Abstract
Ischemic and hemorrhagic stroke can occur in the setting of pediatric trauma, particularly those with head or neck injuries. The risk of stroke appears highest within the first two weeks after trauma. Stroke diagnosis may be challenging due to lack of awareness or concurrent injuries limiting detailed neurologic assessment. Other injuries may also complicate stroke management, with competing priorities for blood pressure, ventilator management, or antithrombotic timing. Here we review epidemiology, clinical presentation and diagnostic approach to blunt arterial injuries including dissection, cerebral sinovenous thrombosis, mineralizing angiopathy, stroke from abusive head trauma, and traumatic hemorrhagic stroke. Due to the complexities and heterogeneity of concomitant injuries in stroke related to trauma, a single pathway for stroke management is impractical. Therefore providers must understand goals and possible costs or consequences of stroke management decisions in order to individualize patient care. We discuss physiologic principles of cerebral perfusion and oxygen delivery, considerations for ventilator strategy when stroke and lung injury are present, and current available evidence of risks and benefits of anticoagulation to provide a framework for multidisciplinary discussions of cerebrovascular injury management in pediatric trauma patients.
Keywords: Child, humans, stroke, cerebrovascular trauma, hemorrhage, craniocerebral trauma, thrombosis, arterial dissection
Introduction
Trauma is the leading cause of death for children greater than one year of age in the United States. 1 Cerebral vascular complications of trauma can dramatically worsen the likelihood of mortality and morbidity in this patient population, as they can lead to ischemic and hemorrhagic stroke. Improved awareness and recognition of these complications is key, as early identification of patients at risk can facilitate timely diagnosis and treatment.
Delayed diagnosis is unfortunately common in pediatric stroke2, 3. Children with trauma may be particularly vulnerable not only due to the general lack of awareness of pediatric stroke, but also due to concurrent injuries or treatment of injuries that can preclude thorough assessment of neurologic status. Examples of such limitations include splinting of broken bones that impede function or examination of the extremities, or sedation requirements to tolerate intubation that impede mental status assessment. Awareness and vigilance are therefore necessary for timely diagnosis of stroke in children with trauma. Literature on the epidemiology of trauma-related stroke in the pediatric population is limited, often leading to lack of consensus among different providers about when or how to screen for cerebral vascular complications. This review will focus on types of vascular injuries and risk factors for stroke in trauma, as well as special considerations for stroke management in the setting of complex competing priorities of other traumatic injuries in the pediatric intensive care unit.
Epidemiology
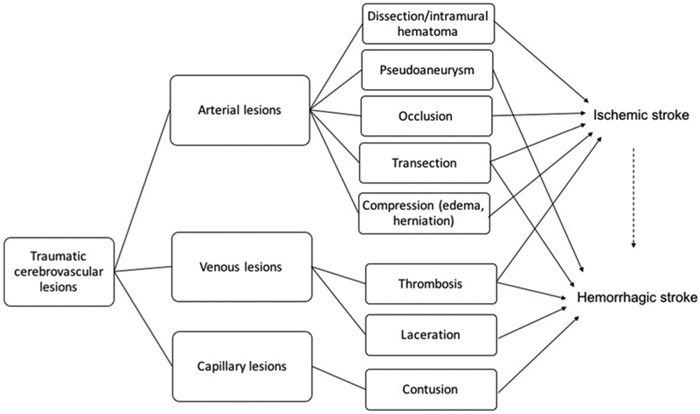
Traumatic cerebral vascular injury can affect venous or arterial systems (Figure 1) and be caused by blunt or penetrating trauma. As injuries from motor vehicle collision are the leading cause of death for children in the United States,4 this review will focus on injuries resulting from blunt trauma, interpreted as all trauma that is non-penetrating and non-thermal. Nevertheless, it is important to note that, though rare, penetrating cerebrovascular injury in pediatric trauma is a challenging entity of its own. Penetrating cerebrovascular injury has been reported in 5.4% of pediatric patients that were specifically screened for penetrating trauma, including gunshot wounds5. The overall incidence of all stroke types in pediatric blunt trauma is not well defined in the literature. In a large database of children and young adult trauma encounters, the 4-week arterial ischemic stroke incidence was 4.0 per 100,000 encounters. In 37% of ischemic stroke cases, the stroke occurred on the day of trauma, and all strokes occurred within 15 days.6 The epidemiology of hemorrhagic stroke and cerebral sinus venous thrombosis (CSVT) in trauma is also not well studied.
Figure 1:
Simplified schematic of traumatic cerebrovascular injuries contributing to pediatric stroke.
Categories of pediatric stroke due to vascular injury in non-penetrating trauma
Blunt arterial cerebrovascular injury
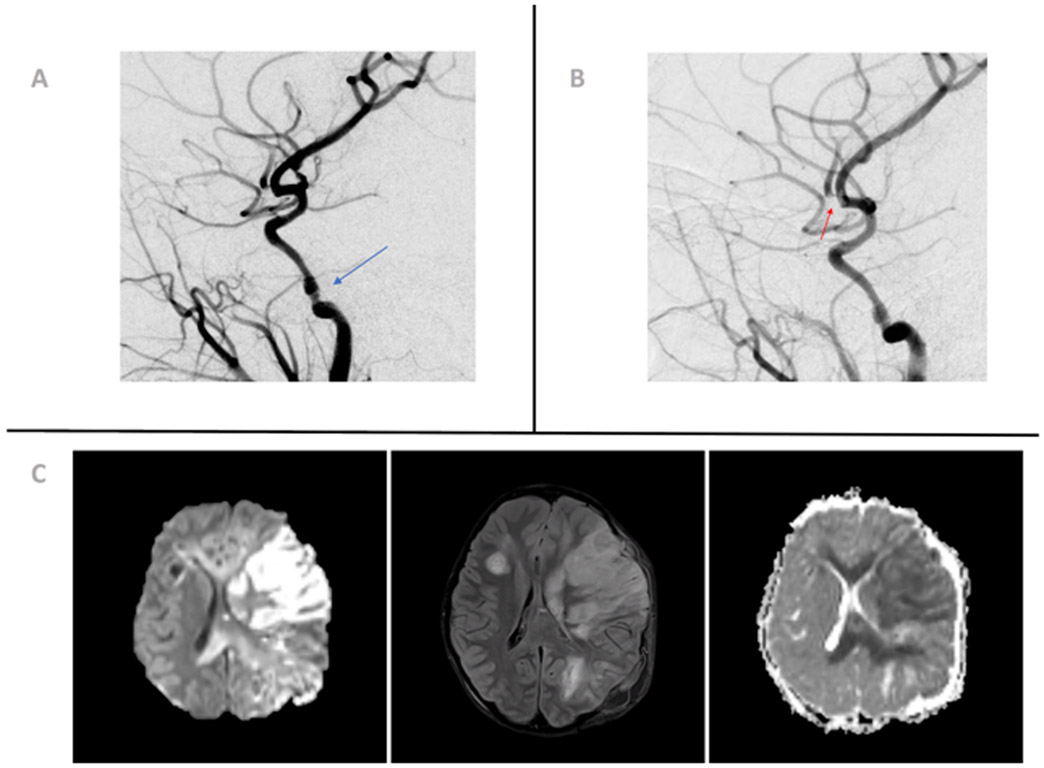
Blunt cerebrovascular injuries (BCVI) to the carotid and vertebral arteries in the setting of blunt trauma include primarily arterial dissections and intramural hematomas, but also potentially more severe injuries such as occlusions, pseudoaneurysms, transections, and carotid/cavernous sinus fistulas.7 For the purposes of this review, we will consider blunt trauma to be all trauma that is non-penetrating and non-thermal. Intramural hematomas and arterial dissections occur when there is disruption of the intima that allows blood to enter the arterial wall with the potential risk of narrowing its caliber leading to stenosis or occlusion. An arterial pseudoaneurysm is a false aneurysm caused by partial rupture of the arterial wall, often after a dissection, or by total rupture of the arterial wall followed by hemorrhage that is ultimately contained by perivascular tissues or thrombus. Arterial transection can lead to blood extravasation and is associated with high morbidity and mortality.8 Arteriovenous fistulas occur when an artery ruptures into a vein, often resulting into impaired venous drainage or, less likely, ischemia via vascular steal. The most widely accepted classification system in BCVI has been proposed by Biffl et al, which grades vessel injury on a 5-level grading scale, from irregularity in vessel wall to arterial transection.9 The incidence of BCVI has been reported to be 0.03-1.3% in children with blunt trauma 10-14 and 3.5% in pediatric traumatic brain injury (TBI)15. Motor vehicle collision is the most prevalent mechanism of injury.11 Figure 2 illustrates and example of BCVI in a patient who was an unrestrained passenger in a motor vehicle collision.
Figure 2:
Angiography and magnetic resonance imaging of a 10-year-old girl presenting after high-speed rollover motor vehicle accident with significant head trauma. (A) Angiographic image showing a 40% narrowing of left internal carotid artery petrous segment (blue arrow), consistent with a Biffl grade 2 injury (dissection or intramural hematoma ≥25% luminal narrowing). (B) Angiographic image showing filling defect in the left middle cerebral artery M2 anterior division (red arrow), most consistent with focal partially occlusive thrombus. (C) Diffusion-weighted imaging, apparent diffusion coefficient, and fluid-attenuated inversion recovery sequences showing extensive cytotoxic edema with associated diffusion restriction involving the left frontoparietal lobe in the middle cerebral artery territory consistent with a recent infarct. The color version of this figure is available in the online edition.
Craniocervical arterial dissection is the most prevalent type of BCVI7, and among the most common cerebral arteriopathies associated with pediatric arterial ischemic stroke16. Over half of all cases of pediatric craniocervical arterial dissection are associated with trauma to the head and neck16-18. In up to 25% of cases, trauma can be minimal such as low impact falls and participation in contact sports16. Though rare, intraoral trauma has also been associated with carotid artery dissection in the pediatric population19, 20. Interestingly, intracranial anterior circulation arterial dissections are common in the pediatric population and relatively uncommon in adults, especially when they are not preceded by significant trauma21. When the posterior circulation is involved, extracranial dissections are more common21.
Though digital subtraction angiography remains the gold-standard, some authors suggest that computed tomographic angiogram (CTA) and magnetic resonance angiogram (MRA) are replacing it as the first-line diagnostic test of choice for pediatric BCVI22-26. While there are established screening strategies in adults 27, 28, ambiguity remains regarding when to screen for pediatric BCVI.10 Several groups have attempted to identify the best scoring systems to predict risk for BCVI in children, whether it is applying adult scoring systems or creating new ones.10-12, 15, 29-31 Table 1 compares proposed pediatric BCVI screening, including the Eastern Association for the Surgery of Trauma criteria, the Denver criteria, the Memphis criteria, the Utah score, and the McGovern score.27-31 In a recent retrospective cohort study of pediatric trauma patients with confirmed BCVI, a comparison between three of the aforementioned scores (EAST, Utah, and Denver) revealed that the Denver criteria appears to have the lowest false negative rate, missing only 2% of patients.10 However, some have expressed concerns the Denver criteria could lead to overscreening in children who may have potential long-term risks from unnecessary radiation exposure.29 In another recent retrospective study, the mechanism of injury was added to the Utah score criteria yielding the McGovern score, which has improved sensitivity compared to EAST, Denver, Memphis, and Utah scoring systems.31 In pediatric blunt cranial trauma, fractures of the petrous temporal bone (carotid canal), focal neurological deficit, stroke, and a GCS score of < 8 have been described as independent risk factors for BCVI.15, 32
Table 1:
Comparison of screening criteria for BCVI that have been used in the pediatric population. These guidelines and scoring systems encompass different risk factors with the goal of aiding in the decision-making process of when to pursue vessel imaging to screen for BCVI. Please refer to references for specifics on each of the different scores, this table attempts to visually display the risk factors included in them.
| EAST27 | Denver28 | Memphis30 | Utah29 | McGovern31 | |
|---|---|---|---|---|---|
| Neurologic abnormality unexplained by imaging | x | x | x | ||
| Focal neurologic deficit | x | x | x | ||
| GCS score of 8 or less | x | x | x | ||
| Lefort II or III facial fractures | x | x | x | ||
| Petrous bone fracture | x | x | x | ||
| Cervical spine fracture | x | x | x | ||
| Stroke on imaging | x | x | x | ||
| Carotid canal fracture | x | x | |||
| Blunt trauma and epistaxis | x | ||||
| Neck/nose/mouth arterial hemorrhage | x | ||||
| Anisocoria | x | ||||
| Cervical bruit | x | ||||
| Diffuse axonal injury | x | ||||
| Basilar skull fracture | x | ||||
| Mechanism of injury (motor-vehicle or automobile-pedestrian accident) | x | ||||
| High energy mechanism with associated featuresa | x |
Mandible fracture, complex skull fracture/basilar skull fracture/occipital condyle fracture, DAI and GCS score of less than 6, near- hanging with anoxic brain injury, clothesline type injury, scalp degloving, TBI with thoracic injury, thoracic vascular injury, blunt cardiac rupture
In children with confirmed BCVI, an ischemic cerebral vascular accident (CVA) is diagnosed in 18%-27% of cases, with one recent retrospective study showing that approximately half of the strokes are present on admission and the remainder occur later during the hospital course.10, 11 Patients with BVCI are frequently started on aspirin, but often in the setting of trauma there are contraindications to both anti-platelet and anticoagulation agents such as concurrent intracranial hemorrhage.10, 11
Cerebral sinovenous thrombosis
Cerebral sinovenous thrombosis (CSVT) may occur in the setting of blunt trauma in children, particularly with concomitant skull fractures.33-39 In patients with skull fractures undergoing dedicated venous imaging, CVST occurs in 20-30% of cases 33, 40. Different proposed mechanisms of etiology include compression of the sinus by adjacent hematomas, obstruction or laceration of the dural sinuses by depressed bony fracture fragments, intramural hemorrhage, injury to the endothelial lining, or thrombus extension from injured emissary veins.39
Clinical findings suspicious for CSVT in a pediatric patient with blunt trauma should lead to further investigation with dedicated venous imaging. Symptoms have been reported up to 5 days after initial trauma, and may either be new symptoms or worsening of existing neurologic symptoms41. Gait ataxia, vomiting, and headache are among the most commonly reported symptoms, but seizures, decreased mental status and focal neurologic deficits may also occur.33, 34, 41 In a trauma patient, headache and vomiting are often nonspecific, with multiple plausible etiologies. In addition to clinical symptoms, CT or MRI findings may prompt further dedicated venous imaging.33 The presence of a skull fracture close to a venous sinus is a common indication for dedicated venous imaging in pediatric blunt trauma,33 but other suspicious radiological findings in non-contrast head CT include high density signal in either cortical veins42, dural sinuses43, or torcular herophili 44; or specific patterns of intraparenchymal hemorrhage and edema. In head CT with contrast (e.g., CT venography), filling defects in the dural venous sinuses or absent cortical veins are indicative of CSVT.41, 45
Mineralizing angiopathy and basal ganglia stroke in minor trauma
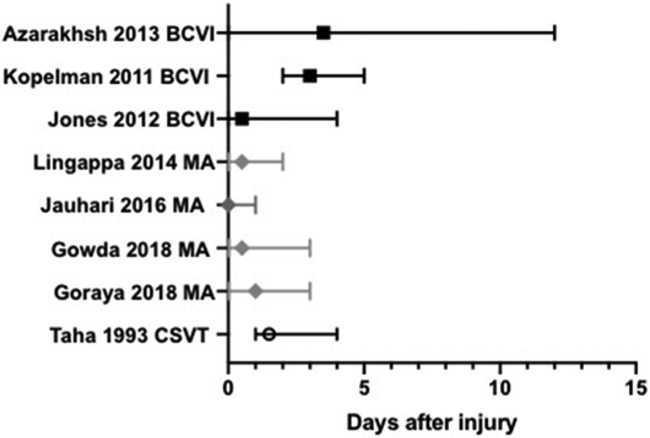
Minor head trauma may cause basal ganglia stroke in young children in the presence of mineralizing angiopathy.46-50 The mean age of presentation is 11-14 months 46, 47, 49, 50 and the majority (63-72%) are boys.46, 49, 50 The most common clinical presentation is acute hemiparesis, although seizures and hemidystonia have also been described 46-49. Onset of symptoms occurs immediately or within first 8 hours after traumatic event in the majority of cases, but hemidystonia can start even up to 48 hours after event (Figure 3). The reported head trauma associated with mineralizing angiopathy often appears to be trivial, such as falling while attempting to stand or walk or rolling off furniture.46, 49
Figure 3:
Timing of stroke or thrombosis occurrence relative to trauma. Median and range shown for reported cases11-13, 41, 46, 47, 49, 50. BCVI = blunt cerebrovascular injury, MA = mineralizing angiopathy, CSVT= cerebral sinovenous thrombosis
The etiology for mineralization of lenticulostriate arteries is not fully known, but may be associated with remote or occult viral infection, such as cytomegalovirus or Epstein-Barr virus.51 It may occur in up to 2% of neonatal intensive care unit patients,52 although most patients reported to have stroke with minor trauma and mineralizing angiopathy are reported to be previously healthy. Cranial sonography may best define abnormal lenticulostriate arteries in infants with open anterior fontanel,46 but mineralizing angiopathy is also suggested by the presence of basal ganglia or lenticulostriate calcification on CT.46, 48, 49 A proposed mechanism of ischemia is that abnormal lenticulostriate vessels are more vulnerable to occlusion by vasospasm or thrombosis following head injury, thus leading to basal ganglia stroke.46, 53 Stroke recurrence has been reported in up to 20% of patients, including contralateral strokes.47, 49, 51 Outcome is typically favorable in those with isolated, unilateral events whereas infants with recurrent, bilateral events tend to have long-term disability46-49.
Abusive head trauma and ischemic stroke
Pediatric abusive head trauma (AHT) is defined as an injury to the skull or intracranial contents of an infant or young child less than 5 years of age secondary to inflicted blunt impact and/or violent shaking 54. It is the leading cause of serious head injury in infants54, 55. The annual incidence of inflicted TBI has been reported to be 24.6- 32.2 per 100,000 children younger than 1 year old 56-58, with infants having a higher incidence than children in their second year of life 57
Studies report Ischemic stroke to be more prevalent in children with AHT than those with accidental head injuries 59, 60, with a reported stroke incidence of 27-28% among children with suspected AHT 54, 60. Strokes in the setting of AHT are more frequently bilateral, multifocal, and associated with overlying subdural hematoma54. If subdural hematomas are present in a child with traumatic intracranial hemorrhage, the final history is more likely to suggest abuse than accidental trauma..61 For strokes occurring in the setting of subdural hematomas, the mechanism is also unclear but likely involves contribution from elevated ICP either locally or globally. When compared to children with AHT without strokes, those with AHT and strokes have more severe injury, longer hospital stays and greater need for neurosurgical interventions54. It is thought that the pathophysiology of stroke in these patients is related to a global oxygen delivery deficit, due to hypoperfusion and/or hypoxia in the setting of hypotension, prolonged apnea, seizures, vasospasm, or choking mechanism of injury in addition to head impact or shaking 54. When traumatic brain injury from AHT causes refractory intracranial hypertension, this can lead to posterior cerebral artery (PCA), anterior cerebral artery (ACA) and superior cerebellar artery (SCA) infarctions 54.
Hemorrhagic stroke
Following trauma, hemorrhagic stroke occurs through direct brain injury from contusions and small vessel rupture, hemorrhagic conversion of an ischemic stroke, or penetrating trauma causing vascular injury. The direct ischemic effects of extra-axial hemorrhage, such as subdural and epidural hematomas in the setting of trauma are not well studied. Furthermore, intracranial hemorrhage may also occur as a consequence of a primary vascular event such as subarachnoid hemorrhage from a ruptured aneurysm. In young children, intracranial hemorrhage may raise suspicion for AHT, as abuse contribute to up to 30% of all traumatic ICH patients under 3 years of age.62 In hemorrhagic strokes in children aged 29 days to 19 years, head or neck trauma was a risk factor present in 4.6% of cases versus none of a control cohort.63 Undiagnosed cerebral aneurysms and traumatic pseudoaneurysms may also cause hemorrhage.
Tranexamic acid (TXA) is a synthetic lysine derivative that inhibits fibrinolysis. Adult studies found TXA decreases mortality risk of traumatic bleeding 64, 65 although its applicability across all trauma centers remains controversial66. There are not current recommendations for use in pediatric trauma. Although there is evidence of safety and effectiveness in children undergoing surgical procedure 67, 68, there is also evidence of increased risk of seizures in children and adults 69, 70 . TXA crosses the blood brain barrier, and may lower seizure threshold through inhibition of gamma-aminobutyric acid (GABA) or glycine receptors, particularly at higher doses66, 71-73. Therefore, TXA safety in children with potentially higher risk of seizures from stroke or traumatic brain injury is unknown. There is currently an ongoing pilot trial that will be the first randomized clinical trial to evaluate the use of TXA in children with severe trauma and hemorrhage, including hemorrhagic brain injury74.
Diagnosis and Management
Diagnosis
The neurodiagnostic approach in a pediatric patient with polytrauma starts with a brief assessment of neurologic function at the end of the primary survey that is repeated during the secondary survey.75 Altered level of consciousness may be due to direct head trauma, as well as global hypoxia or hypoperfusion. Focal neurologic deficits on exam are more suspicious for a focal neurologic injury including BCVI with or without ischemic stroke, intraparenchymal hemorrhage or CVST but may not necessarily be present on initial evaluation in all etiologies. Thus, detailed neurologic examinations throughout the trauma hospitalization are key to early stroke diagnosis. This often is most easily implemented by consultation of providers specializing in the neurologic examination of a child (pediatric neurosurgeons or pediatric neurologists), who can follow the neurologic exam closely for detection of subtle changes, particularly in high risk patients.
Non-contrast head CT is the recommended initial neuroimaging test for patients with suspected head trauma. This study allows timely evaluation for life-threatening complications that might require emergent intervention such as diffuse cerebral edema, ICH with significant mass effect, depressed skull fracture, and hydrocephalus. In patients where BCVI is suspected, CTA of head and neck should be pursued27. The different diagnostic criteria previously described can aid in deciding when screening is warranted.
Neuroimaging should be strongly considered in patients presenting with a focal neurologic deficit or decreased level of consciousness that does not improve with initial medical management, or any patient that develops new neurologic symptoms or signs while hospitalized, even if initial neuroimaging was performed. Mirsky et al provide suggestions for optimal neuroimaging investigation of different stroke types in children the acute setting76, and Nash and Rafay summarize recent literature of different modalities for specifically diagnosing pediatric arterial dissection16 Head CT may demonstrate new ischemia, hemorrhage, or edema; brain MRI can more sensitively assess for and these and other abnormalities, such as microhemorrhages. Further investigation with dedicated venous imaging (MRV/CTV) should be considered when there are clinical and/or radiological findings suggestive of CSVT33. Notably, patients with polytrauma sometimes have delayed access to MRI if there are competing priorities in their care, particularly if their respiratory or hemodynamic status is unstable.
Management
The primary goals for neuroprotection in acute stroke are to optimize cerebral oxygen delivery to prevent tissue within the penumbra from becoming irreversibly injured and prevent complications and new brain injury. Some goals, such as avoiding hypoglycemia and treating fever and seizures, should not have major barriers to implementation, regardless of other comorbid injuries. Other goals, such as allowing for permissive hypertension or starting anticoagulation may be less straightforward in the setting of polytrauma. It is impractical to list all potential injuries with competing priorities, therefore the remainder of this review will focus on the underlying guiding physiologic principles, highlighting a few areas where evidence may have competing interest in order to aid the multidisciplinary conversations among pediatric neurologists, surgeons, and intensivists for the individual care of each patient.
Cerebral perfusion pressure is the pressure gradient between the mean arterial pressure and intracranial pressure (CPP = MAP-ICP). There is evidence in adult patients with acute ischemic stroke that laying the head of bed flat may increase blood flow, particularly ipsilateral to the stroke.77, 78 For this reason, published guidelines for management of acute stroke in children recommend laying head of bed flat.40 However, elevated head of bed has been credited with decreased aspiration and ventilator-associated pneumonia in intubated patients 79. A large randomized trial in adults did not demonstrate significant improvement in outcomes with head of bed flat 80, and elevating the head of bed to 30 degrees may lower ICP in head-injured patients without significant net effects on cerebral perfusion or blood flow 81. Therefore, in trauma patients, particularly those requiring intubation, elevated head of bed to 30 degrees is preferred positioning, even among those with acute ischemic stroke.
Cerebral oxygen delivery is the product of cerebral blood flow and arterial oxygen content. Cerebral blood flow, measured as the local bulk flow rate of blood per unit weight of brain tissue, is tightly regulated by brain metabolism under normal conditions and may be influenced by blood pressure, cardiac output, arterial levels of oxygen and carbon dioxide, and vessel compliance. Normotension and even permissive hypertension are recommended as acute ischemic stroke blood pressure targets. However, these blood pressure targets may not be feasible if patients present with concomitant hemorrhage. Systematic reviews of traumatic hemorrhagic shock found that permissive hypotension was associated with lower 30-day mortality 82, 83, however it is important to note that none of these studies were pediatric - specific. In hemorrhagic stroke, aggressive normotension is preferred, however in the setting of TBI with increased ICP, goal CPP targets are taken into account.
Arterial oxygen content is dependent on the amount of hemoglobin and the percentage of hemoglobin saturated with oxygen. Therefore, attention to both anemia and oximetry is warranted, as arterial oxygen content is more reflective of brain oxygenation than pulse oximetry alone. Although oxygenation is beneficial for all tissues, the ventilator strategy required to maintain normal oxygenation may impact cerebral perfusion. Intubated patients with traumatic pneumothorax or pneumomediastinum may benefit from a low volume lung strategy to minimize air leak, however this often requires higher mean airway pressure, via higher positive end expiratory pressure (PEEP) or use of high frequency oscillatory ventilation. Higher PEEP and mean airway pressure may increase intracranial pressure by decreasing venous return, particularly if pressure is near the inflection point of the intracranial volume/pressure curve. Chen et al.’s recent review describes the physiology of interactions between positive-pressure ventilation and cerebral oxygenation and perfusion.84 Similarly, normocapnia is a goal for acute ischemic stroke, particularly if other head trauma or elevated ICP are present. However, lung injury or aspiration leading to pediatric acute respiratory distress syndrome may occur in polytraumatic injuries, particularly those with lower Glasgow Coma Scores on arrival, impeding this goal.85, 86 Permissive hypercapnia is recommended to minimize ventilator-induced lung injury and improve lung outcomes, although recommendations do note that permissive hypercapnia should generally be avoided in the presence of intracranial hypertension.87 We recommend multidisciplinary discussion of the severity of known lung and brain injury, relative degree of hypercapnia and intracranial hypertension, and alternative options to mitigate lung injury or intracranial hypertension to decide an individual’s target carbon dioxide goal in the presence of competing interests.
The risks and benefits of antithrombotic therapy (antiplatelet therapy or anticoagulation) in a polytrauma patient are difficult to quantify. Children with vasculopathy, including those with arterial dissections and other blunt cerebrovascular injuries, are among the highest risk populations for stroke recurrence, particularly within the first three months.88 Anticoagulation or antiplatelet therapy may reduce stroke risk after BCVI in adults89 although studies are limited to retrospective reviews and thus it is unclear how the factors contributing to the decision to not start therapy influenced patients’ stroke risk8, 16. In the randomized Cervical Artery Dissection in Stroke Study (CADISS), no difference was found in the efficacy of either antiplatelet or anticoagulant drugs at preventing stroke and death in patients with symptomatic extracranial craniocervical dissection, but the incidence of stroke recurrence was rare in both treatment groups90. Data for antithrombotic therapy are less clear in pediatric trauma.10 Table 2 highlights studies and stroke incidence with antithrombotic therapy in BCVI pediatric patients. Furthermore, while the risk of stroke appears highest in the first 2 weeks after BCVI, optimal duration of antithrombotic therapy is unknown.
Table 2:
Antithrombotic treatment and stroke rates in blunt cerebrovascular injury (BCVI)
| Cohort | # total trauma patients |
# patients with vascular injury |
Antithrombotic treatment |
Antithrombotic w stroke |
Not on antithrombotic w stroke |
|---|---|---|---|---|---|
| Azarakhsh 201313 | 5826 | 19 | 5 aspirin 2 low molecular weight heparin |
0/7 (0%) | 2/12 (17%) |
| Cook 201810 | 7440 | 96 | 57 aspirin 5 anticoagulation |
6/57 on aspirin (11%) | 3/34 (9%) |
| Jones 201212 | 14991 | 45 | 44 heparin (aspirin or anticlopidigrel may be substituted at attending discretion, exact number not specified) | 1/44 (2%) | 1/1 (100%) |
| Kopelman 201111 | 1209 | 11 | 2 aspirin | 0/2 (0%) | 3/9 (30%) |
Per the American Heart Association scientific statement on perinatal and childhood stroke, anticoagulation is the mainstay of treatment of pediatric CVST.24 Not treating with anticoagulation has been correlated with a higher risk of thrombus propagation, venous infarction, hemorrhage, and worse outcome91. Although venous hemorrhage caused by CSVT is not a contraindication to anticoagulation, patients with traumatic CSVT may have additional hemorrhages not associated with CSVT, such as an epidural hematoma or bleeding at other sites, or require frequent surgical interventions, which sometimes complicates therapeutic approaches. Whether or not benefits of anticoagulation outweigh risks in traumatic CSVT in children is not well-studied, but small case series suggest that other intracranial hemorrhage may be present in up to 50% of cases 40, and that infarction may still occur despite therapy 33. There have also been small case series showing no major complications of anticoagulation therapy, even in the presence of concomitant ICH,92 as well as spontaneous recanalization in children with traumatic CVST that did not receive anticoagulation.39 It is important to note that the goal of anticoagulation in CSVT is preventing clot propagation, to promote intrinsic process of thrombus resolution. Thus, factors to consider when weighing clinical risks and benefits include thrombus characteristics (i.e. occlusive vs non-occlusive) and location (i.e. proximity of transverse sinus clot propagating into sagittal sinus) and severity of associated neurological impairment. For patients requiring urgent recanalization, or whom fail or cannot receive anticoagulation, endovascular thrombolysis may be considered 93, 94.
Special considerations for deciding management strategies include monitoring for complications of treatment (i.e assess for compartment syndrome and internal bleeding, repeat neuroimaging to identify new ischemia or intracerebral clot propagation), consideration of anticoagulation reversal or other hemorrhage control measures (i.e if patient is on aspirin, what is the quantity of available platelets to transfuse if needed or would bleeding occur at compressible or addressable site), and potential further procedures needed to address other injuries.
Conclusion
Awareness of stroke and daily detailed neurologic examinations in pediatric trauma patients, particularly those with head and neck injuries or BCVI, can promote early stroke identification allowing teams to optimize management. Trauma patients may have concomitant injuries with competing priorities for strategies of care, but thoughtful consideration of underlying pathophysiology can help teams navigate and prioritize care that is individual to each patient.
Acknowledgements
KPG is supported by NINDS K23 NS099472.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Murphy SL, Mathews TJ, Martin JA, Minkovitz CS, Strobino DM. Annual summary of vital statistics: 2013–2014. Pediatrics. 2017;139 [DOI] [PubMed] [Google Scholar]
- 2.Mallick AA, Ganesan V, Kirkham FJ, Fallon P, Hedderly T, McShane T, et al. Diagnostic delays in paediatric stroke. J Neurol Neurosurg Psychiatry. 2015;86:917–921 [DOI] [PubMed] [Google Scholar]
- 3.Rafay MF, Pontigon AM, Chiang J, Adams M, Jarvis DA, Silver F, et al. Delay to diagnosis in acute pediatric arterial ischemic stroke. Stroke. 2009;40:58–64 [DOI] [PubMed] [Google Scholar]
- 4.Roehler DR, Batra EK, Quinlan KP. Comparing the risk of sudden unexpected infant death to common causes of childhood injury death. J Pediatr. 2019 [DOI] [PubMed] [Google Scholar]
- 5.Ravindra VM, Dewan MC, Akbari H, Bollo RJ, Limbrick D, Jea A, et al. Management of penetrating cerebrovascular injuries in pediatric trauma: A retrospective multicenter study. Neurosurgery. 2017;81:473–480 [DOI] [PubMed] [Google Scholar]
- 6.Fox CK, Hills NK, Vinson DR, Numis AL, Dicker RA, Sidney S, et al. Population-based study of ischemic stroke risk after trauma in children and young adults. Neurology. 2017;89:2310–2316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller PR, Fabian TC, Bee TK, Timmons S, Chamsuddin A, Finkle R, et al. Blunt cerebrovascular injuries: Diagnosis and treatment. J Trauma. 2001;51:279–285; discussion 285-276 [DOI] [PubMed] [Google Scholar]
- 8.Biffl WL, Ray CE Jr., Moore EE, Franciose RJ, Aly S, Heyrosa MG, et al. Treatment-related outcomes from blunt cerebrovascular injuries: Importance of routine follow-up arteriography. Ann Surg. 2002;235:699–706; discussion 706-697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biffl WL, Moore EE, Offner PJ, Brega KE, Franciose RJ, Burch JM. Blunt carotid arterial injuries: Implications of a new grading scale. J Trauma. 1999;47:845–853 [DOI] [PubMed] [Google Scholar]
- 10.Cook MR, Witt CE, Bonow RH, Bulger EM, Linnau KF, Arbabi S, et al. A cohort study of blunt cerebrovascular injury screening in children: Are they just little adults? J Trauma Acute Care Surg. 2018;84:50–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kopelman TR, Berardoni NE, O'Neill PJ, Hedayati P, Vail SJ, Pieri PG, et al. Risk factors for blunt cerebrovascular injury in children: Do they mimic those seen in adults? J Trauma. 2011;71:559–564; discussion 564 [DOI] [PubMed] [Google Scholar]
- 12.Jones TS, Burlew CC, Kornblith LZ, Biffl WL, Partrick DA, Johnson JL, et al. Blunt cerebrovascular injuries in the child. Am J Surg. 2012;204:7–10 [DOI] [PubMed] [Google Scholar]
- 13.Azarakhsh N, Grimes S, Notrica DM, Raines A, Garcia NM, Tuggle DW, et al. Blunt cerebrovascular injury in children: Underreported or underrecognized?: A multicenter atomac study. J Trauma Acute Care Surg. 2013;75:1006–1011; discussion 1011-1002 [DOI] [PubMed] [Google Scholar]
- 14.Lew SM, Frumiento C, Wald SL. Pediatric blunt carotid injury: A review of the national pediatric trauma registry. Pediatr Neurosurg. 1999;30:239–244 [DOI] [PubMed] [Google Scholar]
- 15.Ravindra VM, Riva-Cambrin J, Sivakumar W, Metzger RR, Bollo RJ. Risk factors for traumatic blunt cerebrovascular injury diagnosed by computed tomography angiography in the pediatric population: A retrospective cohort study. J Neurosurg Pediatr. 2015;15:599–606 [DOI] [PubMed] [Google Scholar]
- 16.Nash M, Rafay MF. Craniocervical arterial dissection in children: Pathophysiology and management. Pediatr Neurol. 2019;95:9–18 [DOI] [PubMed] [Google Scholar]
- 17.Rafay MF, Armstrong D, Deveber G, Domi T, Chan A, MacGregor DL. Craniocervical arterial dissection in children: Clinical and radiographic presentation and outcome. J Child Neurol. 2006;21:8–16 [DOI] [PubMed] [Google Scholar]
- 18.Pandey AS, Hill E, Al-Holou WN, Gemmete JJ, Chaudhary N, Thompson BG, et al. Management of pediatric craniocervical arterial dissections. Childs Nerv Syst. 2015;31:101–107 [DOI] [PubMed] [Google Scholar]
- 19.Bent C, Shen P, Dahlin B, Coulter K. Blunt intraoral trauma resulting in internal carotid artery dissection and infarction in a child. Pediatr Emerg Care. 2016;32:534–535 [DOI] [PubMed] [Google Scholar]
- 20.Chamoun RB, Mawad ME, Whitehead WE, Luerssen TG, Jea A. Extracranial traumatic carotid artery dissections in children: A review of current diagnosis and treatment options. J Neurosurg Pediatr. 2008;2:101–108 [DOI] [PubMed] [Google Scholar]
- 21.Fullerton HJ, Johnston SC, Smith WS. Arterial dissection and stroke in children. Neurology. 2001;57:1155–1160 [DOI] [PubMed] [Google Scholar]
- 22.Eastman AL, Chason DP, Perez CL, McAnulty AL, Minei JP. Computed tomographic angiography for the diagnosis of blunt cervical vascular injury: Is it ready for primetime? J Trauma. 2006;60:925–929; discussion 929 [DOI] [PubMed] [Google Scholar]
- 23.Stence NV, Fenton LZ, Goldenberg NA, Armstrong-Wells J, Bernard TJ. Craniocervical arterial dissection in children: Diagnosis and treatment. Curr Treat Options Neurol. 2011;13:636–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferriero DM, Fullerton HJ, Bernard TJ, Billinghurst L, Daniels SR, DeBaun MR, et al. Management of stroke in neonates and children: A scientific statement from the american heart association/american stroke association. Stroke. 2019;50:e51–e96 [DOI] [PubMed] [Google Scholar]
- 25.Orlowski HLP, Kansagra AP, Sipe AL, Miller-Thomas MM, Vo KD, Goyal MS. Utility of ct angiography in screening for traumatic cerebrovascular injury. Clin Neurol Neurosurg. 2018;172:27–30 [DOI] [PubMed] [Google Scholar]
- 26.Malhotra A, Wu X, Kalra VB, Schindler J, Matouk CC, Forman HP. Evaluation for blunt cerebrovascular injury: Review of the literature and a cost-effectiveness analysis. AJNR Am J Neuroradiol. 2016;37:330–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bromberg WJ, Collier BC, Diebel LN, Dwyer KM, Holevar MR, Jacobs DG, et al. Blunt cerebrovascular injury practice management guidelines: The eastern association for the surgery of trauma. J Trauma. 2010;68:471–477 [DOI] [PubMed] [Google Scholar]
- 28.Burlew CC, Biffl WL, Moore EE, Barnett CC, Johnson JL, Bensard DD. Blunt cerebrovascular injuries: Redefining screening criteria in the era of noninvasive diagnosis. J Trauma Acute Care Surg. 2012;72:330–335; discussion 336-337, quiz 539 [DOI] [PubMed] [Google Scholar]
- 29.Ravindra VM, Bollo RJ, Sivakumar W, Akbari H, Naftel RP, Limbrick DD Jr., et al. Predicting blunt cerebrovascular injury in pediatric trauma: Validation of the "utah score". J Neurotrauma. 2017;34:391–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller PR, Fabian TC, Croce MA, Cagiannos C, Williams JS, Vang M, et al. Prospective screening for blunt cerebrovascular injuries: Analysis of diagnostic modalities and outcomes. Ann Surg. 2002;236:386–393; discussion 393-385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herbert JP, Venkataraman SS, Turkmani AH, Zhu L, Kerr ML, Patel RP, et al. Pediatric blunt cerebrovascular injury: The mcgovern screening score. J Neurosurg Pediatr. 2018;21:639–649 [DOI] [PubMed] [Google Scholar]
- 32.Imahara SD, Hopper RA, Wang J, Rivara FP, Klein MB. Patterns and outcomes of pediatric facial fractures in the united states: A survey of the national trauma data bank. J Am Coll Surg. 2008;207:710–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hersh DS, Shimony N, Groves ML, Tuite GF, Jallo GI, Liu A, et al. Pediatric cerebral venous sinus thrombosis or compression in the setting of skull fractures from blunt head trauma. J Neurosurg Pediatr. 2018;21:258–269 [DOI] [PubMed] [Google Scholar]
- 34.Awad AW, Bhardwaj R. Acute posttraumatic pediatric cerebral venous thrombosis: Case report and review of literature Surg neurol int. India; 2014:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barbati G, Dalla Monta G, Coletta R, Blasetti AG. Post-traumatic superior sagittal sinus thrombosis. Case report and analysis of the international literature. Minerva Anestesiol. 2003;69:919–925 [PubMed] [Google Scholar]
- 36.Erdogan B, Caner H, Aydin MV, Yildirim T, Kahveci S, Sen O. Hemispheric cerebrovascular venous thrombosis due to closed head injury. Childs Nerv Syst. 2004;20:239–242 [DOI] [PubMed] [Google Scholar]
- 37.Georgoulis G, Alexiou G, Prodromou N. Sigmoid sinus thrombosis as a sequela of head injury in children and its management. World Neurosurg. 2014;81:e7. [DOI] [PubMed] [Google Scholar]
- 38.Holzmann D, Huisman TA, Linder TE. Lateral dural sinus thrombosis in childhood. Laryngoscope. 1999;109:645–651 [DOI] [PubMed] [Google Scholar]
- 39.Huisman TA, Holzmann D, Martin E, Willi UV. Cerebral venous thrombosis in childhood. Eur Radiol. 2001;11:1760–1765 [DOI] [PubMed] [Google Scholar]
- 40.Rivkin MA, Saraiya PV, Woodrow SI. Sinovenous thrombosis associated with skull fracture in the setting of blunt head trauma. Acta Neurochir (Wien). 2014;156:999–1007; discussion 1007 [DOI] [PubMed] [Google Scholar]
- 41.Taha JM, Crone KR, Berger TS, Becket WW, Prenger EC. Sigmoid sinus thrombosis after closed head injury in children. Neurosurgery. 1993;32:541–545; discussion 545-546 [DOI] [PubMed] [Google Scholar]
- 42.Goldberg AL, Rosenbaum AE, Wang H, Kim WS, Lewis VL, Hanley DF. Computed tomography of dural sinus thrombosis. J Comput Assist Tomogr. 1986;10:16–20 [DOI] [PubMed] [Google Scholar]
- 43.Buonanno FS, Moody DM, Ball MR, Laster DW. Computed cranial tomographic findings in cerebral sinovenous occlusion. J Comput Assist Tomogr. 1978;2:281–290 [DOI] [PubMed] [Google Scholar]
- 44.Garetier M, Rousset J, Pearson E, Tissot V, Gentric JC, Nowak E, et al. Value of spontaneous hyperdensity of cerebral venous thrombosis on helical ct. Acta Radiol. 2014;55:1245–1252 [DOI] [PubMed] [Google Scholar]
- 45.Virapongse C, Cazenave C, Quisling R, Sarwar M, Hunter S. The empty delta sign: Frequency and significance in 76 cases of dural sinus thrombosis. Radiology. 1987;162:779–785 [DOI] [PubMed] [Google Scholar]
- 46.Goraya JS, Berry S, Saggar K, Ahluwalia A. Stroke after minor head trauma in infants and young children with basal ganglia calcification: A lenticulostriate vasculopathy? J Child Neurol. 2018;33:146–152 [DOI] [PubMed] [Google Scholar]
- 47.Gowda VK, Manjeri V, Srinivasan VM, Sajjan SV, Benakappa A. Mineralizing angiopathy with basal ganglia stroke after minor trauma: Case series including two familial cases. J Pediatr Neurosci. 2018;13:448–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Toelle SP, Avetisyan T, Kuyumjyan N, Sukhudyan B, Boltshauser E, Hackenberg A. Infantile basal ganglia stroke after mild head trauma associated with mineralizing angiopathy of lenticulostriate arteries: An under recognized entity. Neuropediatrics. 2018;49:262–268 [DOI] [PubMed] [Google Scholar]
- 49.Lingappa L, Varma RD, Siddaiahgari S, Konanki R. Mineralizing angiopathy with infantile basal ganglia stroke after minor trauma. Dev Med Child Neurol. 2014;56:78–84 [DOI] [PubMed] [Google Scholar]
- 50.Jauhari P, Sankhyan N, Khandelwal N, Singhi P. Childhood basal ganglia stroke and its association with trivial head trauma. J Child Neurol. 2016;31:738–742 [DOI] [PubMed] [Google Scholar]
- 51.Yang FH, Wang H, Zhang JM, Liang HY. Clinical features and risk factors of cerebral infarction after mild head trauma under 18 months of age. Pediatr Neurol. 2013;48:220–226 [DOI] [PubMed] [Google Scholar]
- 52.Makhoul IR, Eisenstein I, Sujov P, Soudack M, Smolkin T, Tamir A, et al. Neonatal lenticulostriate vasculopathy: Further characterisation. Arch Dis Child Fetal Neonatal Ed. 2003;88:F410–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nabika S, Kiya K, Satoh H, Mizoue T, Oshita J, Kondo H. Ischemia of the internal capsule due to mild head injury in a child. Pediatr Neurosurg. 2007;43:312–315 [DOI] [PubMed] [Google Scholar]
- 54.Khan NR, Fraser BD, Nguyen V, Moore K, Boop S, Vaughn BN, et al. Pediatric abusive head trauma and stroke. J Neurosurg Pediatr. 2017;20:183–190 [DOI] [PubMed] [Google Scholar]
- 55.Billmire ME, Myers PA. Serious head injury in infants: Accident or abuse? Pediatrics. 1985;75:340–342 [PubMed] [Google Scholar]
- 56.Barlow KM, Minns RA. Annual incidence of shaken impact syndrome in young children Lancet. England; 2000:1571–1572. [DOI] [PubMed] [Google Scholar]
- 57.Keenan HT, Runyan DK, Marshall SW, Nocera MA, Merten DF, Sinal SH. A population-based study of inflicted traumatic brain injury in young children. Jama. 2003;290:621–626 [DOI] [PubMed] [Google Scholar]
- 58.Ellingson KD, Leventhal JM, Weiss HB. Using hospital discharge data to track inflicted traumatic brain injury. Am J Prev Med. 2008;34:S157–162 [DOI] [PubMed] [Google Scholar]
- 59.Kemp AM, Jaspan T, Griffiths J, Stoodley N, Mann MK, Tempest V, et al. Neuroimaging: What neuroradiological features distinguish abusive from non-abusive head trauma? A systematic review. Arch Dis Child. 2011;96:1103–1112 [DOI] [PubMed] [Google Scholar]
- 60.Gilles EE, Nelson MD Jr. Cerebral complications of nonaccidental head injury in childhood. Pediatr Neurol. 1998;19:119–128 [DOI] [PubMed] [Google Scholar]
- 61.Amagasa S, Matsui H, Tsuji S, Uematsu S, Moriya T, Kinoshita K. Characteristics distinguishing abusive head trauma from accidental head trauma in infants with traumatic intracranial hemorrhage in japan. Acute Med Surg. 2018;5:265–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hettler J, Greenes DS. Can the initial history predict whether a child with a head injury has been abused? Pediatrics. 2003;111:602–607 [DOI] [PubMed] [Google Scholar]
- 63.Singhal NS, Hills NK, Sidney S, Fullerton HJ. Role of trauma and infection in childhood hemorrhagic stroke due to vascular lesions. Neurology. 2013;81:581–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Morrison JJ, Dubose JJ, Rasmussen TE, Midwinter MJ. Military application of tranexamic acid in trauma emergency resuscitation (matters) study. Arch Surg. 2012;147:113–119 [DOI] [PubMed] [Google Scholar]
- 65.Shakur H, Roberts I, Bautista R, Caballero J, Coats T, Dewan Y, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (crash-2): A randomised, placebo-controlled trial. Lancet. 2010;376:23–32 [DOI] [PubMed] [Google Scholar]
- 66.Ramirez RJ, Spinella PC, Bochicchio GV. Tranexamic acid update in trauma. Crit Care Clin. 2017;33:85–99 [DOI] [PubMed] [Google Scholar]
- 67.Faraoni D, Goobie SM. The efficacy of antifibrinolytic drugs in children undergoing noncardiac surgery: A systematic review of the literature. Anesth Analg. 2014;118:628–636 [DOI] [PubMed] [Google Scholar]
- 68.Faraoni D, Willems A, Melot C, De Hert S, Van der Linden P. Efficacy of tranexamic acid in paediatric cardiac surgery: A systematic review and meta-analysis. Eur J Cardiothorac Surg. 2012;42:781–786 [DOI] [PubMed] [Google Scholar]
- 69.Maeda T, Sasabuchi Y, Matsui H, Ohnishi Y, Miyata S, Yasunaga H. Safety of tranexamic acid in pediatric cardiac surgery: A nationwide database study. J Cardiothorac Vasc Anesth. 2017;31:549–553 [DOI] [PubMed] [Google Scholar]
- 70.Lin Z, Xiaoyi Z. Tranexamic acid-associated seizures: A meta-analysis. Seizure. 2016;36:70–73 [DOI] [PubMed] [Google Scholar]
- 71.Kratzer S, Irl H, Mattusch C, Burge M, Kurz J, Kochs E, et al. Tranexamic acid impairs gamma-aminobutyric acid receptor type a-mediated synaptic transmission in the murine amygdala: A potential mechanism for drug-induced seizures? Anesthesiology. 2014;120:639–649 [DOI] [PubMed] [Google Scholar]
- 72.Furtmuller R, Schlag MG, Berger M, Hopf R, Huck S, Sieghart W, et al. Tranexamic acid, a widely used antifibrinolytic agent, causes convulsions by a gamma-aminobutyric acid(a) receptor antagonistic effect. J Pharmacol Exp Ther. 2002;301:168–173 [DOI] [PubMed] [Google Scholar]
- 73.Lecker I, Wang DS, Romaschin AD, Peterson M, Mazer CD, Orser BA. Tranexamic acid concentrations associated with human seizures inhibit glycine receptors. J Clin Invest. 2012;122:4654–4666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nishijima DK, VanBuren J, Hewes HA, Myers SR, Stanley RM, Adelson PD, et al. Traumatic injury clinical trial evaluating tranexamic acid in children (tic-toc): Study protocol for a pilot randomized controlled trial. Trials. 2018;19:593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McFadyen JG, Ramaiah R, Bhananker SM. Initial assessment and management of pediatric trauma patients. Int J Crit Illn Inj Sci. 2012;2:121–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mirsky DM, Beslow LA, Amlie-Lefond C, Krishnan P, Laughlin S, Lee S, et al. Pathways for neuroimaging of childhood stroke. Pediatr Neurol. 2017;69:11–23 [DOI] [PubMed] [Google Scholar]
- 77.Wojner-Alexander AW, Garami Z, Chernyshev OY, Alexandrov AV. Heads down: Flat positioning improves blood flow velocity in acute ischemic stroke. Neurology. 2005;64:1354–1357 [DOI] [PubMed] [Google Scholar]
- 78.Olavarria VV, Arima H, Anderson CS, Brunser AM, Munoz-Venturelli P, Heritier S, et al. Head position and cerebral blood flow velocity in acute ischemic stroke: A systematic review and meta-analysis. Cerebrovasc Dis. 2014;37:401–408 [DOI] [PubMed] [Google Scholar]
- 79.Li Bassi G, Torres A. Ventilator-associated pneumonia: Role of positioning. Curr Opin Crit Care. 2011;17:57–63 [DOI] [PubMed] [Google Scholar]
- 80.Anderson CS, Arima H, Lavados P, Billot L, Hackett ML, Olavarria VV, et al. Cluster-randomized, crossover trial of head positioning in acute stroke. N Engl J Med. 2017;376:2437–2447 [DOI] [PubMed] [Google Scholar]
- 81.Feldman Z, Kanter MJ, Robertson CS, Contant CF, Hayes C, Sheinberg MA, et al. Effect of head elevation on intracranial pressure, cerebral perfusion pressure, and cerebral blood flow in head-injured patients. J Neurosurg. 1992;76:207–211 [DOI] [PubMed] [Google Scholar]
- 82.Albreiki M, Voegeli D. Permissive hypotensive resuscitation in adult patients with traumatic haemorrhagic shock: A systematic review. Eur J Trauma Emerg Surg. 2018;44:191–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tran A, Yates J, Lau A, Lampron J, Matar M. Permissive hypotension versus conventional resuscitation strategies in adult trauma patients with hemorrhagic shock: A systematic review and meta-analysis of randomized controlled trials. J Trauma Acute Care Surg. 2018;84:802–808 [DOI] [PubMed] [Google Scholar]
- 84.Chen H, Menon DK, Kavanagh BP. Impact of altered airway pressure on intracranial pressure, perfusion, and oxygenation: A narrative review. Crit Care Med. 2019;47:254–263 [DOI] [PubMed] [Google Scholar]
- 85.de Roulet A, Burke RV, Lim J, Papillon S, Bliss DW, Ford HR, et al. Pediatric trauma-associated acute respiratory distress syndrome: Incidence, risk factors, and outcomes. J Pediatr Surg. 2018 [DOI] [PubMed] [Google Scholar]
- 86.Killien EY, Mills B, Watson RS, Vavilala MS, Rivara FP. Risk factors on hospital arrival for acute respiratory distress syndrome following pediatric trauma. Crit Care Med. 2018;46:e1088–e1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rimensberger PC, Cheifetz IM. Ventilatory support in children with pediatric acute respiratory distress syndrome: Proceedings from the pediatric acute lung injury consensus conference. Pediatr Crit Care Med. 2015;16:S51–60 [DOI] [PubMed] [Google Scholar]
- 88.Fullerton HJ, deVeber GA, Hills NK, Dowling MM, Fox CK, Mackay MT, et al. Inflammatory biomarkers in childhood arterial ischemic stroke: Correlates of stroke cause and recurrence. Stroke. 2016;47:2221–2228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cothren CC, Biffl WL, Moore EE, Kashuk JL, Johnson JL. Treatment for blunt cerebrovascular injuries: Equivalence of anticoagulation and antiplatelet agents. Arch Surg. 2009;144:685–690 [DOI] [PubMed] [Google Scholar]
- 90.Markus HS, Hayter E, Levi C, Feldman A, Venables G, Norris J. Antiplatelet treatment compared with anticoagulation treatment for cervical artery dissection (cadiss): A randomised trial. Lancet Neurol. 2015;14:361–367 [DOI] [PubMed] [Google Scholar]
- 91.Moharir MD, Shroff M, Stephens D, Pontigon AM, Chan A, MacGregor D, et al. Anticoagulants in pediatric cerebral sinovenous thrombosis: A safety and outcome study. Ann Neurol. 2010;67:590–599 [DOI] [PubMed] [Google Scholar]
- 92.Xavier F, Komvilaisak P, Williams S, Kulkarni AV, deVeber G, Moharir MD. Anticoagulant therapy in head injury-associated cerebral sinovenous thrombosis in children. Pediatr Blood Cancer. 2014;61:2037–2042 [DOI] [PubMed] [Google Scholar]
- 93.Mortimer AM, Bradley MD, O'Leary S, Renowden SA. Endovascular treatment of children with cerebral venous sinus thrombosis: A case series. Pediatr Neurol. 2013;49:305–312 [DOI] [PubMed] [Google Scholar]
- 94.Lee SK, Mokin M, Hetts SW, Fifi JT, Bousser MG, Fraser JF. Current endovascular strategies for cerebral venous thrombosis: Report of the snis standards and guidelines committee. J Neurointerv Surg. 2018;10:803–810 [DOI] [PubMed] [Google Scholar]