Abstract
Introduction
Murine models provide evidence that maternal stress during pregnancy can influence placenta morphology and function, including altered expression of genes involved in the maintenance and progression of pregnancy and fetal development. Corresponding research evaluating the impact of maternal stress on placental gene expression in humans is limited. We examined maternal stress in relation to placental expression of 17 candidate genes in a community-based sample.
Methods
Participants included 60 mother-newborn pairs enrolled in the PRogramming of Intergenerational Stress Mechanisms pregnancy cohort based at the Mount Sinai Hospital in New York City. Placentas were collected immediately following delivery and gene expression was measured using a qPCR-based platform. Maternal experiences of traumatic and non-traumatic stress were measured using the Life Stressor Checklist-Revised (LSC-R) administered during a mid-pregnancy interview. We used multivariable linear regression to examine associations between LSC-R scores and expression of each gene in separate models in the sample overall and stratified by fetal sex.
Results
Higher maternal stress was associated with significantly increased placental expression of the nutrient sensor gene OGT, the glucose transporter gene GLUT1, and the hypoxia sensor gene HIF3A. In models stratified by fetal sex, significant associations remained only among males.
Discussion
This study represents one of the most comprehensive examinations of maternal lifetime traumatic and non-traumatic stress in relation to placental gene expression in human tissue. Our findings support that maternal stress may alter sex-specific placental expression of genes involved in critical developmental processes.
Keywords: stress, trauma, pregnancy, placenta, gene expression, mRNA
1. Introduction
Maternal stress has been linked to pregnancy complications, including problems related to placental morphology and function (e.g., pre-eclampsia), pregnancy loss, poor fetal growth, pre-term birth, delivery complications, and neonatal morbidity [1–3]. Evidence also supports that prenatal stress can program plastic fetal systems with long-term consequences for disease risk [4]. In addition, stress-related exposure to excessive glucocorticoids (i.e. cortisol in humans) has been shown to create lasting changes to brain morphology [5], alter offspring stress reactivity [6], attenuate immune system response to challenge [7] and shift the developmental trajectory of other key regulatory systems. Sex differences in fetal development and neonatal risks are also established, including in relation to maternal stress [8]. For example, in addition to a higher rate of miscarriage, boys are more likely to be stillborn, premature, and suffer delivery complications [9–11]. Sexually dimorphic programming of disease susceptibility is further supported by sex biases in the prevalence of many developmental and chronic diseases (e.g., autism [12], asthma [13], cardiovascular [14]). While the mechanisms underlying stress-induced fetal programming are not well elucidated, growing evidence supports a role for the placenta in the transmission of maternal stress to the fetus.
The placenta is a dynamic, sex-specific endocrine and immune-active organ that functions as a protective barrier and conduit for oxygen and nutrient exchange [15, 16]. The placenta can morphologically and functionally adapt to the environment and is poised to respond to changes in the maternal milieu given its position at the maternal-fetal interface [17]. For example, during pregnancy, the placenta integrates with the maternal hypothalamic pituitary adrenal (HPA) stress response axis to influence circulating maternal cortisol levels and regulate fetal exposure to cortisol [18]. Research largely conducted using murine models has shown that elevated maternal stress and/or glucocorticoid exposure can alter expression of placental growth factors, nutrient sensors, nutrient transporters, oxygen sensors, and immune-response molecules with downstream consequences for fetal development; however, corresponding research in humans is limited [19–26].
The placenta shares the sex of the fetus and sex differences in patterns of placental signaling in response to maternal stimuli, including gene expression, have been demonstrated in human and rodent tissue [27, 28]. The influence of prenatal stress on placental gene expression is less well delineated in humans. Here, we leveraged data from an ethnically diverse urban sample of pregnant women to examine maternal self-reported lifetime traumatic and non-traumatic stress ascertained using a standardized instrument in relation to sex-specific placental expression of 17 candidate genes involved in a range of key processes known to influence fetal development and previously found to be stress-sensitive in animal studies.
2. Methods
2.1. Pregnancy cohort
Participants included 60 mother-newborn pairs enrolled in the ongoing PRogramming of Intergenerational Stress Mechanisms (PRISM) pregnancy cohort between January 2016 and April 2019. Participants were recruited from the Mount Sinai Hospital prenatal clinic in New York City. Eligibility criteria included fluency in English or Spanish and age 18 years or older. Exclusion criteria included multiple gestation pregnancy, HIV positive status, intake of ≥7 alcoholic drinks per week prior to pregnancy or any alcohol following pregnancy, or a major documented health condition with the fetus identified during pregnancy or at delivery. Information on race/ethnicity, age, height, weight, education, smoking status, and mode of delivery was collected using standardized questionnaires administered during an in-person study visit conducted at enrollment and/or during the third trimester. Maternal exposure to cigarette smoke was defined as self-reported smoking during pregnancy or exposure to environmental tobacco smoke for one hour or more per week during pregnancy. Gestational age at birth was estimated from 1) last menstrual period, and 2) obstetrical estimates; if the discrepancy between the two sources was greater than two weeks, obstetrical estimates were used. Information on diagnosis with gestation-related health conditions (eclampsia, pre-eclampsia, gestational hypertension, gestational diabetes), chronic health conditions active during pregnancy (chronic hypertension, Types 1 diabetes, Type 2 diabetes) or infections requiring medical treatment during pregnancy (urinary tract or kidney infection, sexually transmitted infection, other infection) was abstracted from the mother’s electronic medical record. Written informed consent was obtained from all participants and all study procedures were approved by the institutional review board at the Icahn School of Medicine at Mount Sinai.
2.2. Maternal lifetime stress
We assessed maternal lifetime stress using the Life Stressor Checklist – Revised (LSC-R), which was administered by trained study staff during a mid-pregnancy, in-person interview. The LSC-R includes questions about the occurrence of 30 traumatic and non-traumatic stressful life events, including experiences with natural disasters, injury and illness, financial problems, violence, and other events. The measure is well suited for use with a pregnant sample as it has a focus on experiences relevant to women, such as abortion and sexual assault. For each endorsed event, the mother is also asked to rate how much the event affected her life in the previous year using a five-point intensity scale (1= “not at all or never” to 5= “extremely”). We summed the 5-point responses across the 30 events to create a score ranging from 0–150 that reflects the mother’s subjective rating of how much the endorsed stressors affected her life in the previous year.
2.3. Placenta collection and processing
Placenta samples were collected at delivery, positioned with the fetal side facing up, and biopsied (approximately 1–2 cm3) from each of four quadrants at the midpoint between the cord insertion site and the outer edge approximately 1–1.5 cm below the fetal membrane to avoid membrane contamination. The membrane was then removed and samples were cut into smaller pieces (approximately 0.1 cm3) before being placed into 5 ml of RNAlater™RNA Stabilization Reagent (Qiagen) and stored at −4°C for ≤24 h; excess RNAlater was then removed and samples were stored at −80°C.
2.4. Selection of candidate genes
We a priori selected 17 genes previously reported to be stress-sensitive in experimental animal studies (Table 1). These candidate genes play roles in nutrient sensing (OGT, mTOR, DEPTOR) [19, 22, 26, 29], nutrient transport and growth (GLUT1, GLUT3, GLUT4, SLC38A1, SLC38A2, IGF2, IGF2R) [19, 21, 23–25, 30], responses to hypoxia (HIF1A, HIF3A) [24], inflammation (IL6, IL1B) [20], and maternal-fetal stress transmission (HSD11B2, NR3C1, NR3C2) [19, 21, 23, 25, 30–33]).
Table 1.
Candidate placental genes.
| Symbol | Name | Function |
|---|---|---|
| OGT | O-Linked N-Acetylglucosamine Transferase | Glycosyltransferase |
| mTOR | Mammalian Target of Rapamycin | Cellular response to nutrient deprivation |
| DEPTOR | DEP Domain Containing mTOR Interacting Protein | Regulates mTOR signaling |
| GLUT1 | Solute Carrier Family 2 Member 1 | Glucose transporter |
| GLUT3 | Solute Carrier Family 2 Member 3 | Glucose transporter |
| GLUT4 | Solute Carrier Family 2 Member 4 | Glucose transporter (insulin-regulated) |
| IGF2 | Insulin Like Growth Factor 2 | Growth promoter |
| IGF2R | Insulin Like Growth Factor 2 Receptor | IGF2 receptor |
| SLC38A1 | Solute Carrier Family 38 Member 1 | Amino acid (glutamine) transporter |
| SLC38A2 | Solute Carrier Family 38 Member 2 | Amino acid transporter (sodium-dependent) |
| HIF1A | Hypoxia Inducible Factor 1 Subunit Alpha | Regulates hypoxia-inducible gene expression |
| HIF3A | Hypoxia Inducible Factor 3 Subunit Alpha | Regulates hypoxia-inducible gene expression |
| IL6 | Interleukin 6 | Pro-inflammatory cytokine |
| IL1B | Interleukin 1 Beta | Pro-inflammatory cytokine |
| HSD11B2 | Hydroxysteroid 11-Beta Dehydrogenase 2 | Conversion of cortisol to cortisone |
| NR3C1 | Nuclear Receptor Subfamily 3 Group C Member 1 | Glucocorticoid receptor |
| NR3C2 | Nuclear Receptor Subfamily 3 Group C Member 2 | Mineralocorticoid receptor |
2.5. RNA isolation and quantitative PCR
RNA was extracted from placental biopsies using the Maxwell simplyRNA Tissue Kit (Promega #AS1280) following the manufacturer’s instructions. Quantity and purity of extracted RNA was assessed with a Nanodrop spectrophotometer (Thermo Electron North America, Madison, WI, USA) and samples were stored at −80°C until use. Placental RNA was reverse transcribed using the RT2 First Strand Kit (Cat #33040, QIAGEN) following the manufacturer’s protocol and a custom RT2 Profiler PCR Array (QIAGEN) was carried out. The qPCR reactions were conducted on a LightCycler 480 II Instrument (Roche Diagnostics Corporation, Indianapolis, IN) using the following thermocycling conditions: an initial denaturation step at 95°C for 10 min, followed by 45 cycles at 95°C for 15 s, and 60°C at 1 min. All samples were assayed in triplicate and Ct values within a sample were averaged. Seven samples were removed due to quality control failure leaving a final sample size of 53 participants (28 females, 25 males). Differences in sample content were accounted for by normalizing the data against the geometric mean of standard housekeeping genes (ACTB, GAPDH); the housekeeping genes were not significantly associated with maternal stress (p=0.69) or fetal sex (p=0.45) as determined by linear regression. mRNA expression was determined using the relative quantification 2−ΔCt approach [34] and values were log2-transformed to achieve a normal distribution before statistical analyses.
2.6. Statistical analysis
We first ran t-tests to determine whether placental gene expression significantly varied by sex. We also examined whether expression varied by 1) maternal chronic or gestation-related health complications during pregnancy, or 2) maternal infection during pregnancy. Next, we considered the role of maternal stress by regressing mRNA level on LSC-R scores in the sample overall and stratified by sex. We controlled for multiple comparisons using the FDR adjustment approach. We log2-transformed LSC-R scores to achieve normal residuals, therefore, we interpret effect estimates as the percent change in expression per 10% increase in maternal stress (i.e. LSC-R score). We examined both unadjusted models and models adjusted for maternal age (continuous in years), race/ethnicity (Black vs. Hispanic vs. White/other), education (less than high school degree vs. high school degree or equivalent), pre-pregnancy BMI (continuous in kg/m2), cigarette smoke exposure during pregnancy (any versus none as previously defined), newborn gestational age at delivery (continuous in weeks), and mode of delivery (vaginal vs. cesarean section). All statistical analyses were conducted using SAS software (version 9.4 SAS Institute, Cary, N.C., USA) or RStudio (version 3.6.2, The R Foundation).
3. Results
3.1. Sample characteristics
Fetal sex was approximately evenly distributed by design (n=28, 53% females; n=25, 47% males). The average maternal age at delivery was 28 years and the majority of women were Black (60%) or Hispanic (30%). Approximately one third of mothers reported exposure to cigarette smoke during pregnancy and 40% had less than a high school education. The majority of mothers delivered vaginally (60%) and the average newborn gestational age was 39 weeks; five babies were born before 37 weeks gestation. The range of LSC-R scores was 1–51 and the median±interquartile range was 9±9. LSC-R scores were not significantly different between mothers carrying a male versus female (t(51)=0.98, p=0.33). Likewise, no covariate considered in analyses significantly varied by fetal sex (all p-value <0.05, Table 2).
Table 2.
Characteristics of the study sample. Numbers are mean±standard deviation or frequency (%).
| All (n=53, 100%) | Male (n=25, 47%) | Female (n=28, 53%) | P-valuea | |
|---|---|---|---|---|
| Maternal age (years) | 28.5±6.1 | 27.7±6.7 | 29.2±5.6 | 0.375 |
| Race/ethnicity | 0.820 | |||
| White/other | 5 (10) | 3 (13) | 2 (7) | |
| Black | 30 (60) | 12 (52) | 18 (67) | |
| Hispanic | 15 (30) | 8 (35) | 7 (26) | |
| Education | 0.610 | |||
| High school or less | 21 (40) | 9 (37) | 12 (43) | |
| More than high school | 31 (60) | 15 (63) | 16 (57) | |
| Pre-pregnancy body mass indexb | 27.0±9.2 | 25.8±7.7 | 29.7±13.4 | 0.081 |
| Cigarette smoke exposure | 19 (36) | 7 (28) | 12 (43) | 0.260 |
| Mode of delivery | 0.102 | |||
| Vaginal | 32 (60) | 18 (72) | 14 (50) | |
| Cesarean section | 21 (40) | 7 (28) | 14 (50) | |
| Gestational age at birth (weeks) | 38.7±2.3 | 38.9±2.3 | 38.5±2.4 | 0.474 |
| Life Stressor Checklist-Revised scoreb | 9.0±9.0 | 9.0±9.0 | 9.0±12.5 | 0.457 |
P-value are from t-tests (continuous variables) or Chi-Square tests (categorical variables) for differences by sex.
Values are median±interquartile range and p-value is from Wilcoxon Rank Sum test.
3.2. Impact of fetal sex on placental mRNA expression
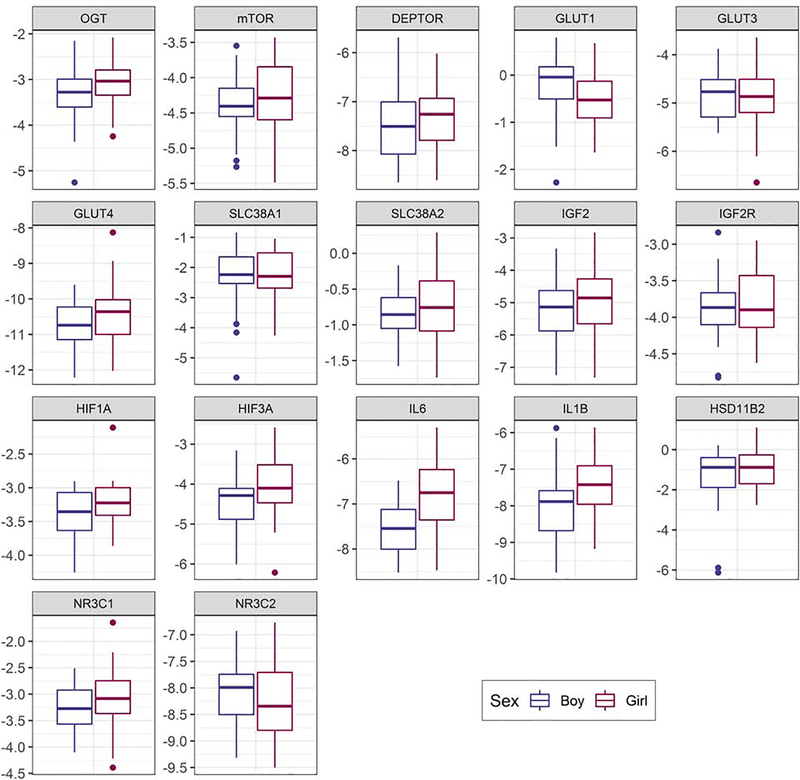
Figure 1 and Supplemental Table 1 present placental gene expression results stratified by sex. As illustrated, several genes showed basal differences (p<0.10) in expression, including: GLUT1 (p=0.073), IL6 (p=0.0002), IL1B (p=0.012), HIF1A (p=0.067), and HIF3A (p=0.059). In all cases except GLUT1, expression was higher among female compared to male placentas.
Figure 1.
Box and whisker plots of sex-specific placental expression of 17 genes. Boxes on the left (blue) represent male data and boxes on the right (pink) represent female data. The y-axis is log2 gene expression.
3.3. Impact of maternal health on placental mRNA expression
Supplemental Tables 2 and 3 present placental gene expression results stratified by whether the mother had: 1) a gestation-related or chronic health condition active during pregnancy (n=13), or 2) an infection requiring medical treatment during pregnancy (n=13). We observed no significant differences in expression of candidate placental genes by these factors.
3.3. Associations between maternal stress and placental mRNA expression
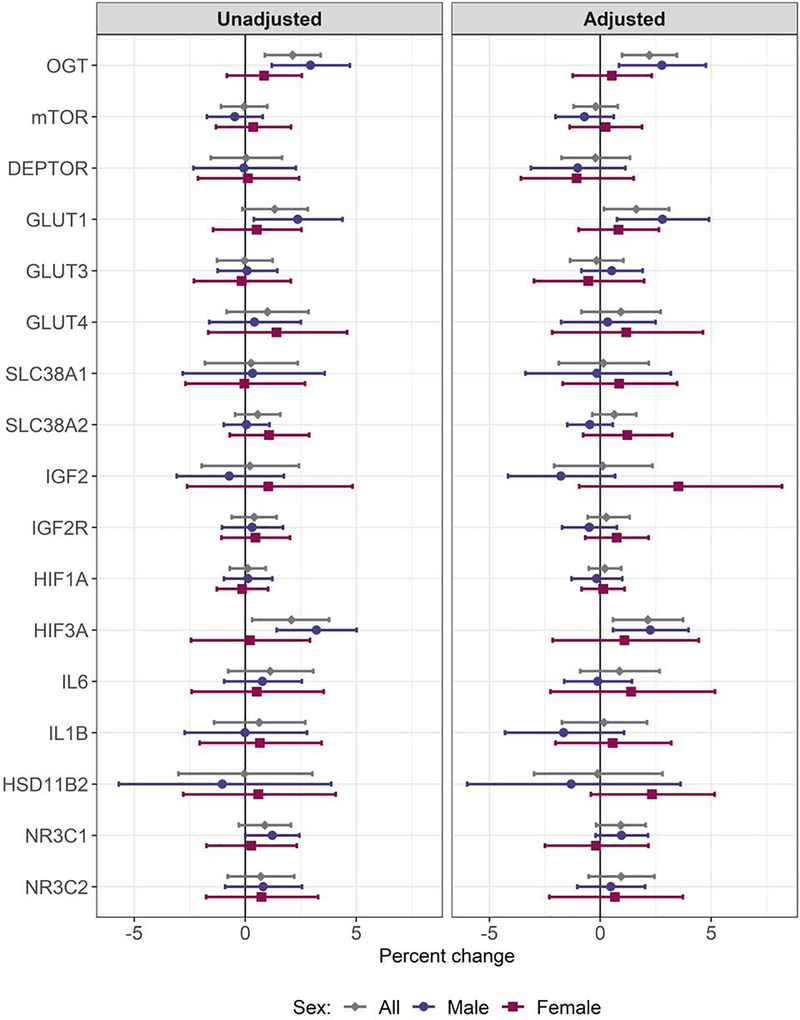
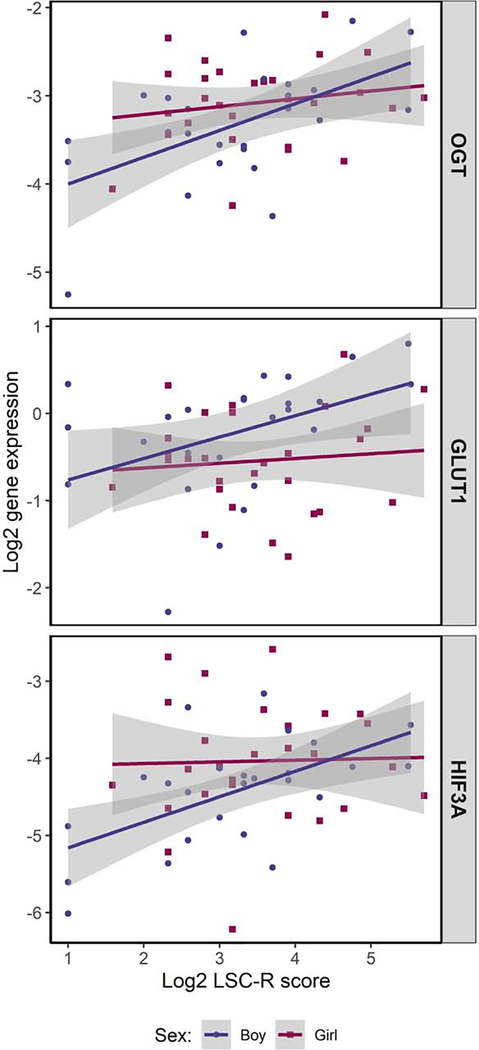
In the sample overall, higher maternal stress was associated with upregulation of OGT (unadjusted: percent change per 10% increase in LSC-R = 2.13%, nominal p-value: 0.0008, FDR p-value: 0.014; adjusted: 2.21%, nominal: 0.0004, FDR: 0.007) and HIF3A (unadjusted: 2.08%, nominal: 0.014, FDR: 0.122; adjusted: 2.14%, nominal: 0.007, FDR: 0.063); in adjusted models, we also observed a significant association with GLUT1 (1.62%, nominal: 0.029, FDR: 0.167). When considering models among male and female placentas separately, we observed significant associations only among male placentas. Specifically, among males higher maternal stress was associated with significantly higher placental expression of OGT (2.94%, nominal: 0.0009, FDR: 0.008), GLUT1 (2.37%, nominal: 0.018, FDR: 0.104), and HIF3A (3.21%, nominal: 0.0004, FDR: 0.007) in unadjusted models (Supplemental Table 4). Findings were similar in adjusted models: OGT (2.78%, nominal: 0.005, FDR: 0.049), GLUT1 (2.81%, nominal: 0.007, FDR: 0.049), and HIF3A (2.26%, nominal: 0.009, FDR: 0.049) (Supplemental Table 5). The unadjusted association with NR3C1 was marginally significant (1.22%, nominal: 0.051, FDR: 0.215), however, significance was lost after controlling for multiple comparisons, as well as covariates (0.96%, nominal: 0.108, FDR: 0.459). We did not observe significant associations between maternal stress and gene expression among female placentas; however, the relationship between maternal stress and placental gene expression diverged by sex for HSD11B2, IGF2 and IL1B. In these cases, higher stress was associated with a trend towards lower expression in males and higher expression in females. Figure 2 presents the percent change in placental gene expression per 10% increase in maternal LSC-R scores overall and stratified by fetal sex for each candidate gene and Figure 3 presents scatter plots of associations that reached statistical significance.
Figure 2.
Overall and sex-specific associations between maternal stress and expression of 17 candidate placental genes. The marker indicates the percent change in expression per 10% increase in stress score and bars reflect the 95% confidence limits.
Figure 3.
Scatter plots of maternal stress, as indicated by Life Stressor Checklist - Revised (LSC-R) scores, and placental expression of OGT, GLUT1, and HIF3A, stratified by fetal sex.
4. Discussion
We examined the relationship between maternal lifetime stress and placental expression of candidate genes previously shown to be stress-sensitive in experimental animal studies among a sample of women enrolled in an urban pregnancy cohort. Among male placentas, higher maternal stress was associated with significantly increased expression of placental OGT, GLUT1, and HIF3A, which play key roles in nutrient sensing, glucose transport, and cellular responses to hypoxia, respectively. We did not find significant associations between maternal stress and placental expression of any candidate genes among females. While previous research investigating these relationships in humans is limited, a number of prior studies have been conducted using murine models with mixed results. Our finding of an association between maternal stress and altered levels of placental OGT corroborates results from several recent studies conducted in mice and rats indicating OGT is stress sensitive [19, 22, 26, 35], however, the direction of effect has varied across species and by whether mRNA or protein levels were considered. Similarly, two previous studies conducted in rats have shown GLUT1 to be sensitive to stressors administered late during pregnancy, however, while these studies identified a negative association, we found increased maternal stress was associated with upregulated GLUT1 [19, 23]. It is notable that the association we observed was among male placentas only, whereas prior research in rats has not considered sex specific relationships. Less research has examined maternal stress in relation to placental expression of HIF hypoxia sensors. Consistent with our findings, the only prior study to investigate this relationship found male, but not female, placentas of mice exposed to chronic variable stress during pregnancy showed a significant upregulation of HIF3A [24]. The following discussion provides more detail on each of the candidate genes selected and compares our findings to previously conducted research.
Nutrient sensors
OGT is a widely expressed enzyme that catalyzes the post-translational modification of target proteins with a single moiety of N-acetyl glucosamine (N-GlcNAc) through O-linked glycosylation (O-GlcNAcylation), which alters target protein stability, degradation, and transcriptional activity [36]. In the placenta, O-GlcNAcylation is important for nutrient sensing, growth signaling, and regulation of oxygen tension, among other functions [37]. OGT is X-linked and in murine models it has been shown to escape X-inactivation, resulting in placental levels that are twice as high in females compared to males. One previous study measured OGT in human placenta tissue (n=4) enriched for fetal (XY) or maternal (XX) contributions and found expression was lower among XY samples [22]. Our results are consistent with this finding, however, the difference in expression between male and female placentas was not statistically significant. Previous research (Supplemental Table 6) examining dams exposed to corticosterone during pregnancy via an implanted pump found OGT protein levels were significantly higher among male, but not female, placentas of treated compared to control mice [26]. Yet, other research conducted using murine models has found placental Ogt is downregulated following maternal exposure to chronic daily stress [22] or physiological stressors [19] during pregnancy. Targeted deletion of placental Ogt in mice has also been shown to recapitulate offspring phenotypes characteristic of prenatal stress exposure [35].
In contrast to the significant association we detected between stress and OGT in male placentas, we did not find a relationship between maternal stress and mTOR or DEPTOR expression. mTOR and DEPTOR are components of a key nutrient-sensing signaling pathway that is thought to facilitate supply and demand relationships between mother and fetus [38, 39]. Research in rodents has shown this pathway, which is in part regulated by the IGF system, is stress-sensitive [29], however, we are not aware of other research in humans that has investigated these associations (Supplemental Table 6).
Nutrient transporters
Glucose and amino acids are the primary energy substrates utilized by the developing fetus and are shuttled across trophoblast and endothelial cell membranes by glucose (GLUT1, GLUT3, GLUT4) and amino acid transporters (SLC38A1, SLC38A2), which are in part modulated by the IGF system. GLUT1 is the primary isoform involved in placental glucose transport throughout pregnancy and is considered rate limiting [40]. GLUT3 is a high-affinity isoform that is thought to enable sufficient glucose uptake early during pregnancy, when placenta circulation is incomplete and glucose concentrations in poorly-exchanging extracellular fluids are limited [41]. GLUT4 is insulin responsive during the first trimester and is hypothesized to enhance placental glucose uptake in response to insulin signals of maternal nutrient surplus [41]. Prior research in humans and rodents suggests the GLUT transporter family is particularly sensitive to disruption by adverse conditions, including dietary deficiencies and psychosocial stress [23, 24]. We found a significant association between maternal stress and increased expression of GLUT1 among male placentas; however, we did not detect associations with GLUT3 or GLUT4 among either sex. Glucose transporters are regulated in part by glucocorticoids and previous research supports that altered expression of glucose transporters following cortisol exposure may mediate the well-established association between maternal stress and reduced fetal growth [42]. For example, administration of synthetic glucocorticoids to pregnant dams has been shown to result in a dose-dependent decline in fetal weight and a dose-dependent increase in Glut1 and Glut3 expression [43]. It is plausible that the increased placental GLUT1 expression we observed in relation to stress reflects a reflexive increase in fetal glucose availability to counter the growth restricting effects of excessive stress-related cortisol exposure. Previous studies conducted in rodents have reported a mix of positive and negative associations between maternal stress and expression of glucose transporters (Supplemental Table 6). In a study of male rats, restraint stress during late pregnancy was associated with significantly decreased Glut1, but increased Glut3 and Glut4 expression, compared to control males [23]. In a separate study, placentas from male, but not female, mice showed increased Glut4 expression in response to chronic variable stress [24]. In contrast, physiological stress during pregnancy has been associated with reduced Glut1, Glut2, and Glut4 mRNA levels in rats, however, sex-specific associations were not examined [19].
While we did not detect significant associations between maternal stress and SLC38A1 or SLC38A2, in sex-stratified models we observed a positive trend between stress and expression of these amino acid transporters among female placenta only. This is in contrast to a previous mouse study that detected an inverse association between physiological stress and Slc38a2 levels in male and female placentas considered together [19]. We also did not detect significant associations between maternal stress and expression of IGF2 or its receptor IGF2R, which play important roles in regulating nutrient transport and have previously been identified as sex-differential and stress-sensitive, albeit with mixed results across studies [19, 21, 25]. In one previous human study, maternal anxiety and depressed mood, correlates of psychosocial stress, were associated with significantly increased female placental expression of IGF2 [30]. Maternal depressed mood was also associated with increased IGF2R expression among female placentas [30].
Hypoxia sensors
We detected a significant positive association between maternal stress and HIF3A expression among male placentas; we did not detect a significant association with HIF1A among either sex. Prior to complete development of the functioning placenta, pregnancy take place under low-oxygen conditions that promote trophoblast differentiation. As pregnancy progresses, connections with the maternal system are established that enable improved oxygen transfer. HIF1A and HIF3A play key roles in regulating development across this shifting oxygen gradient. Specifically, in the early hypoxic uterine environment, HIF1A and HIF3A are stable and regulate hypoxia-responsive target genes that promote cell survival. Under normoxic conditions, as occurs later during pregnancy, HIFs are rapidly degraded and transcriptional activity is lost. Our finding of higher HIF3A expression at term among higher stress placentas may reflect an underlying relationship between stress and increased hypoxia. In turn, hypoxia during later pregnancy has been associated with adverse pregnancy outcomes, including fetal growth restriction and low birthweight [44]. Elevated levels of placental HIFs beyond the first trimester have also been associated with pre-eclampsia, pregnancy loss, and fetal growth restriction [45], with limited research supporting sexually dimorphic relationships [46]. In the only other study to our knowledge that has investigated expression of HIF3A in placenta tissue, mice exposed to chronic variable stress early during pregnancy showed significant upregulation of Hif3a among male, but not female, placenta [24].
Pro-inflammatory cytokines
Despite extensive research supporting that stress can precipitate immune system changes, including activation of pro-inflammatory cytokines, we did not detect significant associations between maternal stress and placental expression of IL6 or IL1B. This is consistent with a previous mouse study that did not detect significant associations between chronic variable stress administered during pregnancy and Il6 [24], but in contrast to a second study that detected increased Il6 and Il1b among male placentas from dams exposed to chronic variable stress during early pregnancy only [20]. Notably, we did find that levels of IL6 and IL1B were significantly higher among female compared to male placentas, supporting that differences in immune responses may contribute to sex-biased pregnancy complications and later disease susceptibility.
Stress-response system
Maternal cortisol binds to placental glucocorticoid (NR3C1) and mineralocorticoid (NR3C2) receptors, which translocate into the nucleus and act as transcription factors to control development of fetal systems and promote intra-uterine growth [47]. Cortisol can passively diffuse across the placenta, however, fetal exposure is limited by the action of HSD11B2, which catalyzes the conversion of glucocorticoids to inactive metabolites (i.e. cortisol to cortisone) [48]. We did not detect significant associations between maternal stress and these (HSD11B2, NR3C1, or NR3C2) components of the placenta stress response system, although the association with NR3C1 among males approached significance. Additionally, while associations with HSD11B2 were not significant, the direction of effect diverged by sex, such that higher stress was associated with decreased expression among males, but increased expression among females. Several studies conducted using murine models have shown that stress down regulates Hsd11b2 expression [19, 23, 25, 31], yet other research has failed to detect significant associations [21, 49] and only two studies have examined sex-specific effects with conflicting results [23, 25]. In humans, prenatal negative life events have been linked to decreased placental HSD11B2 expression among white women, but not among Black or Hispanic women. Several other studies have examined maternal symptoms of anxiety and/or depression in relation to placental HSD11B2 expression with mixed results [30, 32, 50–52] (Supplemental Table 7).
Less research has examined stress in relation to NR3C1 and NR3C2 expression. In rodents, physical stress during pregnancy has been associated with decreased expression of both receptors [19], whereas, direct cortisol administration has been associated with increased Nr3c1 expression among males, but not females [21]. This finding is consistent with the positive, albeit marginally significant, association we detected between maternal stress and NR3C1 expression among males only. In humans, anxious and/or depressive symptomology has been associated with increased placental NR3C1 and NR3C2 expression [33, 50]; although the only study reporting sex-specific associations found an inverse relationship among females [30] (Supplemental Table 7).
Strengths and Limitations
This study has a number of strengths. Foremost, the majority of prior research in this area has examined the impact of stress on placental gene expression in murine models, for which considerable differences exist in placenta morphology and function, including differences in the stress barrier [17]. For example, the cortisol inactivating enzyme HSD11B2 ceases to be produced in the murine placenta around mid-gestation, but is highly expressed in humans throughout pregnancy [53]. Additionally, while the majority of previous studies that have been conducted in humans have examined only 1–4 genes, we were able to consider a wider range of genes with roles that span several important developmental processes. We evaluated maternal stress using a comprehensive instrument that considers a breadth of traumatic and non-traumatic stressful experiences and evaluates both objective and perceived domains. It is also notable that our study sample is diverse, with the majority of mother-infant pairs self-reporting Black or Hispanic race/ethnicity. Despite bearing a greater burden of stress exposure, minority women remain understudied in much biomedical and public health research [54].
Some limitations are also worth noting. As with most human research, placenta samples were collected at term, thus we were unable to evaluate whether the impact of maternal stress on placenta gene expression varies across the course of pregnancy or is heightened during gestational windows corresponding to the development of specific prenatal systems. Notably, recent research examining extracellular vesicles of placental origin and their cargo, which can be isolated from maternal blood throughout pregnancy, provides an emerging avenue that may enable consideration of mid-gestational placental functioning in future studies [55]. Importantly, the findings in this study are based on a limited sample size. It will be critical for future studies to validate the results reported here in larger samples. Future research is also needed to better understand the mechanisms, such as the role of epigenetic programming, through which maternal stress influences gene expression.
5. Conclusion
We found maternal history of traumatic and non-traumatic stress was associated with sex-specific changes in placental expression of key nutrient sensors, nutrient transporters, and regulators of oxygen tension. These intra-uterine adaptations may play a role in long-term programming effects and contribute to the sex bias observed in many developmental and chronic diseases. Understanding the mechanisms by which potentially modifiable factors, such as stress, program the propensity for future disease could inform strategies for improving pregnancy outcomes and developmental trajectories.
Supplementary Material
Highlights.
Placental expression of GLUT1, IL6, IL1B, HIF1A, and HIF3A is sex-differential.
Maternal stress is associated with placental upregulation of OGT, GLUT1 and HIF3A.
When considering sex, significant associations remain only among males.
The placenta may be a key determinant of sexually dimorphic prenatal programming.
Acknowledgements
We thank Dr. Qian Li and Yula Ma for assistance conducting the gene expression assay.
Funding
This work was supported by the National Institutes of Health [grant numbers: P30 ES023515, R01 HL114396, R01 HL095606, and UG3 OD023337]. During the preparation of this manuscript, WJC was supported by T32 HD049311.
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Cook N, Ayers S, Horsch A, Maternal posttraumatic stress disorder during the perinatal period and child outcomes: A systematic review, J Affect Disord 225 (2018) 18–31. [DOI] [PubMed] [Google Scholar]
- [2].Dunkel Schetter C, Tanner L, Anxiety, depression and stress in pregnancy: implications for mothers, children, research, and practice, Curr Opin Psychiatry 25(2) (2012) 141–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Van den Bergh BRH, van den Heuvel MI, Lahti M, Braeken M, de Rooij SR, Entringer S, Hoyer D, Roseboom T, Raikkonen K, King S, Schwab M, Prenatal developmental origins of behavior and mental health: The influence of maternal stress in pregnancy, Neurosci Biobehav Rev (2017). [DOI] [PubMed] [Google Scholar]
- [4].Entringer S, Buss C, Wadhwa PD, Prenatal stress, development, health and disease risk: A psychobiological perspective-2015 Curt Richter Award Paper, Psychoneuroendocrinology 62 (2015) 366–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Buss C, Davis EP, Shahbaba B, Pruessner JC, Head K, Sandman CA, Maternal cortisol over the course of pregnancy and subsequent child amygdala and hippocampus volumes and affective problems, Proc Natl Acad Sci U S A 109(20) (2012) E1312–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Field T, Diego M, Hernandez-Reif M, Figueiredo B, Deeds O, Ascencio A, Schanberg S, Kuhn C, Comorbid depression and anxiety effects on pregnancy and neonatal outcome, Infant Behav Dev 33(1) (2010) 23–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Coe C, All roads lead to psychoneuroimmunology, in: Suls JM, Davidson KW, Kaplan RM (Eds.), Handbook of health psychology and behavioral medicine, Guilford Press, New York, 2010, pp. xv, 608 p. [Google Scholar]
- [8].Bruckner TA, Nobles J, Intrauterine stress and male cohort quality: the case of September 11, 2001, Soc Sci Med 76(1) (2013) 107–14. [DOI] [PubMed] [Google Scholar]
- [9].Engel PJ, Smith R, Brinsmead MW, Bowe SJ, Clifton VL, Male sex and pre-existing diabetes are independent risk factors for stillbirth, Aust N Z J Obstet Gynaecol 48(4) (2008) 375–83. [DOI] [PubMed] [Google Scholar]
- [10].O’Driscoll DN, McGovern M, Greene CM, Molloy EJ, Gender disparities in preterm neonatal outcomes, Acta Paediatr (2018). [DOI] [PubMed] [Google Scholar]
- [11].Vu HD, Dickinson C, Kandasamy Y, Sex Difference in Mortality for Premature and Low Birth Weight Neonates: A Systematic Review, Am J Perinatol 35(8) (2018) 707–715. [DOI] [PubMed] [Google Scholar]
- [12].Werling DM, Geschwind DH, Sex differences in autism spectrum disorders, Curr Opin Neurol 26(2) (2013) 146–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Shah R, Newcomb DC, Sex Bias in Asthma Prevalence and Pathogenesis, Front Immunol 9 (2018) 2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Mosca L, Barrett-Connor E, Wenger NK, Sex/gender differences in cardiovascular disease prevention: what a difference a decade makes, Circulation 124(19) (2011) 2145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Costa MA, The endocrine function of human placenta: an overview, Reprod Biomed Online 32(1) (2016) 14–43. [DOI] [PubMed] [Google Scholar]
- [16].Pavlov O, Pavlova O, Ailamazyan E, Selkov S, Characterization of cytokine production by human term placenta macrophages in vitro, Am J Reprod Immunol 60(6) (2008) 556–67. [DOI] [PubMed] [Google Scholar]
- [17].Rosenfeld CS, Sex-Specific Placental Responses in Fetal Development, Endocrinology 156(10) (2015) 3422–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Mastorakos G, Ilias I, Maternal and fetal hypothalamic-pituitary-adrenal axes during pregnancy and postpartum, Ann N Y Acad Sci 997 (2003) 136–49. [DOI] [PubMed] [Google Scholar]
- [19].Briffa JF, Hosseini SS, Tran M, Moritz KM, Cuffe JSM, Wlodek ME, Maternal growth restriction and stress exposure in rats differentially alters expression of components of the placental glucocorticoid barrier and nutrient transporters, Placenta 59 (2017) 30–38. [DOI] [PubMed] [Google Scholar]
- [20].Bronson SL, Bale TL, Prenatal stress-induced increases in placental inflammation and offspring hyperactivity are male-specific and ameliorated by maternal antiinflammatory treatment, Endocrinology 155(7) (2014) 2635–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cuffe JS, O’Sullivan L, Simmons DG, Anderson ST, Moritz KM, Maternal corticosterone exposure in the mouse has sex-specific effects on placental growth and mRNA expression, Endocrinology 153(11) (2012) 5500–11. [DOI] [PubMed] [Google Scholar]
- [22].Howerton CL, Morgan CP, Fischer DB, Bale TL, O-GlcNAc transferase (OGT) as a placental biomarker of maternal stress and reprogramming of CNS gene transcription in development, Proc Natl Acad Sci U S A 110(13) (2013) 5169–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Mairesse J, Lesage J, Breton C, Breant B, Hahn T, Darnaudery M, Dickson SL, Seckl J, Blondeau B, Vieau D, Maccari S, Viltart O, Maternal stress alters endocrine function of the feto-placental unit in rats, Am J Physiol Endocrinol Metab 292(6) (2007) E1526–33. [DOI] [PubMed] [Google Scholar]
- [24].Mueller BR, Bale TL, Sex-specific programming of offspring emotionality after stress early in pregnancy, J Neurosci 28(36) (2008) 9055–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Pankevich DE, Mueller BR, Brockel B, Bale TL, Prenatal stress programming of offspring feeding behavior and energy balance begins early in pregnancy, Physiol Behav 98(1–2) (2009) 94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Pantaleon M, Steane SE, McMahon K, Cuffe JSM, Moritz KM, Placental O-GlcNAc-transferase expression and interactions with the glucocorticoid receptor are sex specific and regulated by maternal corticosterone exposure in mice, Sci Rep 7(1) (2017) 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Clifton VL, Review: Sex and the human placenta: mediating differential strategies of fetal growth and survival, Placenta 31 Suppl (2010) S33–9. [DOI] [PubMed] [Google Scholar]
- [28].Gabory A, Roseboom TJ, Moore T, Moore LG, Junien C, Placental contribution to the origins of sexual dimorphism in health and diseases: sex chromosomes and epigenetics, Biol Sex Differ 4(1) (2013) 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Mparmpakas D, Zachariades E, Goumenou A, Gidron Y, Karteris E, Placental DEPTOR as a stress sensor during pregnancy, Clin Sci (Lond) 122(7) (2012) 349–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Mina TH, Raikkonen K, Riley SC, Norman JE, Reynolds RM, Maternal distress associates with placental genes regulating fetal glucocorticoid exposure and IGF2: Role of obesity and sex, Psychoneuroendocrinology 59 (2015) 112–22. [DOI] [PubMed] [Google Scholar]
- [31].Jensen Pena C, Monk C, Champagne FA, Epigenetic effects of prenatal stress on 11beta-hydroxysteroid dehydrogenase-2 in the placenta and fetal brain, PLoS One 7(6) (2012) e39791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].O’Donnell KJ, Bugge Jensen A, Freeman L, Khalife N, O’Connor TG, Glover V, Maternal prenatal anxiety and downregulation of placental 11beta-HSD2, Psychoneuroendocrinology 37(6) (2012) 818–26. [DOI] [PubMed] [Google Scholar]
- [33].Reynolds RM, Pesonen AK, O’Reilly JR, Tuovinen S, Lahti M, Kajantie E, Villa PM, Laivuori H, Hamalainen E, Seckl JR, Raikkonen K, Maternal depressive symptoms throughout pregnancy are associated with increased placental glucocorticoid sensitivity, Psychol Med 45(10) (2015) 2023–30. [DOI] [PubMed] [Google Scholar]
- [34].Livak KJ, Schmittgen TD, Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method, Methods 25(4) (2001) 402–8. [DOI] [PubMed] [Google Scholar]
- [35].Howerton CL, Bale TL, Targeted placental deletion of OGT recapitulates the prenatal stress phenotype including hypothalamic mitochondrial dysfunction, Proc Natl Acad Sci U S A 111(26) (2014) 9639–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Holt GD, Hart GW, The subcellular distribution of terminal N-acetylglucosamine moieties. Localization of a novel protein-saccharide linkage, O-linked GlcNAc, J Biol Chem 261(17) (1986) 8049–57. [PubMed] [Google Scholar]
- [37].Hart B, Morgan E, Alejandro EU, Nutrient sensor signaling pathways and cellular stress in fetal growth restriction, J Mol Endocrinol 62(2) (2019) R155–R165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Roos S, Powell TL, Jansson T, Placental mTOR links maternal nutrient availability to fetal growth, Biochem Soc Trans 37(Pt 1) (2009) 295–8. [DOI] [PubMed] [Google Scholar]
- [39].Jansson T, Powell TL, IFPA 2005 Award in Placentology Lecture. Human placental transport in altered fetal growth: does the placenta function as a nutrient sensor? -- a review, Placenta 27 Suppl A (2006) S91–7. [DOI] [PubMed] [Google Scholar]
- [40].Illsley NP, Glucose transporters in the human placenta, Placenta 21(1) (2000) 14–22. [DOI] [PubMed] [Google Scholar]
- [41].Illsley NP, Baumann MU, Human placental glucose transport in fetoplacental growth and metabolism, Biochim Biophys Acta Mol Basis Dis (2018) 165359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Hahn T, Barth S, Graf R, Engelmann M, Beslagic D, Reul JM, Holsboer F, Dohr G, Desoye G, Placental glucose transporter expression is regulated by glucocorticoids, J Clin Endocrinol Metab 84(4) (1999) 1445–52. [DOI] [PubMed] [Google Scholar]
- [43].Langdown ML, Sugden MC, Enhanced placental GLUT1 and GLUT3 expression in dexamethasone-induced fetal growth retardation, Mol Cell Endocrinol 185(1–2) (2001) 109–17. [DOI] [PubMed] [Google Scholar]
- [44].Jang EA, Longo LD, Goyal R, Antenatal maternal hypoxia: criterion for fetal growth restriction in rodents, Front Physiol 6 (2015) 176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Patel J, Landers K, Mortimer RH, Richard K, Regulation of hypoxia inducible factors (HIF) in hypoxia and normoxia during placental development, Placenta 31(11) (2010) 951–7. [DOI] [PubMed] [Google Scholar]
- [46].Hemmings DG, Williams SJ, Davidge ST, Increased myogenic tone in 7-month-old adult male but not female offspring from rat dams exposed to hypoxia during pregnancy, Am J Physiol Heart Circ Physiol 289(2) (2005) H674–82. [DOI] [PubMed] [Google Scholar]
- [47].Seckl JR, Meaney MJ, Glucocorticoid programming, Ann N Y Acad Sci 1032 (2004) 63–84. [DOI] [PubMed] [Google Scholar]
- [48].Benediktsson R, Calder AA, Edwards CR, Seckl JR, Placental 11 beta-hydroxysteroid dehydrogenase: a key regulator of fetal glucocorticoid exposure, Clin Endocrinol (Oxf) 46(2) (1997) 161–6. [DOI] [PubMed] [Google Scholar]
- [49].Welberg LA, Thrivikraman KV, Plotsky PM, Chronic maternal stress inhibits the capacity to up-regulate placental 11beta-hydroxysteroid dehydrogenase type 2 activity, J Endocrinol 186(3) (2005) R7–R12. [DOI] [PubMed] [Google Scholar]
- [50].Capron LE, Ramchandani PG, Glover V, Maternal prenatal stress and placental gene expression of NR3C1 and HSD11B2: The effects of maternal ethnicity, Psychoneuroendocrinology 87 (2018) 166–172. [DOI] [PubMed] [Google Scholar]
- [51].Ponder KL, Salisbury A, McGonnigal B, Laliberte A, Lester B, Padbury JF, Maternal depression and anxiety are associated with altered gene expression in the human placenta without modification by antidepressant use: implications for fetal programming, Dev Psychobiol 53(7) (2011) 711–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Seth S, Lewis AJ, Saffery R, Lappas M, Galbally M, Maternal Prenatal Mental Health and Placental 11beta-HSD2 Gene Expression: Initial Findings from the Mercy Pregnancy and Emotional Wellbeing Study, Int J Mol Sci 16(11) (2015) 27482–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Brown RW, Diaz R, Robson AC, Kotelevtsev YV, Mullins JJ, Kaufman MH, Seckl JR, The ontogeny of 11 beta-hydroxysteroid dehydrogenase type 2 and mineralocorticoid receptor gene expression reveal intricate control of glucocorticoid action in development, Endocrinology 137(2) (1996) 794–7. [DOI] [PubMed] [Google Scholar]
- [54].Konkel L, Racial and Ethnic Disparities in Research Studies: The Challenge of Creating More Diverse Cohorts, Environ Health Perspect 123(12) (2015) A297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Jin J, Menon R, Placental exosomes: A proxy to understand pregnancy complications, Am J Reprod Immunol 79(5) (2018) e12788. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.