogrel versus aspirin alone after coronary artery bypass grafting: the clopidogrel after surgery for coronary artery disease (CASCADE) Trial. Circulation 2010;122:2680–7. [DOI] [PubMed] [Google Scholar]
- 27.Agarwal N, Mahmoud AN, Patel NK et al. Meta-Analysis of Aspirin Versus Dual Antiplatelet Therapy Following Coronary Artery Bypass Grafting. Am J Cardiol 2018;121:32–40. [DOI] [PubMed] [Google Scholar]
- 28.Nocerino AG, Achenbach S, Taylor AJ. Meta-analysis of effect of single versus dual antiplatelet therapy on early patency of bypass conduits after coronary artery bypass grafting. Am J Cardiol 2013;112:1576–9. [DOI] [PubMed] [Google Scholar]
- 29.Deo SV, Dunlay SM, Shah IK et al. Dual anti-platelet therapy after coronary artery bypass grafting: is there any benefit? A systematic review and meta-analysis. J Card Surg 2013;28:109–16. [DOI] [PubMed] [Google Scholar]
- 30.Yanagawa B, Ruel M, Bonneau C et al. Dual antiplatelet therapy use by Canadian cardiac surgeons. J Thorac Cardiovasc Surg 2015;150:1548–54 e3. [DOI] [PubMed] [Google Scholar]
- 31.Mori M, Shioda K, Yun JJ, Mangi AA, Darr U, Geirsson A. Pattern and predictors of dual antiplatelet use after coronary artery bypass graft surgery. J Thorac Cardiovasc Surg 2018;155:632–638. [DOI] [PubMed] [Google Scholar]
- 32.Biancari F, Mariscalco G, Gherli R et al. Variation in preoperative antithrombotic strategy, severe bleeding, and use of blood products in coronary artery bypass grafting: results from the multicentre E-CABG registry. Eur Heart J Qual Care Clin Outcomes 2018;4:246–257. [DOI] [PubMed] [Google Scholar]
- 33.Likosky DS, Wallace AS, Prager RL et al. Sources of Variation in Hospital-Level Infection Rates After Coronary Artery Bypass Grafting: An Analysis of The Society of Thoracic Surgeons Adult Heart Surgery Database. The Annals of thoracic surgery 2015;100:1570–5; discussion 1575–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Likosky DS, Zhang M, Paone G et al. Impact of institutional culture on rates of transfusions during cardiovascular procedures: The Michigan experience. Am Heart J 2016;174:1–6. [DOI] [PubMed] [Google Scholar]
- 35.Yeh RW, Secemsky EA, Kereiakes DJ et al. Development and Validation of a Prediction Rule for Benefit and Harm of Dual Antiplatelet Therapy Beyond 1 Year After Percutaneous Coronary Intervention. Jama 2016;315:1735–49. [DOI] [PMC free article] [PubMed] [Google Scholar]


